The Use of Percutaneous Thermal Sensing Microchips for Body Temperature Measurements in Horses Prior to, during and after Treadmill Exercise
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Microchipping
2.3. Central Venous Temperature (TCV) Probe Insertion
2.4. Rectal Temperature (TR) Probe Placement
2.5. Data Collection
2.5.1. Exercise Program
2.5.2. Temperature Acquisition
2.5.3. Data Processing
2.6. Statistical Analysis
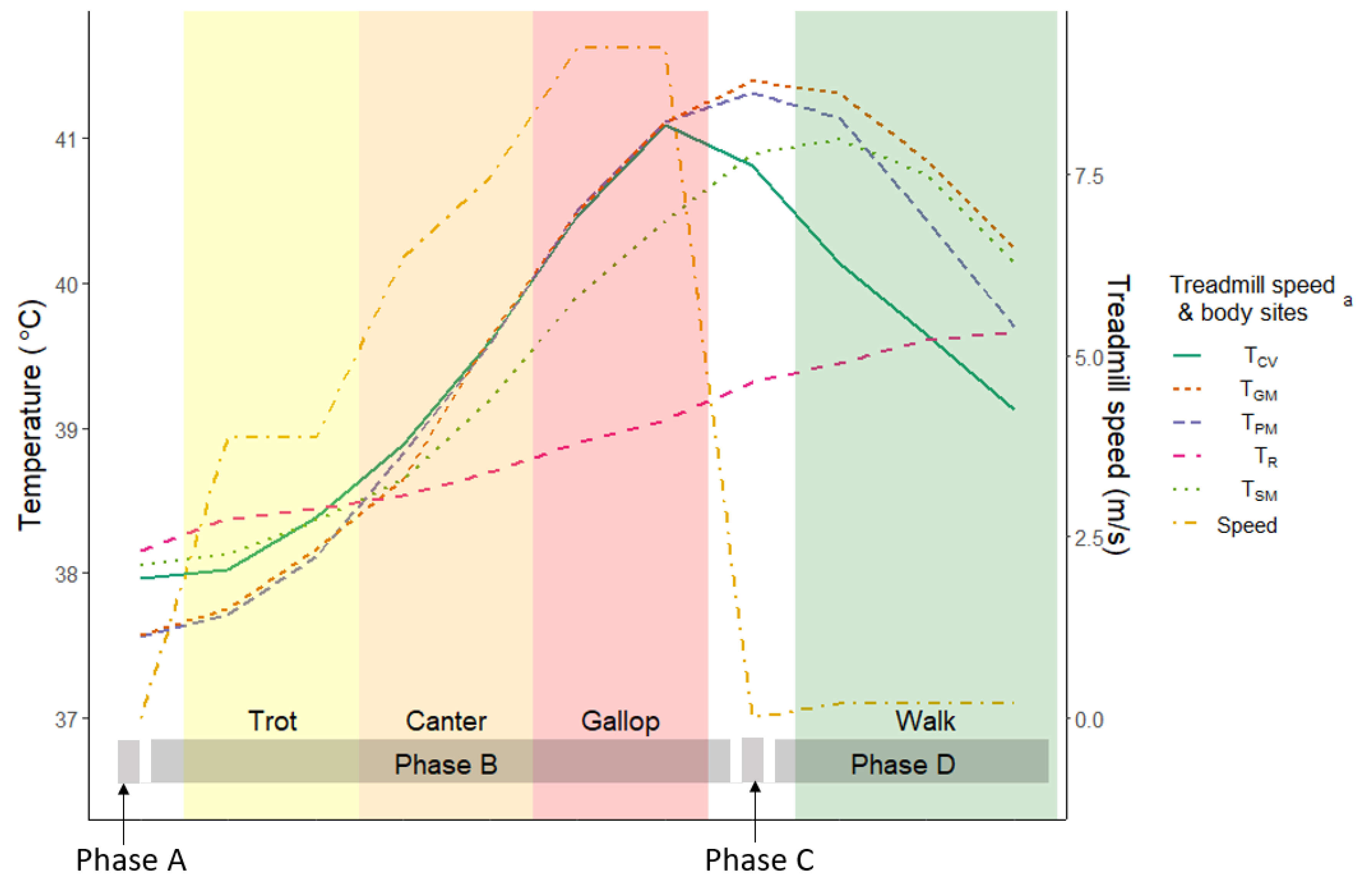
3. Results
3.1. Results of the Preliminary Study (n = 2)
3.2. Results of the Final Study (n = 8)
Body Temperatures and Its Paired Analysis
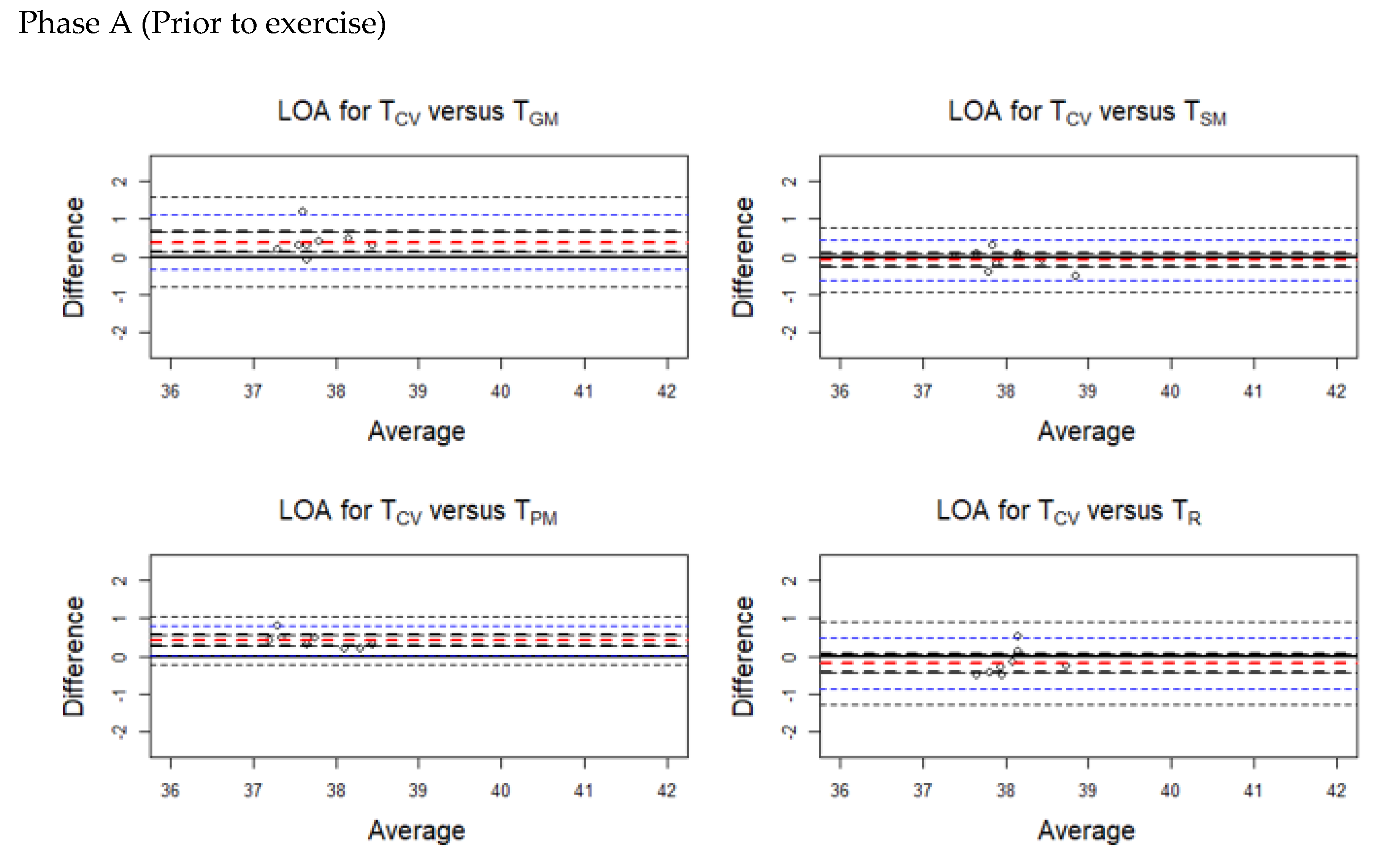
3.3. Results of Limit of Agreement (LOA)
3.4. Results of Repeated Measured Correlation (rmc) during Phases B and D
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Repeated-Measures Correlation Coefficient (rrm)
Appendix A.2. Linear Mixed-Effects Model
| Normality Test | ||
|---|---|---|
| Statistic | p-Value | |
| Phase A (Prior to exercise) | ||
| TCV | 0.969 | 0.893 |
| TPM | 0.904 | 0.316 |
