No Worm Is an Island; The Influence of Commensal Gut Microbiota on Cyathostomin Infections
Abstract
:Simple Summary
Abstract
1. Introduction
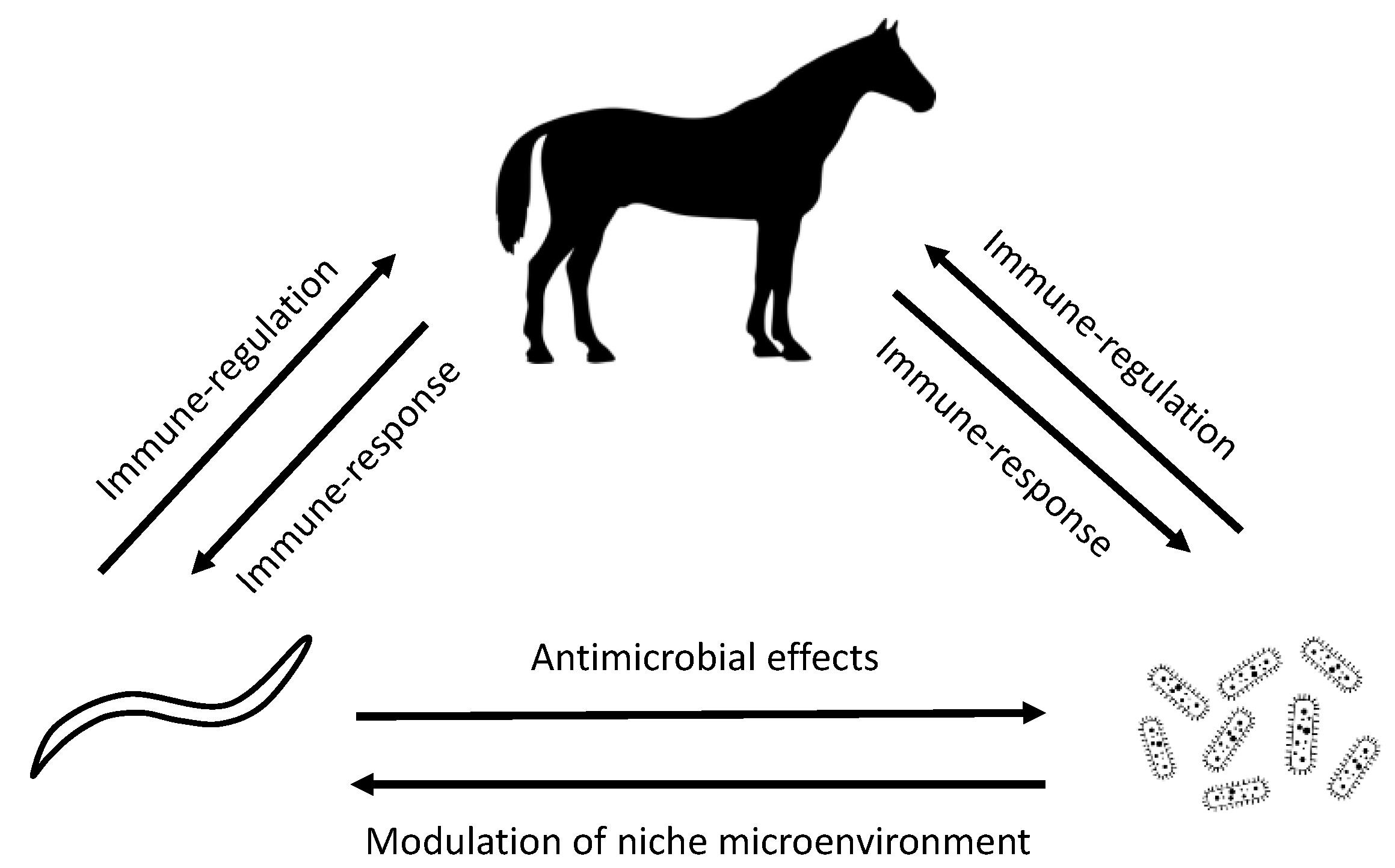
2. Evidence for Cyathostomin–Microbiome Interactions in Horses
2.1. Early Life Programming of Host Immunity by Microbiome
2.2. Impact of Acute Cyathostomin Infection on the Gut Microbiome
2.3. The Relationship between the Gut Microbiome and Immunoregulation in Chronic Cyathostomin Infection
3. The Role of Equine Gut Microbiota in Acute Larval Cyathostominosis
3.1. The Role of the Gut Microbiota in the Initiation of ALC
3.2. The Role of Gut Microbiota in the Pathophysiology of ALC
4. Future Therapeutic Options Based on Microbiota Modulation
4.1. Microbiota Modulating Tools
4.2. Scope for Prevention of Cyathostomin Infection
4.3. Scope for Treatment of Cyathostomin Infection
4.4. Considering the Microbiota and Overall Gut Health in Long-Term Management of Cyathostomins
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roshan, N.; Clancy, A.K.; Borody, T.J. Faecal Microbiota Transplantation is Effective for the Initial Treatment of Clostridium difficile Infection: A Retrospective Clinical Review. Infect. Dis. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Haifer, C.; Leong, R.W.; Paramsothy, S. The role of faecal microbiota transplantation in the treatment of inflammatory bowel disease. Curr. Opin. Pharmacol. 2020, 55, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Edwards, J.F.; Cohen, N.D. Chronic idiopathic inflammatory bowel diseases of the horse. J. Vet. Intern. Med. 2000, 14, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kemper, D.L.; Perkins, G.A.; Schumacher, J.; Edwards, J.F.; Valentine, B.A.; Divers, T.J.; Cohen, N.D. Equine lymphocytic-plasmacytic enterocolitis: A retrospective study of 14 cases. Equine Vet. J. Suppl. 2000, 32, 108–112. [Google Scholar] [CrossRef]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.J.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM consensus statement on equine metabolic syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef]
- Bond, S.; Leguillette, R.; Richard, E.A.; Couetil, L.; Lavoie, J.P.; Martin, J.G.; Pirie, R.S. Equine asthma: Integrative biologic relevance of a recently proposed nomenclature. J. Vet. Intern. Med. 2018, 32, 2088–2098. [Google Scholar] [CrossRef] [Green Version]
- Peachey, L.E.; Molena, R.A.; Jenkins, T.P.; Di Cesare, A.; Traversa, D.; Hodgkinson, J.E.; Cantacessi, C. The relationships between faecal egg counts and gut microbial composition in UK Thoroughbreds infected by cyathostomins. Int. J. Parasitol. 2018, 48, 403–412. [Google Scholar] [CrossRef]
- Peachey, L.E.; Castro, C.; Molena, R.A.; Jenkins, T.P.; Griffin, J.L.; Cantacessi, C. Dysbiosis associated with acute helminth infections in herbivorous youngstock—Observations and implications. Sci. Rep. 2019, 9, 11121. [Google Scholar] [CrossRef] [Green Version]
