Feeding Tall Fescue Seed Reduces Ewe Milk Production, Lamb Birth Weight and Pre-Weaning Growth Rate
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Ewe Genotyping
2.3. Blood Samples
2.4. Doppler Ultrasound
2.5. Lambing and Milk Production
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, S.P.; Hoveland, C.S.; Clark, E.M.; Davis, N.D.; Smith, L.A.; Grimes, H.W.; Holliman, J.L. Association of an Endophytic Fungus with Fescue Toxicity in Steers Fed Kentucky 31 Tall Fescue Seed or Hay. J. Anim. Sci. 1982, 55, 1259–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuedemann, J.A.; Hoveland, C.S. Fescue Endophyte: History and Impact on Animal Agriculture. J. Prod. Agric. 1988, 1, 39–44. [Google Scholar] [CrossRef]
- Peters, C.W.; Grigsby, K.N.; Aldrich, C.G.; Paterson, J.A.; Lipsey, R.J.; Kerley, M.S.; Garner, G.B. Performance, forage utilization, and ergovaline consumption by beef cows grazing endophyte fungus-infected tall fescue, endophyte fungus-free tall fescue, or orchardgrass pastures1. J. Anim. Sci. 1992, 70, 1550–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, J.K.; Thompson, J.F.N. Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 1992, 70, 1594–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, C.A.; Charlton, N.D.; Takach, J.E.; Swoboda, G.A.; Trammell, M.A.; Huhman, D.V.; Hopkins, A.A. Characterization of Epichloà coenophiala within the US: Are all tall fescue endophytes created equal? Front. Chem. 2014, 2, 95. [Google Scholar] [CrossRef] [Green Version]
- Watson, R.H.; McCann, M.A.; Parish, J.A.; Hoveland, C.S.; Thompson, F.N.; Bouton, J.H. Productivity of cow–calf pairs grazing tall fescue pastures infected with either the wild-type endophyte or a nonergot alkaloid-producing endophyte strain, AR5421,2. J. Anim. Sci. 2004, 82, 3388–3393. [Google Scholar] [CrossRef]
- Duckett, S.K.; Andrae, J.G.; Pratt, S.L. Exposure to ergot alkaloids during gestation reduces fetal growth in sheep. Front. Chem. 2014, 2, 68. [Google Scholar] [CrossRef] [Green Version]
- Britt, J.L.; Greene, M.A.; Bridges, W.C.; Klotz, J.L.; Aiken, G.E.; Andrae, J.G.; Pratt, S.L.; Long, N.M.; Schrick, F.N.; Strickland, J.R.; et al. Ergot alkaloid exposure during gestation alters. I. Maternal characteristics and placental development of pregnant ewes1. J. Anim. Sci. 2019, 97, 1874–1890. [Google Scholar] [CrossRef]
- Greene, M.A.; Britt, J.L.; Powell, R.R.; Feltus, F.A.; Bridges, W.C.; Bruce, T.; Klotz, J.L.; Miller, M.F.; Duckett, S.K. Ergot alkaloid exposure during gestation alters: 3. Fetal growth, muscle fiber development, and miRNA transcriptome1. J. Anim. Sci. 2019, 97, 3153–3168. [Google Scholar] [CrossRef]