| TGM | 0.984 | 0.981 |
| TSM | 0.929 | 0.506 |
| TR | 0.765 | 0.012 * |
| Phase B (Exercise on the treadmill) | ||
| TCV | 0.918 | 0.413 |
| TPM | 0.841 | 0.167 |
| TGM | 0.945 | 0.657 |
| TSM | 0.946 | 0.674 |
| TR | 0.958 | 0.795 |
| Phase C (Immediately after treadmill) | ||
| TCV | 0.93 | 0.512 |
| TPM | 0.941 | 0.621 |
| TGM | 0.942 | 0.633 |
| TSM | 0.792 | 0.023 * |
| TR | 0.983 | 0.975 |
| Phase D (Cool-down walk) | ||
| TCV | 0.921 | 0.438 |
| TPM | 0.914 | 0.385 |
| TGM | 0.943 | 0.637 |
| TSM | 0.941 | 0.618 |
| TR | 0.899 | 0.281 |
| All the data (Phases A–D) | ||
| TCV | 0.94 | 0.076 |
| TPM | 0.931 | 0.057 |
| TGM | 0.905 | 0.008 * |
| TSM | 0.94 | 0.077 |
| TR | 0.954 | 0.184 |
| LOA | Normality Test | ||||
|---|---|---|---|---|---|
| Mean Bias | LL | UL | Statistic | p-Value | |
| Phase A (Prior to exercise) | |||||
| TPM | 0.40 | 0.261 | 0.539 | 0.89 | 0.22 |
| TGM | 0.388 | 0.13 | 0.645 | 0.83 | 0.07 |
| TSM | −0.088 | −0.274 | 0.099 | 0.96 | 0.85 |
| TR | −0.195 | −0.438 | 0.048 | 0.87 | 0.14 |
| Phase B (Exercise on the treadmill) | |||||
