Tetrazolium Salt WST-8 as a Novel and Reliable Chromogenic Indicator for the Assessment of Boar Semen Quality
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Semen Collection and Preparation for the WST-8 Assay
2.2. WST-8 Assay
2.3. Sperm Functional Integrity Analyses by Flow Cytometry
2.4. Computer-Assisted Sperm Analysis for Motility-Related Sperm Parameters
2.5. Statistical Analysis
3. Results
3.1. Flow Cytometry and CASA Analyses Showed Consistent and Positive Correlations among Different Sperm Sample Preparations
3.2. Positive Correlation between the WST-8 Reduction Rate and Boar Sperm Parameters
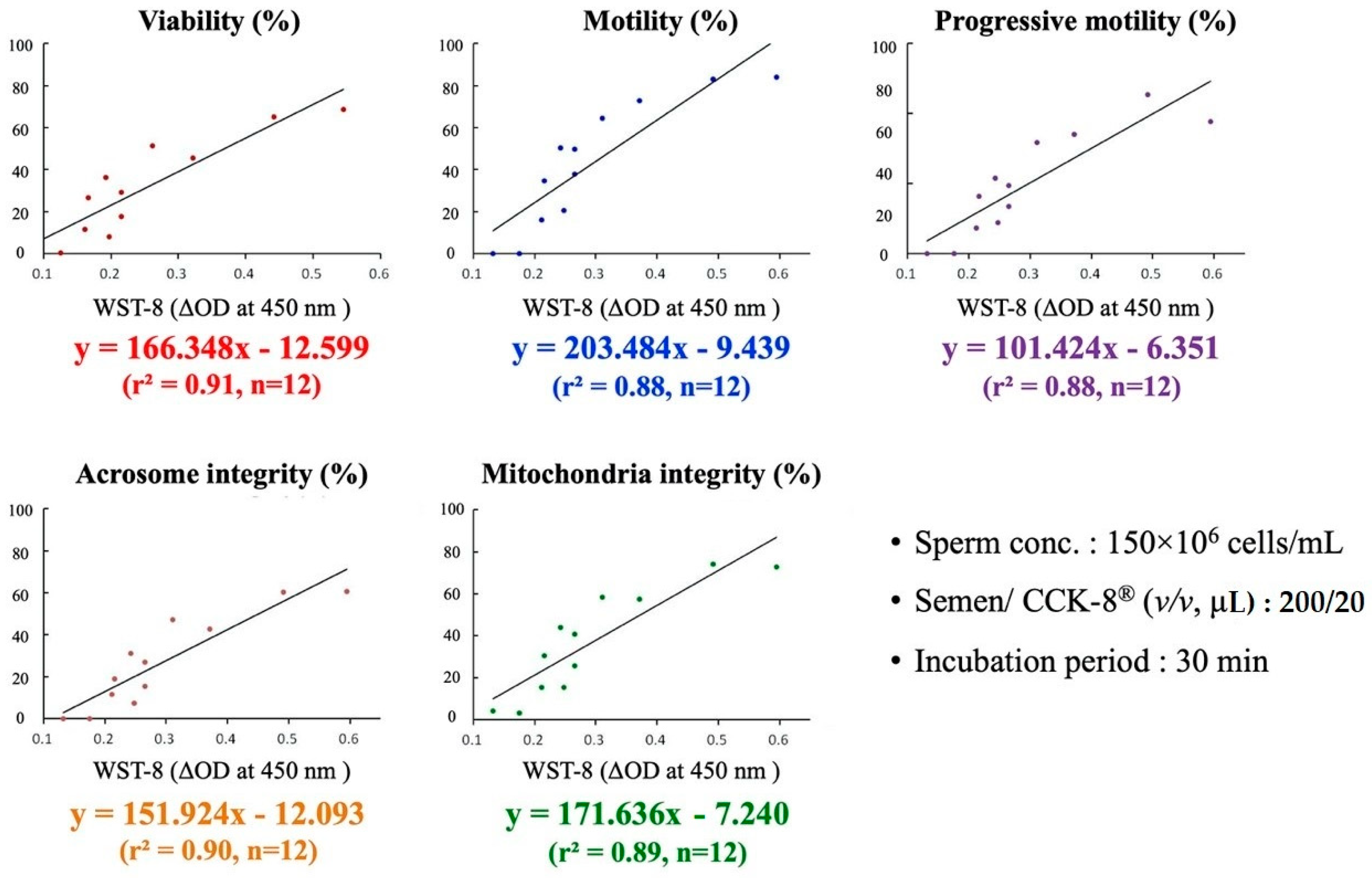
3.3. Models and Formula for Interpolation of Boar Semen Quality Using the WST-8 Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knox, R.V. Artificial insemination in pigs today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef]
- Barbarosie, C.; Agarwal, A.; Henkel, R. Diagnostic value of advanced semen analysis in evaluation of male infertility. Andrologia 2020, e13625. [Google Scholar] [CrossRef]
- Love, C.C. Modern Techniques for Semen Evaluation. Vet. Clin. N. Am. Equine Pract. 2016, 32, 531–546. [Google Scholar] [CrossRef]
- Moce, E.; Graham, J.K. In vitro evaluation of sperm quality. Anim. Reprod. Sci. 2008, 105, 104–118. [Google Scholar] [CrossRef]
- Sutovsky, P. New Approaches to Boar Semen Evaluation, Processing and Improvement. Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Moraes, C.R.; Meyers, S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef]
- Marques, D.B.D.; Lopes, M.S.; Broekhuijse, M.; Guimaraes, S.E.F.; Knol, E.F.; Bastiaansen, J.W.M.; Silva, F.F.; Lopes, P.S. Genetic parameters for semen quality and quantity traits in five pig lines. J. Anim. Sci. 2017, 95, 4251–4259. [Google Scholar] [CrossRef]
- Amann, R.P.; Waberski, D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology 2014, 81, 5–17.e3. [Google Scholar] [CrossRef]
- Van der Horst, G.; Maree, L.; du Plessis, S.S. Current perspectives of CASA applications in diverse mammalian spermatozoa. Reprod. Fertil. Dev. 2018, 30, 875–888. [Google Scholar] [CrossRef]