- Walshe, N.; Mulcahy, G.; Crispie, F.; Cabrera-Rubio, R.; Cotter, P.; Jahns, H.; Duggan, V. Outbreak of acute larval cyathostominosis—A “perfect storm” of inflammation and dysbiosis. Equine Vet. J. 2020. [Google Scholar] [CrossRef]
- Wang, M.; Wu, L.; Weng, R.; Zheng, W.; Wu, Z.; Lv, Z. Therapeutic potential of helminths in autoimmune diseases: Helminth-derived immune-regulators and immune balance. Parasitol. Res. 2017, 116, 2065–2074. [Google Scholar] [CrossRef]
- Weinstock, J.V. Do We Need Worms to Promote Immune Health? Clin. Rev. Allergy Immunol. 2015, 49, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Jiang, J.; She, X.; Niu, Y.; Ming, Y. The Potential Role of Schistosome-Associated Factors as Therapeutic Modulators of the Immune System. Infect. Immunol. 2020, 88. [Google Scholar] [CrossRef] [PubMed]
- Charabati, M.; Donkers, S.J.; Kirkland, M.C.; Osborne, L.C. A critical analysis of helminth immunotherapy in multiple sclerosis. Mult. Scler. 2020, 26, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, M.J.; Ardeshir, A.; Kanwar, B.; Mirpuri, J.; Gundra, U.M.; Leung, J.M.; Wiens, K.E.; Vujkovic-Cvijin, I.; Kim, C.C.; Yarovinsky, F.; et al. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog. 2012, 8, e1003000. [Google Scholar] [CrossRef]
- Ruyssers, N.E.; De Winter, B.Y.; De Man, J.G.; Loukas, A.; Pearson, M.S.; Weinstock, J.V.; Van den Bossche, R.M.; Martinet, W.; Pelckmans, P.A.; Moreels, T.G. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm. Bowel Dis. 2009, 15, 491–500. [Google Scholar] [CrossRef]
- Love, S.; Murphy, D.; Mellor, D. Pathogenicity of cyathostome infection. Vet. Parasitol. 1999, 85, discussion 113–121, 121–122, 215–225. [Google Scholar] [CrossRef]
- Lichtenfels, J.R.; Kharchenko, V.A.; Krecek, R.C.; Gibbons, L.M. An annotated checklist by genus and species of 93 species level names for 51 recognized species of small strongyles (Nematoda: Strongyloidea: Cyathostominea) of horses, asses and zebras of the world. Vet. Parasitol. 1998, 79, 65–79. [Google Scholar] [CrossRef]
- Clark, A.; Salle, G.; Ballan, V.; Reigner, F.; Meynadier, A.; Cortet, J.; Koch, C.; Riou, M.; Blanchard, A.; Mach, N. Strongyle Infection and Gut Microbiota: Profiling of Resistant and Susceptible Horses Over a Grazing Season. Front. Physiol. 2018, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Walshe, N.; Duggan, V.; Cabrera-Rubio, R.; Crispie, F.; Cotter, P.; Feehan, O.; Mulcahy, G. Removal of adult cyathostomins alters faecal microbiota and promotes an inflammatory phenotype in horses. Int. J. Parasitol. 2019, 49, 489–500. [Google Scholar] [CrossRef]