| TPM | 0.199 | −0.058 | 0.297 | 0.95 | 0.72 |
| TGM | 0.076 | −0.052 | 0.204 | 0.90 | 0.26 |
| TSM | 0.254 | −0.09 | 0.599 | 0.92 | 0.46 |
| TR | 0.647 | 0.488 | 0.813 | 0.97 | 0.86 |
| Phase C (Immediately after treadmill) | |||||
| TPM | −0.5 | −0.851 | −0.149 | 0.90 | 0.31 |
| TGM | −0.575 | −0.787 | −0.363 | 0.94 | 0.60 |
| TSM | −0.075 | −0.691 | 0.541 | 0.80 | 0.03 |
| TR | 1.496 | 1.131 | 1.861 | 0.91 | 0.33 |
| Phase D (Cool−down walk) | |||||
| TPM | −0.768 | −1.23 | −0.307 | 0.98 | 0.96 |
| TGM | −1.172 | −1.515 | −0.829 | 0.88 | 0.19 |
| TSM | −1.018 | −1.537 | −0.499 | 0.97 | 0.85 |
| TR | 0.034 | −0.217 | 0.286 | 0.91 | 0.32 |
| Mean Bias | Lower Limit | Upper Limit | |
|---|---|---|---|
| TPM | −0.319 | −1.078 | 0.441 |
| TGM | −0.459 | −1.078 | 0.441 |
| TSM | −0.26 | −1.653 | 1.134 |
| TR | 0.483 | −0.787 | 1.799 |
References
- Racing Australia. A Guide to the Racing Industry in Australia 2017–2018. Available online: http://publishingservices.risa.com.au/otherpublications/Fact%20Book%202017-2018/ (accessed on 31 March 2020).