- Yaniz, J.L.; Silvestre, M.A.; Santolaria, P.; Soler, C. CASA-Mot in mammals: An update. Reprod. Fertil. Dev. 2018, 30, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Bucher, K.; Malama, E.; Siuda, M.; Janett, F.; Bollwein, H. Multicolor flow cytometric analysis of cryopreserved bovine sperm: A tool for the evaluation of bull fertility. J. Dairy Sci. 2019, 102, 11652–11669. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pastor, F.; Mata-Campuzano, M.; Alvarez-Rodriguez, M.; Alvarez, M.; Anel, L.; de Paz, P. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. 2010, 45 (Suppl. 2), 67–78. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Nizanski, W.; Lukaszewicz, E. Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology 2010, 74, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Petrunkina, A.M.; Harrison, R.A. Fluorescence technologies for evaluating male gamete (dys)function. Reprod. Domest. Anim. 2013, 48 (Suppl. 1), 11–24. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Aziz, D.M. Assessment of bovine sperm viability by MTT reduction assay. Anim. Reprod. Sci. 2006, 92, 1–8. [Google Scholar] [CrossRef]
- Aziz, D.M.; Ahlswede, L.; Enbergs, H. Application of MTT reduction assay to evaluate equine sperm viability. Theriogenology 2005, 64, 1350–1356. [Google Scholar] [CrossRef]
- Yu, J.F.; Lai, Y.H.; Wang, T.E.; Wei, Y.S.; Chang, Y.J.; Li, S.H.; Chin, S.C.; Joshi, R.; Chang, H.W.; Tsai, P.S. The effects of type I collagenase on the degelification of chimpanzee (Pan troglodytes) semen plug and sperm quality. BMC Vet. Res. 2018, 14, 58. [Google Scholar] [CrossRef]
- Ishiyama, M.; Miyazono, Y.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 1997, 44, 1299–1305. [Google Scholar] [CrossRef]
- Kairo, S.K.; Bedwell, J.; Tyler, P.C.; Carter, A.; Corbel, M.J. Development of a tetrazolium salt assay for rapid determination of viability of BCG vaccines. Vaccine 1999, 17, 2423–2428. [Google Scholar] [CrossRef]
- Lin, H.L.; Liaw, R.B.; Chen, Y.H.; Kang, T.C.; Lin, D.Y.; Chen, L.R.; Wu, M.C. Evaluation of cockerel spermatozoa viability and motility by a novel enzyme based cell viability assay. Br. Poult. Sci. 2019, 60, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Chamchoy, K.; Pakotiprapha, D.; Pumirat, P.; Leartsakulpanich, U.; Boonyuen, U. Application of WST-8 based colorimetric NAD(P)H detection for quantitative dehydrogenase assays. BMC Biochem. 2019, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, M.J. WST-8 analysis of cell viability during osteogenesis of human mesenchymal stem cells. Methods Mol. Biol. 2011, 740, 21–25. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018. [Google Scholar] [CrossRef]