- Klotz, J.L.; Britt, J.L.; Miller, M.F.; Snider, M.A.; Aiken, G.E.; Long, N.M.; Pratt, S.L.; Andrae, J.G.; Duckett, S.K. Ergot alkaloid exposure during gestation alters: II. Uterine and umbilical artery vasoactivity1. J. Anim. Sci. 2019, 97, 1891–1902. [Google Scholar] [CrossRef] [Green Version]
- Sibley, D.R.; Creese, I. Interactions of ergot alkaloids with anterior pituitary D-2 dopamine receptors. Mol. Pharmacol. 1983, 23, 585–593. [Google Scholar] [PubMed]
- Strickland, J.R.; Cross, D.L.; Jenkins, T.C.; Petroski, R.J.; Powell, R.G. The effect of alkaloids and seed extracts of endophyte-infected tall fescue on prolactin secretion in an in vitro rat pituitary perfusion system. J. Anim. Sci. 1992, 70, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.R.; Cross, D.L.; Birrenkott, G.P.; Grimes, L.W. Effect of ergovaline, loline, and dopamine antagonists on rat pituitary cell prolactin release in vitro. Am. J. Vet. Res. 1994, 55, 716–721. [Google Scholar] [PubMed]
- Berde, B. Ergot compounds: A synopsis. Adv. Biochem. Psychopharmacol. 1980, 23, 3–23. [Google Scholar]
- Weber, H.P. Ergot compounds: A synopsis. In Ergot Compounds and Brain Function: Nueroendocrine and Neuropsychiatric Aspects; Goldstein, M., Lieberman, A., Calne, D.B., Thorner, M.O., Eds.; Raven Press: New York, NY, USA, 1980; pp. 25–34. [Google Scholar]
- Strickland, J.R.; Looper, M.L.; Matthews, J.C.; Rosenkrans, C.F., Jr.; Flythe, M.D.; Brown, K.R. Board-invited review: St. Anthony’s Fire in livestock: Causes, mechanisms, and potential solutions. J. Anim. Sci. 2011, 89, 1603–1626. [Google Scholar] [CrossRef] [PubMed]
- Aiken, G.E.; Kirch, B.H.; Strickland, J.R.; Bush, L.P.; Looper, M.L.; Schrick, F.N. Hemodynamic responses of the caudal artery to toxic tall fescue in beef heifers1. J. Anim. Sci. 2007, 85, 2337–2345. [Google Scholar] [CrossRef] [Green Version]
- Klotz, J.L.; Kirch, B.; Aiken, G.E.; Bush, L.P.; Strickland, J.R. Bioaccumulation of ergovaline in bovine lateral saphenous veins in vitro1,2. J. Anim. Sci. 2009, 87, 2437–2447. [Google Scholar] [CrossRef] [Green Version]
- Akersr, M.R.; Tucker, A.H.; Bauman, D.E.; Capuco, A.V.; Goodman, G.T. Prolactin Regulation of Milk Secretion and Biochemical Differentiation of Mammary Epithelial Cells in Periparturient Cows. Endocrinology 1981, 109, 23–30. [Google Scholar] [CrossRef]
- Akers, R.M.; Bauman, D.E.; Goodman, G.T.; Capuco, A.V.; Tucker, H.A. Prolactin Regulation of Cytological Differentiation of Mammary Epithelial Cells in Periparturient Cows. Endocrinology 1981, 109, 31–40. [Google Scholar] [CrossRef]