- Racing Australia. A Guide to the Racing Industry in Australia 2018–2019. Available online: http://publishingservices.risa.com.au/otherpublications/FactBook2018-2019/ (accessed on 31 March 2020).
- Racing NSW. Racing in Hot Weather. Available online: http://www.racingnsw.com.au/site/_content/document/00000048-source.pdf (accessed on 6 October 2020).
- Racing Victoria. Hot Weather Racing Policy. Available online: http://www.rv.racing.com/notices/2014-07-07/hot-weather-policy (accessed on 6 October 2020).
- Racing Queensland. Animal Welfare Policy. Available online: http://www.racingqueensland.com.au/Animal_welfare_policy_1.pdf.aspx (accessed on 6 October 2020).
- Racing and Wagering Western Australia. Racing horses in Hot Weather. Available online: http://www.rwwa.com.au/home/thoroughbredpolicy29.pdf (accessed on 6 October 2020).
- Thoroughbred Racing Northern Territory. Racing Horses in Hot Weather. Policy no.014. Available online: www.trnt.org.au/racing-info/policies (accessed on 6 October 2020).
- Hodgson, D.R. Chapter 8—Thermoregulation. In The Athletic Horse, 2nd ed.; Hodgson, D.R., McKeever, K.H., McGowan, C.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2014; pp. 108–124. [Google Scholar] [CrossRef]
- Marlin, D.J.; Scott, C.M.; Schroter, R.C.; Mills, P.C.; Harris, R.C.; Harris, P.A.; Orme, C.E.; Roberts, C.A.; Marr, C.M.; Dyson, S.J.; et al. Physiological responses in nonheat acclimated horses performing treadmill exercise in cool (20 degrees C/40%RH), hot dry (30 degrees C/40%RH) and hot humid (30 degrees C/80%RH) conditions. Equine Vet. J. 1996, 28, 70–84. [Google Scholar] [CrossRef]
- Jones, J.H.; Carlson, G.P. Estimation of metabolic energy cost and heat production during a 3-day-event. Equine Vet. J. 1995, 27, 23–30. [Google Scholar] [CrossRef]
- Lindinger, M.I. Exercise in the heat: Thermoregulatory limitations to performance in humans and horses. Can. J. Appl. Physiol. 1999, 24, 152–163. [Google Scholar] [CrossRef]
- Geor, R.J.; McCutcheon, L.J.; Ecker, G.L.; Lindinger, M.I. Heat storage in horses during submaximal exercise before and after humid heat acclimation. J. Appl. Physiol. 2000, 89, 2283–2293. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, L.J.; Geor, R.J. Equine Sports Medicine and Surgery, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 901–918. [Google Scholar] [CrossRef]
- McDonald, R.E.; Fleming, R.I.; Beeley, J.G.; Bovell, D.L.; Lu, J.R.; Zhao, X.; Cooper, A.; Kennedy, M.W. Latherin: A surfactant protein of horse sweat and saliva (Latherin Protein of horses). PLoS ONE 2009, 4, e5726. [Google Scholar] [CrossRef]
- Guthrie, A.J.; Lund, R.J. Thermoregulation: Base mechanisms and hyperthermia. Vet. Clin. N. Am. Equine Pract. 1998, 14, 45–59. [Google Scholar] [CrossRef]
- Kennedy, M.W. Latherin and other biocompatible surfactant proteins. Biochem. Soc. Trans. 2011, 39, 1017. [Google Scholar] [CrossRef] [Green Version]