- Cordelli, E.; Eleuteri, P.; Leter, G.; Rescia, M.; Spano, M. Flow cytometry applications in the evaluation of sperm quality: Semen analysis, sperm function and DNA integrity. Contraception 2005, 72, 273–279. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.; Sostaric, E.; Feitsma, H.; Gadella, B.M. Relationship of flow cytometric sperm integrity assessments with boar fertility performance under optimized field conditions. J. Anim. Sci. 2012, 90, 4327–4336. [Google Scholar] [CrossRef]
- Van den Berg, B.M. Microscopic analysis of MTT stained boar sperm cells. Open Vet. J. 2015, 5, 58–63. [Google Scholar]
- Byun, J.; Choo, S.; Kim, H.; Kim, Y.; Hwang, Y.; Kim, D. Evaluation of boar sperm viability by mtt reduction assay in beltsville thawing solution extender. Asian-Australas. J. Anim. Sci. 2008, 21, 494–498. [Google Scholar] [CrossRef]
- Ginouves, M.; Carme, B.; Couppie, P.; Prevot, G. Comparison of tetrazolium salt assays for evaluation of drug activity against Leishmania spp. J. Clin. Microbiol. 2014, 52, 2131–2138. [Google Scholar] [CrossRef]
- Prabst, K.; Engelhardt, H.; Ringgeler, S.; Hubner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [CrossRef]
- Hossain, M.S.; Johannisson, A.; Wallgren, M.; Nagy, S.; Siqueira, A.P.; Rodriguez-Martinez, H. Flow cytometry for the assessment of animal sperm integrity and functionality: State of the art. Asian J. 2011, 13, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Yu-Chia Chang, J.-F.Y.; Wang, T.-E.; Chin, S.-C.; Wei, Y.-S.; Chen, T.-Y.; Tsai, P.-S. Investigation of epididymal proteins and general sperm membrane characteristics of Formosan pangolin (Manis pentadactyla pentadactyla). BMC Zool. 2020, 5, 15. [Google Scholar]

| Active/Compromised Sperm Ratio | Flow Cytometry Assessment | CASA Assessment | |||
|---|---|---|---|---|---|
| Viability (%) | Acrosome Integrity (%) | Mitochondrial Integrity (%) | Motile Sperm (%) | Progressive Sperm (%) | |
| 0/10 | 0.40 ± 0.11 a | 0.23 ± 0.18 a | 5.50 ± 1.87 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 2/8 | 12.47 ± 1.32 b | 9.88 ± 1.17 b | 16.62 ± 2.30 b | 18.95 ± 1.50 b | 8.18 ± 0.52 b |
| 4/6 | 24.03 ± 2.21 c | 18.83 ± 0.98 c | 29.86 ± 1.83 c | 35.35 ± 1.92 c | 16.17 ± 0.64 c |
| 6/4 | 36.50 ± 3.03 d | 30.10 ± 1.31 d | 44.60 ± 1.75 d | 52.05 ± 2.39 d | 23.72 ± 1.33 d |
| 8/2 | 52.33 ± 2.73 e | 46.03 ± 1.56 e | 58.78 ± 0.65 e | 67.37 ± 2.63 e | 32.92 ± 1.18 e |
| 10/0 | 72.05 ± 3.06 f | 62.65 ± 1.47 f | 73.80 ± 0.95 f | 81.37 ± 2.42 f | 40.75 ± 2.02 f |
| Parameters | Correlation Coefficients (r) | |||
|---|---|---|---|---|
| Sperm Concentration (106 cells/mL) | ||||
| 300 | 150 | 75 | 37.5 | |
| 10 min | ||||