- McCann, J.S.; Caudle, A.B.; Thompson, F.N.; Stuedemann, J.A.; Heusner, G.L.; Thompson, J.D.L. Influence of endophyte-infected tall fescue on serum prolactin and progesterone in gravid mares1. J. Anim. Sci. 1992, 70, 217–223. [Google Scholar] [CrossRef]
- Émile, J.C.; Bony, S.; Ghesquière, M. Influence of consumption of endophyte-infested tall fescue hay on performance of heifers and lambs. J. Anim. Sci. 2000, 78, 358–364. [Google Scholar] [CrossRef]
- Campbell, B.T.; Kojima, C.J.; Cooper, T.A.; Bastin, B.C.; Wojakiewicz, L.; Kallenbach, R.L.; Schrick, F.N.; Waller, J.C. A Single Nucleotide Polymorphism in the Dopamine Receptor D2 Gene May Be Informative for Resistance to Fescue Toxicosis in Angus-Based Cattle. Anim. Biotechnol. 2013, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Aiken, G.E.; Strickland, J.R.; Looper, M.L.; Bush, L.P.; Schrick, F.N. Hemodynamics are altered in the caudal artery of beef heifers fed different ergot alkaloid concentrations1. J. Anim. Sci. 2009, 87, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Chestnut, A.; Erickson, B.; Kelly, F. Effects of Prepartum Consumption of Endophyte-Infested Tall Fescue on Serum Prolactin and Subsequent Milk Production of Holstein Cows. J. Dairy Sci. 1993, 76, 1928–1933. [Google Scholar] [CrossRef]
- Duckett, S.K.; Furusho-Garcia, I.F.; Rico, J.E.; McFadden, J.W. Flaxseed Oil or n-7 Fatty Acid-Enhanced Fish Oil Supplementation Alters Fatty Acid Composition, Plasma Insulin and Serum Ceramide Concentrations, and Gene Expression in Lambs. Lipids 2019, 54, 389–399. [Google Scholar] [CrossRef]
- Holtenius, P.; Holtenius, K. A model to estimate insulin sensitivity in dairy cows. Acta Vet. Scand. 2007, 49, 29. [Google Scholar] [CrossRef] [Green Version]
- Matheson, S.M.; Rooke, J.A.; McIlvaney, K.; Jack, M.; Ison, S.; Bünger, L.; Dwyer, C.M. Development and validation of on-farm behavioural scoring systems to assess birth assistance and lamb vigour. Animal 2011, 5, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Mavrogenis, A.P.; Papachristoforu, C.; Lysandrides, P.; Roushias, A. Environmental and genetic factors affected udder characteristics and milk production in Chios sheep. Genet. Sel. Evol. 1988, 20, 477–488. [Google Scholar] [CrossRef]
- Cardellino, R.A.; Benson, M.E. Lactation curves of commercial ewes rearing lambs. J. Anim. Sci. 2002, 80, 23–27. [Google Scholar] [CrossRef]