- Divers, T.J. Chapter 33—Temperature-related problems: Hypothermia and hyperthermia. In Equine Emergencies, 4th ed.; Orsini, J.A., Divers, T.J., Eds.; W.B. Saunders: St. Louis, MO, USA, 2014; pp. 573–577. [Google Scholar] [CrossRef]
- Hodgson, D.R.; Davis, R.E.; McConaghy, F.F. Thermoregulation in the horse in response to exercise. Br. Vet. J. 1994, 150, 219–235. [Google Scholar] [CrossRef]
- CouroucÉ-Malblanc, A.; Hodgson, D.R. Chapter 29—Clinical exercise testing. In The Athletic Horse, 2nd ed.; Hodgson, D.R., McKeever, K.H., McGowan, C.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2014; pp. 366–378. [Google Scholar] [CrossRef]
- Morgan, K.; Funkquist, P.; Nyman, G. The effect of coat clipping on thermoregulation during intense exercise in trotters. Equine Vet. J. 2002, 34, 564–567. [Google Scholar] [CrossRef]
- Morgan, K. Effects of short-term changes in ambient air temperature or altered insulation in horses. J. Therm. Biol. 1997, 22, 187–194. [Google Scholar] [CrossRef]
- Green, A.R.; Gates, R.S.; Lawrence, L.M. Measurement of horse core body temperature. J. Therm. Biol. 2005, 30, 370–377. [Google Scholar] [CrossRef]
- Brownlow, M.; Dart, A.; Jeffcott, L. Exertional heat illness: A review of the syndrome affecting racing Thoroughbreds in hot and humid climates. Aust. Vet. J. 2016, 94, 240–247. [Google Scholar] [CrossRef]
- Brownlow, M. Exertional heat illness in thoroughbred racehorses: Observations and treatment in the field. Proc. Int. Conf. Racing Anal. Vet. 2014, 20, 13–22. [Google Scholar]
- Gagnon, D.; Lemire, B.B.; Casa, D.J.; Kenny, G.P. Cold-water immersion and the treatment of hyperthermia: Using 38.6 °C as a safe rectal temperature cooling limit. J. Athl. Train. 2010, 45, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Casa, J.D.; McDermott, P.B.; Lee, C.E.; Yeargin, W.S.; Armstrong, E.L.; Maresh, M.C. Cold water immersion: The gold standard for exertional heatstroke treatment. Exerc. Sport Sci. Rev. 2007, 35, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Gaudio, F.G.; Grissom, C.K. Cooling methods in heat stroke. J. Emerg. Med. 2016, 50, 607–616. [Google Scholar] [CrossRef]
- Barwood, M.; Davey, S.; House, J.; Tipton, M. Post-exercise cooling techniques in hot, humid conditions. Eur. J. Appl. Physiol. 2009, 107, 385–396. [Google Scholar] [CrossRef]
- Bongers, C.C.W.G.; Hopman, M.T.E.; Eijsvogels, T.M.H. Cooling interventions for athletes: An overview of effectiveness, physiological mechanisms, and practical considerations. Temperature 2017, 4, 60–78. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, M.; Arfuso, F.; Giudice, E.; Abbate, F.; Longo, F.; Piccione, G. Core and surface temperature modification during road transport and physical exercise in horse after acupuncture needle stimulation. J. Equine Vet. Sci. 2017, 55, 84–89. [Google Scholar] [CrossRef]
- Verdegaal, E.; Jonas, S.; Caraguel, C.; Franklin, S. Real-time monitoring of the core body temperature of endurance horses during field exercise. Equine Vet. J. 2014, 46, 19–20. [Google Scholar] [CrossRef]