- Meier, S.; Paterson, A.; Cantacessi, C.; Scotti, R.; Peachey, L. A good workman never blames his tools; accounting for technical variation in a meta-analysis of equine 16S microbiome data. Unpubl. Data 2020. [Google Scholar]
- O’Donnell, M.M.; Harris, H.M.; Jeffery, I.B.; Claesson, M.J.; Younge, B.; O’Toole, P.W.; Ross, R.P. The core faecal bacterial microbiome of Irish Thoroughbred racehorses. Lett. Appl. Microbiol. 2013, 57, 492–501. [Google Scholar] [CrossRef]
- Costa, M.C.; Weese, J.S. Understanding the Intestinal Microbiome in Health and Disease. Vet. Clin. N. Am. Equine Pract. 2018, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Quercia, S.; Freccero, F.; Castagnetti, C.; Soverini, M.; Turroni, S.; Biagi, E.; Rampelli, S.; Lanci, A.; Mariella, J.; Chinellato, E.; et al. Early colonisation and temporal dynamics of the gut microbial ecosystem in Standardbred foals. Equine Vet. J. 2018. [Google Scholar] [CrossRef]
- Costa, M.C.; Stampfli, H.R.; Allen-Vercoe, E.; Weese, J.S. Development of the faecal microbiota in foals. Equine Vet. J. 2016, 48, 681–688. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, U.; Henderson, J.D.; Furtado, K.L.; Pedroja, M.; Elenamarie, O.; Mora, A.; Pechanec, M.Y.; Maga, E.A.; Mienaltowski, M.J. Utilizing the fecal microbiota to understand foal gut transitions from birth to weaning. PLoS ONE 2019, 14, e0216211. [Google Scholar] [CrossRef] [Green Version]
- Lindenberg, F.; Krych, L.; Kot, W.; Fielden, J.; Frokiaer, H.; van Galen, G.; Nielsen, D.S.; Hansen, A.K. Development of the equine gut microbiota. Sci. Rep. 2019, 9, 14427. [Google Scholar] [CrossRef]
- Francis-Smith, K.; Wood-Gush, D.G. Coprophagia as seen in thoroughbred foals. Equine Vet. J. 1977, 9, 155–157. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440 e1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentine, G.; Chu, D.M.; Stewart, C.J.; Aagaard, K.M. Relationships Between Perinatal Interventions, Maternal-Infant Microbiomes, and Neonatal Outcomes. Clin. Perinatol. 2018, 45, 339–355. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019. [Google Scholar] [CrossRef] [PubMed]
- Wohlfender, F.D.; Barrelet, F.E.; Doherr, M.G.; Straub, R.; Meier, H.P. Diseases in neonatal foals. Part 1: The 30 day incidence of disease and the effect of prophylactic antimicrobial drug treatment during the first three days post partum. Equine Vet. J. 2009, 41, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Tennent-Brown, B. Plasma therapy in foals and adult horses. Compend. Contin. Educ. Vet. 2011, 33, E1–E4. [Google Scholar]
- Metcalf, J.L.; Song, S.J.; Morton, J.T.; Weiss, S.; Seguin-Orlando, A.; Joly, F.; Feh, C.; Taberlet, P.; Coissac, E.; Amir, A.; et al. Evaluating the impact of domestication and captivity on the horse gut microbiome. Sci. Rep. 2017, 7, 15497. [Google Scholar] [CrossRef] [Green Version]