- Monroe, J.; Cross, D.; Hudson, L.; Hendricks, D.; Kennedy, S.; Bridges, W. Effect of selenium and endophyte-contaminated fescue on performance and reproduction in mares. J. Equine Vet. Sci. 1988, 8, 148–153. [Google Scholar] [CrossRef]
- Strahan, S.R.; Hemken, R.W.; Jackson, J.A., Jr.; Buckner, R.C.; Bush, L.P.; Siegel, M.R. Performance of Lactating Dairy Cows Fed Tall Fescue Forage. J. Dairy Sci. 1987, 70, 1228–1234. [Google Scholar] [CrossRef]
- Essig, H.W.; Aremu, B.; Cantrell, C.E.; Boyd, M.E.; Withers, F.T., Jr. Impacts of Endophyte-Infected Fescue on Cow/Calf Production1. Prof. Anim. Sci. 1993, 9, 64–69. [Google Scholar] [CrossRef]
- Lyons, P.C.; Plattner, R.D.; Bacon, C.W. Occurrence peptide and clavinet ergot alkaloids in tall fescue grass. Science 1986, 232, 487–489. [Google Scholar] [CrossRef] [Green Version]
- Foote, A.P.; Harmon, D.L.; Brown, K.R.; Strickland, J.R.; McLeod, K.R.; Bush, L.P.; Klotz, J.L. Constriction of bovine vasculature caused by endophyte-infected tall fescue seed extract is similar to pure ergovaline. J. Anim. Sci. 2012, 90, 1603–1609. [Google Scholar] [CrossRef]
- Koontz, A.F.; Bush, L.P.; Klotz, J.L.; McLeod, K.R.; Schrick, F.N.; Harmon, D.L. Evaluation of a ruminally dosed tall fescue seed extract as a model for fescue toxicosis in steers. J. Anim. Sci. 2012, 90, 914–921. [Google Scholar] [CrossRef]
- Burke, J.; Jackson, W.; Robson, G. Seasonal changes in body weight and condition, and pregnancy and lambing rates of sheep on endophyte-infected tall fescue in the south-eastern United States. Small Rumin. Res. 2002, 44, 141–151. [Google Scholar] [CrossRef]
- Aiken, G.E.; Flythe, M.D. Vasoconstrictive responses by the carotid and auricular arteries in goats to ergot alkaloid exposure. Front. Chem. 2014, 2, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiken, G.E.; Sutherland, B.; Fletcher, L. Haemodynamics of lambs grazing perennial ryegrass (Lolium perenne L.) either infected with AR6 novel, wild-type endophyte, or endophyte-free. N. Z. Vet. J. 2011, 59, 179–184. [Google Scholar] [CrossRef]
- McDowell, K.J.; Moore, E.; Parks, A.G.; Bush, L.P.; Horohov, D.W.; Lawrence, L.M. Vasoconstriction in horses caused by endophyte-infected tall fescue seed is detected with Doppler ultrasonography. J. Anim. Sci. 2013, 91, 1677–1684. [Google Scholar] [CrossRef]
- Klotz, J.L.; Aiken, G.E.; Bussard, J.R.; Foote, A.P.; Harmon, D.L.; Goff, B.M.; Schrick, F.; Strickland, J.R. Vasoactivity and Vasoconstriction Changes in Cattle Related to Time off Toxic Endophyte-Infected Tall Fescue. Toxins 2016, 8, 271. [Google Scholar] [CrossRef] [Green Version]