| Viability (%) | 0.48 | −0.61 | −0.18 | −0.16 |
| Acrosome integrity (%) | 0.54 | −0.49 | −0.31 | −0.16 |
| Mitochondrial integrity (%) | 0.30 | −0.86 * | −0.62 | −0.40 |
| Motile sperm (%) | 0.52 | −0.46 | −0.23 | −0.15 |
| Progressive sperm (%) | 0.51 | −0.45 | −0.22 | −0.16 |
| 20 min | ||||
| Viability (%) | 0.81 * | −0.60 | −0.06 | −0.06 |
| Acrosome integrity (%) | 0.88 * | −0.49 | −0.20 | −0.12 |
| Mitochondrial integrity (%) | 0.81 * | −0.90 * | −0.58 | 0.41 |
| Motile sperm (%) | 0.81 * | −0.48 | −0.11 | −0.11 |
| Progressive sperm (%) | 0.78 * | −0.49 | −0.10 | −0.10 |
| 30 min | ||||
| Viability (%) | 0.87 * | −0.28 | 0.10 | −0.10 |
| Acrosome integrity (%) | 0.92 * | −0.19 | −0.04 | −0.08 |
| Mitochondrial integrity (%) | 0.85 * | −0.88 * | 0.42 | 0.23 |
| Motile sperm (%) | 0.84 * | −0.20 | 0.08 | −0.04 |
| Progressive sperm (%) | 0.82 * | −0.21 | 0.09 | −0.03 |
| 40 min | ||||
| Viability (%) | 0.89 * | −0.04 | 0.32 | 0.08 |
| Acrosome integrity (%) | 0.91 * | 0.05 | 0.30 | 0.09 |
| Mitochondrial integrity (%) | 0.85 * | −0.89 * | 0.53 | 0.45 |
| Motile sperm (%) | 0.82 * | 0.03 | 0.45 | 0.12 |
| Progressive sperm (%) | 0.82 * | 0.02 | 0.45 | 0.12 |
| 50 min | ||||
| Viability (%) | 0.89 * | 0.36 | 0.34 | 0.39 |
| Acrosome integrity (%) | 0.89 * | 0.42 | 0.42 | 0.56 |
| Mitochondrial integrity (%) | 0.85 * | −0.47 | 0.34 | 0.60 |
| Motile sperm (%) | 0.80 * | 0.36 | 0.50 | 0.59 |
| Progressive sperm (%) | 0.81 * | 0.36 | 0.49 | 0.60 |
| 60 min | ||||
| Viability (%) | 0.88 * | 0.51 | 0.61 | 0.75 * |
| Acrosome integrity (%) | 0.87 * | 0.56 | 0.68 | 0.78 * |
| Mitochondrial integrity (%) | 0.84 * | −0.09 | 0.85* | 0.72 |
| Motile sperm (%) | 0.78 * | 0.50 | 0.74 * | 0.84 * |
| Progressive sperm (%) | 0.79 * | 0.50 | 0.74 * | 0.85 * |
| Correlation Coefficients (r) | ||||
|---|---|---|---|---|
| Sperm Concentration (106 cells/mL) | 300 | 150 | ||
| Semen/CCK-8® (v/v, μL) | 200/20 | 200/10 | 200/20 | 200/10 |
| 10 min | ||||
| Viability (%) | 0.88 * | 0.90 * | 0.42 | −0.14 |
| Acrosome integrity (%) | 0.87 * | 0.88 * | 0.41 | −0.12 |
| Mitochondrial activity (%) | 0.83 * | 0.84 * | 0.44 | −0.09 |
| Motile sperm (%) | 0.78 * | 0.81 * | 0.49 | 0.01 |
| Progressive sperm (%) | 0.81 * | 0.84 * | 0.44 | −0.12 |
| 20 min | ||||
| Viability (%) | 0.93 * | 0.95 * | 0.66 | 0.22 |
| Acrosome integrity (%) | 0.96 * | 0.95 * | 0.65 | 0.23 |
| Mitochondrial activity (%) | 0.95 * | 0.94 * | 0.66 | 0.24 |
| Motile sperm (%) | 0.92 * | 0.91 * | 0.68 | 0.31 |