- Knutie, S.A.; Wilkinson, C.L.; Kohl, K.D.; Rohr, J.R. Early-life disruption of amphibian microbiota decreases later-life resistance to parasites. Nat. Commun. 2017, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, L.A.; Smith, K.A.; Filbey, K.J.; Harcus, Y.; Hewitson, J.P.; Redpath, S.A.; Valdez, Y.; Yebra, M.J.; Finlay, B.B.; Maizels, R.M. Commensal-pathogen interactions in the intestinal tract: Lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes 2014, 5, 522–532. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, T.P.; Formenti, F.; Castro, C.; Piubelli, C.; Perandin, F.; Buonfrate, D.; Otranto, D.; Griffin, J.L.; Krause, L.; Bisoffi, Z.; et al. A comprehensive analysis of the faecal microbiome and metabolome of Strongyloides stercoralis infected volunteers from a non-endemic area. Sci. Rep. 2018, 8, 15651. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Tang, M.S.; Lim, Y.A.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J.; et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl. Trop. Dis. 2014, 8, e2880. [Google Scholar] [CrossRef]
- Davidson, A.J.; Hodgkinson, J.E.; Proudman, C.J.; Matthews, J.B. Cytokine responses to Cyathostominae larvae in the equine large intestinal wall. Res. Vet. Sci. 2005, 78, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Edwards, G.B.; Proudman, C.J.; Cripps, P.J.; Matthews, J.B. Cytokine mRNA expression pattern in horses with large intestinal disease. Res. Vet. Sci. 2002, 72, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W.F.; Song, Y.; Wang, A.J.; Smith, A.; Grinchuk, V.; Mongodin, E.; Pei, C.; Ma, B.; Lu, N.; Urban, J.F., Jr.; et al. Type 2 immunity-dependent reduction of segmented filamentous bacteria in mice infected with the helminthic parasite Nippostrongylus brasiliensis. Microbiome 2015, 3, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancroft, A.J.; Hayes, K.S.; Grencis, R.K. Life on the edge: The balance between macrofauna, microflora and host immunity. Trends Parasitol. 2012, 28, 93–98. [Google Scholar] [CrossRef]
- Brundler, P.; Frey, C.F.; Gottstein, B.; Nussbaumer, P.; Neuhaus, S.; Gerber, V. Lower shedding of strongylid eggs by Warmblood horses with recurrent airway obstruction compared to unrelated healthy horses. Vet. J. 2011, 190, e12–e15. [Google Scholar] [CrossRef]
- Neuhaus, S.; Bruendler, P.; Frey, C.F.; Gottstein, B.; Doherr, M.G.; Gerber, V. Increased parasite resistance and recurrent airway obstruction in horses of a high-prevalence family. J. Vet. Intern. Med. 2010, 24, 407–413. [Google Scholar] [CrossRef]
- Gibson, T.E. The effect of repeated anthelmintic treatment with phenothiazine on the faecal egg counts of housed horses, with some observations on the life cycle of Trichonema spp. in the horse. J. Helminthol. 1953, 27, 29–40. [Google Scholar] [CrossRef]