- Dühlmeier, R.; Fluegge, I.; Schwert, B.; Ganter, M. Insulin Sensitivity during Late Gestation in Ewes Affected by Pregnancy Toxemia and in Ewes with High and Low Susceptibility to this Disorder. J. Vet. Intern. Med. 2013, 27, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Holloway, J.W.; Butts, W.T., Jr. Phenotype× nutritional environment interactions in forage intake and efficiency of Angus cows grazing fescue-legume or fescue pastures. J. Anim. Sci. 1983, 56, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Paterson, J.; Forcherio, C.; Larson, B.; Samford, M.; Kerley, M. The effects of fescue toxicosis on beef cattle productivity. J. Anim. Sci. 1995, 73, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.L.; Peck, K.N.; Forella, M.E.; Fox, A.R.; Govoni, K.E.; Zinn, S.A. The effects of poor maternal nutrition during gestation on postnatal growth and development of lambs. J. Anim. Sci. 2016, 94, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Anthony, R.V.; Scheaffer, A.N.; Wright, C.D.; Regnault, T.R.H. Ruminant models of prenatal growth restriction. Reproduction 2003, 61, 183–194. [Google Scholar] [CrossRef]
- Pillai, S.M.; Jones, A.K.; Hoffman, M.L.; McFadden, K.K.; Reed, S.A.; Zinn, S.A.; Govoni, K.E. Fetal and organ development at gestational days 45, 90, 135 and at birth of lambs exposed to under- or over-nutrition during gestation. Transl. Anim. Sci. 2017, 1, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Bond, J.; Lynch, G.P.; Bolt, D.J.; Hawk, H.W.; Jackson, C., Jr.; Wall, R.J. Reproductive performance and lamb weight gains for ewes grazing fungus-infected tall fescue. Nutr. Rep. Int. 1988, 37, 1099–1115. [Google Scholar]
- Rattray, P.V.; Garrett, W.N.; East, N.E.; Hinman, N. Growth, Development and Composition of the Ovine Conceptus and Mammary Gland during Pregnancy. J. Anim. Sci. 1974, 38, 613–626. [Google Scholar] [CrossRef]
- Brown, M.A.; Brown, A.H., Jr.; Jackson, W.G.; Miesner, J.R. Milk production in Angus, Brahman, and Reciprocal-cross cows grazing common bermudagrass or endophyte-infected tall fescue. J. Anim. Sci. 1996, 74, 2058–2066. [Google Scholar] [CrossRef] [Green Version]
- Lean, I.J. Association between feeding perennial ryegrass (Lolium perenne cultivar Grasslands impact) containing high concentrations of ergovaline, and health and productivity in a herd of lactating dairy cows. Aust. Vet. J. 2001, 79, 262–264. [Google Scholar] [CrossRef]