- Teunissen, L.P.J.; Daanen, H.A.M. Infrared thermal imaging of the inner canthus of the eye as an estimator of body core temperature. J. Med. Eng. Technol. 2011, 35, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Rey, B.; Fuller, A.; Hetem, R.S.; Lease, H.M.; Mitchell, D.; Meyer, L.C.R. Microchip transponder thermometry for monitoring core body temperature of antelope during capture. J. Therm. Biol. 2016, 55, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousuke, M.; Masa-Aki, O. Patterns of rectal temperature and shipping fever incidence in horses transported over long-distances. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Robinson, T.R.; Hussey, S.B.; Hill, A.E.; Heckendorf, C.C.; Stricklin, J.B.; Traub-Dargatz, J.L. Comparison of temperature readings from a percutaneous thermal sensing microchip with temperature readings from a digital rectal thermometer in equids. J. Am. Vet. Med. Assoc. 2008, 233, 613–617. [Google Scholar] [CrossRef]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary applications of infrared thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef]
- Goodwin, S. Comparison of body temperatures of goats, horses, and sheep measured with a tympanic infrared thermometer, an implantable microchip transponder, and a rectal thermometer. Contem. Top. Lab. Anim. Sci. 1998, 37, 51–55. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Garrett, P.D. Anatomy of the dorsoscapular ligament of horses. J. Am. Vet. Med. Assoc. 1990, 196, 446–448. [Google Scholar]
- Guard, W.F. Fistula of the withers. N. Am. Vet. 1932, 13, 19–23. [Google Scholar]
- Marlin, D.J.; Scott, C.M.; Roberts, C.A.; Casas, I.; Holah, G.; Schroter, R.C. Post exercise changes in compartimentai body temperature accompanying intermittent cold water cooling in the hyperthermic horse. Equine Vet. J. 1998, 30, 28–34. [Google Scholar] [CrossRef]
- Lamb, D.R.; Gisolfi, C.V.; Nadel, E.R. Exercise, Heat, and Thermoregulation; Brown & Benchmark: Cinccinati, OH, USA, 1993. [Google Scholar]
- Ferguson, R.A.; Ball, D.; Sargeant, A.J. Effect of muscle temperature on rate of oxygen uptake during exercise in humans at different contraction frequencies. J. Exp. Biol. 2002, 205, 981–987. [Google Scholar]
- Karvonen Äikäs, E.; Piironen, P.; Ruosteenoja, R. Intramuscular, rectal and oesophageal temperature during exercise. Acta Physiol. Scand. 1962, 54, 366–370. [Google Scholar] [CrossRef]
- Glen, P.K.; Ollie, J. Sex differences in postexercise esophageal and muscle tissue temperature response. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, 1632–1640. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, S.H.; Ferguson, R.A.; Gerrett, N.; Hupperets, M.; Hodder, S.G.; Havenith, G. Reducing muscle temperature drop after warm-up improves sprint cycling performance. Med. Sci. Sports Exerc. 2013, 45, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giesbrecht, G.G.; Bristow, G.K. A second postcooling afterdrop: More evidence for a convective mechanism. J. Appl. Physiol. 1992, 73, 1253–1258. [Google Scholar] [CrossRef]