| Progressive sperm (%) | 0.94 * | 0.93 * | 0.68 | 0.22 |
| 30 min | ||||
| Viability (%) | 0.94 * | 0.94 * | 0.91 * | 0.69 |
| Acrosome integrity (%) | 0.97 * | 0.96 * | 0.90 * | 0.70 |
| Mitochondrial activity (%) | 0.97 * | 0.95 * | 0.89 * | 0.69 |
| Motile sperm (%) | 0.96 * | 0.93 * | 0.88 * | 0.70 |
| Progressive sperm (%) | 0.966 * | 0.945 * | 0.881 * | 0.684 |
| 40 min | ||||
| Viability (%) | 1.00 * | 1.00 * | 0.89 * | 0.81 * |
| Acrosome integrity (%) | 1.00 * | 1.00 * | 0.89 * | 0.80 * |
| Mitochondrial activity (%) | 1.00 * | 1.00 * | 0.87 * | 0.78 * |
| Motile sperm (%) | 1.00 * | 1.00 * | 0.85 * | 0.78 * |
| Progressive sperm (%) | 1.00 * | 1.00 * | 0.86 * | 0.77 * |
| 50 min | ||||
| Viability (%) | 1.00 * | 1.00 * | 0.90 * | 0.82 * |
| Acrosome integrity (%) | 1.00 * | 1.00 * | 0.91 * | 0.82 * |
| Mitochondrial activity (%) | 1.00 * | 1.00 * | 0.88 * | 0.79 * |
| Motile sperm (%) | 1.00 * | 1.00 * | 0.87 * | 0.77 * |
| Progressive sperm (%) | 1.00 * | 1.00 * | 0.89 * | 0.78 * |
| 60 min | ||||
| Viability (%) | 1.00 * | 1.00 * | 0.91 * | 0.83 * |
| Acrosome integrity (%) | 1.00 * | 1.00 * | 0.92 * | 0.83 * |
| Mitochondrial activity (%) | 1.00 * | 1.00 * | 0.90 * | 0.81 * |
| Motile sperm (%) | 1.00 * | 1.00 * | 0.88 * | 0.79 * |
| Progressive sperm (%) | 1.00 * | 1.00 * | 0.91 * | 0.80 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Wu, C.-P.; Lin, H.-L.; Liaw, R.-B.; Lai, Y.-Y.; Wu, M.-C.; Chen, L.-R.; Jason Tsai, P.-S. Tetrazolium Salt WST-8 as a Novel and Reliable Chromogenic Indicator for the Assessment of Boar Semen Quality. Animals 2020, 10, 2293. https://doi.org/10.3390/ani10122293
Chen Y-H, Wu C-P, Lin H-L, Liaw R-B, Lai Y-Y, Wu M-C, Chen L-R, Jason Tsai P-S. Tetrazolium Salt WST-8 as a Novel and Reliable Chromogenic Indicator for the Assessment of Boar Semen Quality. Animals. 2020; 10(12):2293. https://doi.org/10.3390/ani10122293
Chicago/Turabian StyleChen, Yu-Hsin, Chean-Ping Wu, Hsiu-Lien Lin, Ren-Bao Liaw, Yung-Yu Lai, Ming-Che Wu, Lih-Ren Chen, and Pei-Shiue Jason Tsai. 2020. "Tetrazolium Salt WST-8 as a Novel and Reliable Chromogenic Indicator for the Assessment of Boar Semen Quality" Animals 10, no. 12: 2293. https://doi.org/10.3390/ani10122293
APA StyleChen, Y.-H., Wu, C.-P., Lin, H.-L., Liaw, R.-B., Lai, Y.-Y., Wu, M.-C., Chen, L.-R., & Jason Tsai, P.-S. (2020). Tetrazolium Salt WST-8 as a Novel and Reliable Chromogenic Indicator for the Assessment of Boar Semen Quality. Animals, 10(12), 2293. https://doi.org/10.3390/ani10122293