- Chamley, W.A.; Buckmaster, J.M.; Cerini, M.E.; Cumming, I.A.; Goding, J.R.; Obst, J.M.; Williams, A.; Winfield, C. Changes in the Levels of Progesterone, Corticosteroids, Estrone, Estradiol-17 β, Luteinizing Hormone, and Prolactin in the Peripheral Plasma of the Ewe during Late Pregnancy and at Parturition. Biol. Reprod. 1973, 9, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooley, R.D.; Campbell, J.J.; Findlay, J.K. The importance of prolactin for lactation in the ewe. J. Endocrinol. 1978, 79, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R. Mammary Gland Growth in Sheep. J. Anim. Sci. 1975, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Plaut, K.; Bauman, D.; Agergaard, N.; Akers, R. Effect of exogenous prolactin administration on lactational performance of dairy cows. Domest. Anim. Endocrinol. 1987, 4, 279–290. [Google Scholar] [CrossRef]
- Bequette, B.J.; Backwell, F.R.C.; Crompton, L.A. Current Concepts of Amino Acid and Protein Metabolism in the Mammary Gland of the Lactating Ruminant. J. Dairy Sci. 1998, 81, 2540–2559. [Google Scholar] [CrossRef]
- Louey, S.; Cock, M.L.; Harding, R. Long Term Consequences of Low Birthweight on Postnatal Growth, Adiposity and Brain Weight at Maturity in Sheep. J. Reprod. Dev. 2005, 51, 59–68. [Google Scholar] [CrossRef] [Green Version]

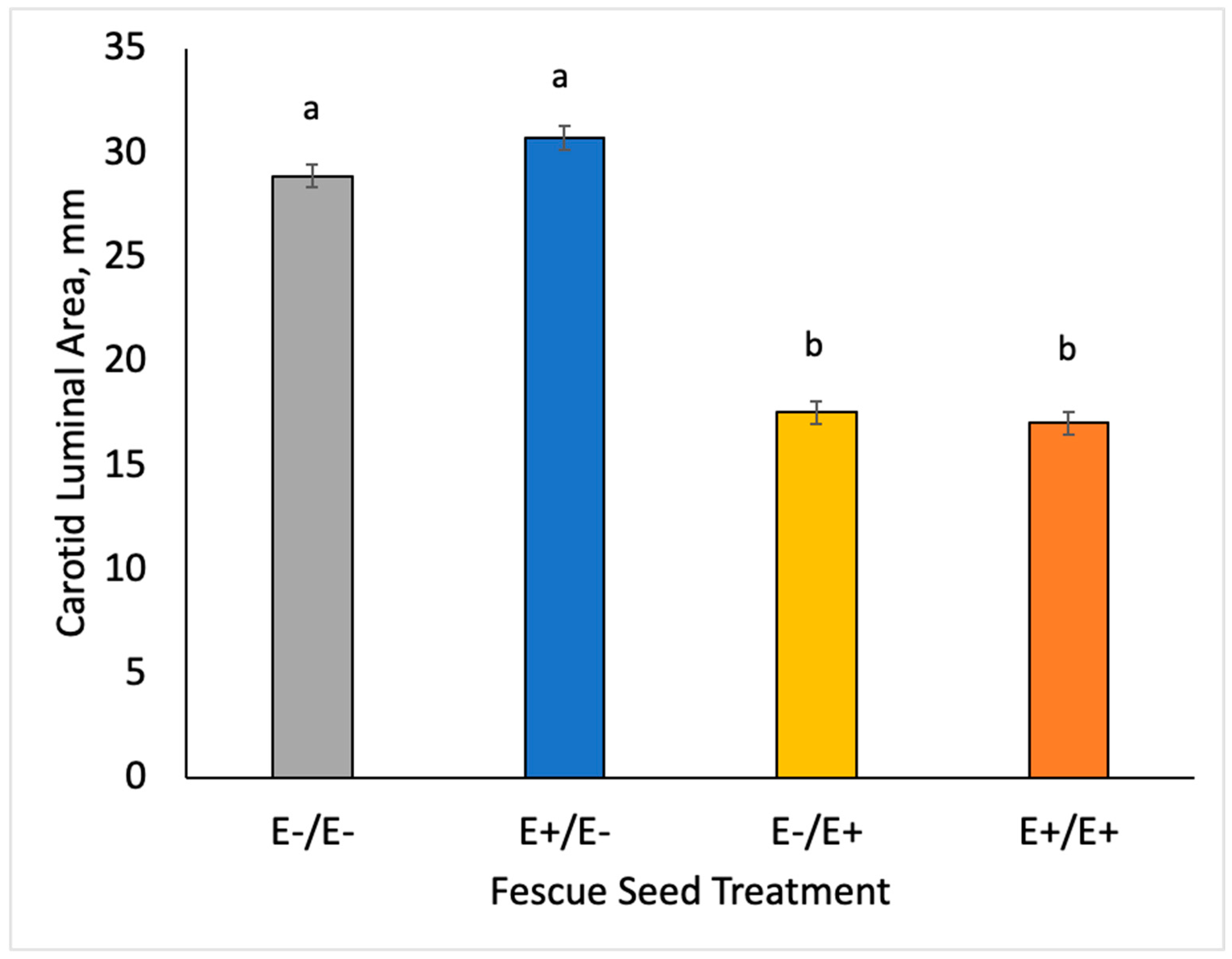




| Ewe Performance | Maternal DRD2 Genotype | Fescue Seed MID | Fescue Seed LATE | Standard | ||||
|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | E− | E+ | E− | E+ | Error (SE) | |
| Ewes (n) | 12 | 26 | 14 | 24 | 28 | 24 | 28 | |
| BW change, kg | ||||||||
| d 28–85 | 5.23 | 2.94 | 5.07 | 5.10 | 3.72 | 4.93 | 3.90 | 4.32 |
| d 86–136 | 20.67 | 19.00 | 16.68 | 19.00 | 18.56 | 21.34 c | 16.23 d | 6.01 |
| d 28–136 | 26.68 | 22.81 | 22.76 | 25.48 | 22.69 | 27.87 a | 20.29 b | 6.61 |
| DMI, kg/d | ||||||||
| d 28–85 | 1.93 | 1.94 | 1.88 | 1.91 | 1.92 | 1.94 | 1.89 | 0.080 |
| d 86–136 | 2.69 | 2.72 | 2.64 | 2.67 | 2.69 | 2.67 | 2.69 | 0.24 |
| d 28–136 | 2.35 | 2.37 | 2.29 | 2.32 | 2.35 | 2.35 | 2.33 | 0.15 |
| ADG, g/d | ||||||||
| d 28–85 | 93.2 | 52.5 | 90.4 | 91.0 | 66.4 | 98.0 | 69.5 | 76.99 |
| d 86–136 | 430.0 | 395.4 | 347.2 | 395.5 | 386.3 | 444.1 c | 337.7 d | 125.15 |
| d 28–136 | 246.5 | 210.6 | 210.6 | 235.6 | 209.7 | 257.8 a | 187.6 b | 61.11 |
| Ewe Parturition Variables | Maternal DRD2 Genotype | Fescue Seed MID | Fescue Seed LATE | Standard Error | ||||
|---|---|---|---|---|---|---|---|---|
| Per Ewe Basis | AA | AG | GG | E− | E+ | E− | E+ | SE |
| Ewes (n) | 12 | 26 | 14 | 24 | 28 | 24 | 28 | |
| Est. lamb no. | 1.79 | 1.82 | 1.68 | 1.80 | 1.73 | 1.70 | 1.83 | 0.43 |