- Timothy, I.M.; Kevin, E.E.; Hageman, K.S.; David, C.P. Altered regional blood flow responses to submaximal exercise in older rats. J. Appl. Physiol. 2004, 96, 81–88. [Google Scholar] [CrossRef]
- Auclair-Ronzaud, J.; Benoist, S.; Dubois, C.; Frejaville, M.; Jousset, T.; Jaffrézic, F.; Wimel, L.; Chavatte-Palmer, P. No-contact microchip monitoring of body temperature in yearling horses. J. Equine Vet. Sci. 2020, 86. [Google Scholar] [CrossRef]
- Kenny, G.P.; Reardon, F.D.; Zaleski, W.; Reardon, M.L.; Haman, F.; Ducharme, M.B. Muscle temperature transients before, during, and after exercise measured using an intramuscular multisensor probe. J. Appl. Physiol. 2003, 94, 2350–2357. [Google Scholar] [CrossRef] [Green Version]
- Ducharme, M.B.; Tikuisis, P. Role of blood as heat source or sink in human limbs during local cooling and heating. J. Appl. Physiol. 1994, 76, 2084–2094. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ohmura, H.; Mukai, K.; Shiose, T.; Takahashi, T. A comparison of five cooling methods in hot and humid environments in thoroughbred horses. J. Equine Vet. Sci. 2020, 91, 103130. [Google Scholar] [CrossRef]
- Geor, R.J.; McCutcheon, L.J.; Ecker, G.L.; Lindinger, M.I. Thermal and cardiorespiratory responses of horses to submaximal exercise under hot and humid conditions. Equine Vet. J. 1995, 27, 125–132. [Google Scholar] [CrossRef]
- Hales, J.R.S.; Bell, A.W.; Fawcett, A.A.; King, R.B. Redistribution of cardiac output and skin Ava activity in sheep during exercise and heat stress. J. Therm. Biol. 1984, 9, 113–116. [Google Scholar] [CrossRef]
- Johnson, J.M. Exercise in a hot environment: The skin circulation. Scand. J. Med. Sci. Sports. 2010, 20, 29–39. [Google Scholar] [CrossRef]
- Gerber, M.I.; Swinker, A.M.; Staniar, W.B.; Werner, J.R.; Jedrzejewski, E.A.; Macrina, A.L. Health factors associated with microchip insertion in horses. J. Equine Vet. Sci. 2012, 32, 177–182. [Google Scholar] [CrossRef]
- Stein, F.J.; Geller, S.C.; Carter, J.C. Evaluation of microchip migration in horses, donkeys, and mules. J. Am. Vet. Med. Assoc. 2003, 223, 1316–1319. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated measures correlation. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Mukaka, M.M. Statistics Corner: A guide to appropriate use of Correlation coefficient in medical research. Malawi Med. J. 2015, 24, 69–71. [Google Scholar]
- Stewart, A.J.; Welles, E.G.; Salazar, T. Fungal infections of the upper respiratory tract. Compend. Equine 2008, 3, 208–220. [Google Scholar]






| Summary Stat | Correlation (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | CC | Correlation (r) | p-Value | LL | UL | |
| Phase A (Prior to exercise) | |||||||||
| TCV | 37.96 | 0.41 | 37.40 | 38.60 | 1.09 | 1 | <0.001 | ||
| TPM | 37.56 | 0.55 | 36.90 | 38.30 | 1.47 | 0.95 | <0.001 | 0.76 | 0.99 |