| Gestation, d | 145.5 | 145.0 | 146.3 | 145.0 | 146.2 | 146.5 | 144.7 | 2.67 |
| Ewe difficulty | 1.54 | 1.40 | 1.44 | 1.40 | 1.52 | 1.50 | 1.41 | 0.92 |
| Lambs born | 1.93 | 1.58 | 1.68 | 1.75 | 1.70 | 1.82 | 1.64 | 0.63 |
| Lambs alive | 1.94 | 1.55 | 1.36 | 1.74 | 1.50 | 1.65 | 1.58 | 0.72 |
| Lamb vigor | 1.87 | 1.89 | 2.08 | 2.01 | 1.88 | 2.02 | 1.87 | 0.75 |
| Lamb sex ratio | 1.44 | 1.46 | 1.48 | 1.59 | 1.33 | 1.48 | 1.44 | 0.45 |
| Birth weight, kg | 8.52 | 8.52 | 8.98 | 8.84 | 8.52 | 9.14 a | 8.21 b | 0.94 |
| Crown-rump, cm | 54.72 | 55.30 | 57.71 | 56.31 | 55.51 | 56.91 | 54.91 | 3.14 |
| Lamb Growth | Maternal DRD2 Genotype | Fescue Seed MID | Fescue Seed LATE | Standard Error | ||||
|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | E− | E+ | E− | E+ | SE | |
| Ewes (n) | 12 | 26 | 14 | 24 | 28 | 24 | 28 | |
| Lamb BW change, kg/ewe | ||||||||
| d 28 | 10.84 b | 14.19 a | 8.97 b | 12.31 g | 10.36 h | 11.82 | 10.85 | 2.88 |
| d 56 | 11.35 c,d | 12.80 c | 9.75 d | 12.22 g | 10.38 h | 11.93 | 10.67 | 3.40 |
| d 75 (wean) | 6.64 d | 8.56 c | 7.90 d | 8.10 | 7.31 | 7.81 | 7.59 | 2.36 |
| Weaning wt, kg/ewe | 37.10 d | 43.74 c | 35.20 c,d | 41.25 e | 36.11 f | 40.32 i | 37.04 j | 5.30 |
| Lamb ADG, g·ewe−1·d−1 | ||||||||
| Birth to d 28 | 431.7 b | 535.8 a | 361.3 b | 485.3 e | 400.6 f | 469.2 | 425.8 | 120.5 |
| d 28 to 56 | 395.0 a,b | 460.7 a | 347.4 b | 435.0 c | 367.1 d | 431.0 | 380.0 | 120.4 |
| d 56 to 75 | 352.5 | 407.6 | 387.2 | 403.0 | 361.8 | 397.4 | 367.4 | 122.6 |
| Overall | 389.7 b | 466.8 a | 360.4 b | 438.5 e | 372.7 f | 423.8 | 387.5 | 71.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Britt, J.L.; Greene, M.A.; Wilbanks, S.A.; Bertrand, J.K.; Klotz, J.L.; Bridges, W., Jr.; Aiken, G.; Andrae, J.G.; Duckett, S.K. Feeding Tall Fescue Seed Reduces Ewe Milk Production, Lamb Birth Weight and Pre-Weaning Growth Rate. Animals 2020, 10, 2291. https://doi.org/10.3390/ani10122291
Britt JL, Greene MA, Wilbanks SA, Bertrand JK, Klotz JL, Bridges W Jr., Aiken G, Andrae JG, Duckett SK. Feeding Tall Fescue Seed Reduces Ewe Milk Production, Lamb Birth Weight and Pre-Weaning Growth Rate. Animals. 2020; 10(12):2291. https://doi.org/10.3390/ani10122291
Chicago/Turabian StyleBritt, Jessica L., Maslyn A. Greene, Sarah A. Wilbanks, J. Keith Bertrand, James L. Klotz, William Bridges, Jr., Glen Aiken, John G. Andrae, and Susan K. Duckett. 2020. "Feeding Tall Fescue Seed Reduces Ewe Milk Production, Lamb Birth Weight and Pre-Weaning Growth Rate" Animals 10, no. 12: 2291. https://doi.org/10.3390/ani10122291