| TGM | 37.58 | 0.41 | 37.00 | 38.30 | 1.08 | 0.59 | 0.12 | −0.20 | 0.91 |
| TSM | 38.05 | 0.54 | 37.40 | 39.10 | 1.43 | 0.87 | <0.001 | 0.44 | 0.98 |
| TR | 38.09 | 0.19 | 37.89 | 38.87 | 0.49 | 0.31 | 0.45 | −0.50 | 0.83 |
| Phase B (Exercise on the treadmill) | |||||||||
| TCV | 39.31 | 0.32 | 38.91 | 39.75 | 0.82 | 1 | <0.001 | ||
| TPM | 39.17 | 0.43 | 38.68 | 39.58 | 1.10 | 0.81 | 0.10 | −0.25 | 0.99 |
| TGM | 39.23 | 0.33 | 38.72 | 39.78 | 0.84 | 0.84 | 0.01 | 0.33 | 0.97 |
| TSM | 39.05 | 0.73 | 37.82 | 40.37 | 1.87 | 0.83 | 0.01 | 0.31 | 0.97 |
| TR | 38.66 | 0.42 | 38.06 | 39.46 | 1.10 | 0.83 | 0.01 | 0.30 | 0.97 |
| Phase C (Immediately after exercise) | |||||||||
| TCV | 40.81 | 0.14 | 40.60 | 41.00 | 0.33 | 1 | <0.001 | ||
| TPM | 41.31 | 0.48 | 40.50 | 41.90 | 1.15 | −0.09 | 0.83 | −0.75 | 0.66 |
| TGM | 41.39 | 0.22 | 41.10 | 41.80 | 0.54 | −0.42 | 0.30 | −0.87 | 0.41 |
| TSM | 41.00 | 0.28 | 38.80 | 42.00 | 0.67 | 0.62 | 0.10 | −0.16 | 0.92 |
| TR | 39.32 | 0.53 | 38.53 | 40.20 | 1.34 | 0.13 | 0.76 | −0.63 | 0.76 |
| Phase D (Cool-down walk) | |||||||||
| TCV | 39.59 | 0.20 | 39.31 | 40.00 | 0.50 | 1 | <0.001 | ||
| TPM | 40.36 | 0.74 | 38.91 | 41.29 | 1.84 | 0.5 | 0.20 | −0.31 | 0.89 |
| TGM | 40.77 | 0.38 | 40.11 | 41.25 | 0.92 | −0.43 | 0.29 | −0.87 | 0.39 |
| TSM | 40.61 | 0.70 | 39.40 | 41.61 | 1.72 | −0.11 | 0.79 | −0.76 | 0.64 |
| TR | 39.56 | 0.35 | 39.12 | 39.99 | 0.88 | 0.21 | 0.62 | −0.58 | 0.8 |
| All the data (Phases A–D) | |||||||||
| TCV | 39.42 | 1.07 | 37.40 | 41.00 | 2.70 | 1 | <0.001 | ||
| TPM | 39.65 | 1.59 | 36.90 | 41.90 | 4.00 | 0.94 | <0.001 | 0.87 | 0.97 |
| TGM | 39.95 | 2.53 | 37.00 | 41.80 | 6.34 | 0.90 | <0.001 | 0.80 | 0.95 |
| TSM | 39.65 | 1.37 | 37.40 | 42.00 | 3.46 | 0.82 | <0.001 | 0.67 | 0.91 |
| TR | 38.92 | 0.68 | 37.89 | 40.20 | 1.75 | 0.71 | <0.001 | 0.47 | 0.85 |
| rmc | p-Value | Lower Limit | Upper Limit | |
|---|---|---|---|---|
| TPM | 0.93 | <0.001 | 0.88 | 0.93 |
| TGM | 0.88 | <0.001 | 0.84 | 0.91 |
| TSM | 0.73 | <0.001 | 0.62 | 0.79 |
| TR | 0.51 | <0.001 | 0.43 | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Zsoldos, R.R.; Woldeyohannes, S.M.; Gaughan, J.B.; Sole Guitart, A. The Use of Percutaneous Thermal Sensing Microchips for Body Temperature Measurements in Horses Prior to, during and after Treadmill Exercise. Animals 2020, 10, 2274. https://doi.org/10.3390/ani10122274
Kang H, Zsoldos RR, Woldeyohannes SM, Gaughan JB, Sole Guitart A. The Use of Percutaneous Thermal Sensing Microchips for Body Temperature Measurements in Horses Prior to, during and after Treadmill Exercise. Animals. 2020; 10(12):2274. https://doi.org/10.3390/ani10122274
Chicago/Turabian StyleKang, Hyungsuk, Rebeka R. Zsoldos, Solomon M. Woldeyohannes, John B. Gaughan, and Albert Sole Guitart. 2020. "The Use of Percutaneous Thermal Sensing Microchips for Body Temperature Measurements in Horses Prior to, during and after Treadmill Exercise" Animals 10, no. 12: 2274. https://doi.org/10.3390/ani10122274