- Peregrine, A.S.; McEwen, B.; Bienzle, D.; Koch, T.G.; Weese, J.S. Larval cyathostominosis in horses in Ontario: An emerging disease? Can. Vet. J. 2006, 47, 80–82. [Google Scholar]
- Giles, C.J.; Urquhart, K.A.; Longstaffe, J.A. Larval cyathostomiasis (immature trichonema-induced enteropathy): A report of 15 clinical cases. Equine Vet. J. 1985, 17, 196–201. [Google Scholar] [CrossRef]
- Mair, T.S. Outbreak of larval cyathostomiasis among a group of yearling and two-year-old horses. Vet. Rec. 1994, 135, 598–600. [Google Scholar]
- Reilly, G.A.; Cassidy, J.P.; Taylor, S.M. Two fatal cases of diarrhoea in horses associated with larvae of the small strongyles. Vet. Rec. 1993, 132, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.W.; Mair, T.S.; Hillyer, M.H.; Love, S. Epidemiological risk factors associated with a diagnosis of clinical cyathostomiasis in the horse. Equine Vet. J. 1995, 27, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.R.; French, D.D.; Klei, T.R. Seasonal transmission of gastrointestinal parasites of equids in southern Louisiana. J. Parasitol. 2001, 87, 1371–1378. [Google Scholar] [CrossRef]
- Eysker, M.; Mirck, M.H. The distribution of inhibited early third stage Cyathostominae larvae in the large intestine of the horse. Z. Parasitenkd. 1986, 72, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Smets, K.; Shaw, D.J.; Deprez, P.; Vercruysse, J. Diagnosis of larval cyathostominosis in horses in Belgium. Vet. Rec. 1999, 144, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Deprez, P.; Vercruysse, J. Treatment and follow-up of clinical cyathostominosis in horses. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 527–529. [Google Scholar] [CrossRef]
- Eysker, M.; Boersema, J.H.; Kooyman, F.N. Seasonally inhibited development of cyathostomine nematodes in Shetland ponies in The Netherlands. Vet. Parasitol. 1990, 36, 259–264. [Google Scholar] [CrossRef]
- Ogbourne, C.P. Epidemiological studies on horses infected with nematodes of the family Trichonematidae (Witenberg, 1925). Int. J. Parasitol. 1975, 5, 667–672. [Google Scholar] [CrossRef]
- Mair, T.S. Recurrent diarrhoea in aged ponies associated with larval cyathostomiasis. Equine Vet. J. 1993, 25, 161–163. [Google Scholar] [CrossRef]
- Murphy, D.; Love, S. The pathogenic effects of experimental cyathostome infections in ponies. Vet. Parasitol. 1997, 70, 99–110. [Google Scholar] [CrossRef]
- Ramanan, D.; Bowcutt, R.; Lee, S.C.; Tang, M.S.; Kurtz, Z.D.; Ding, Y.; Honda, K.; Gause, W.C.; Blaser, M.J.; Bonneau, R.A.; et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 2016, 352, 608–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glendinning, L.; Nausch, N.; Free, A.; Taylor, D.W.; Mutapi, F. The microbiota and helminths: Sharing the same niche in the human host. Parasitology 2014, 141, 1255–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, T.P.; Peachey, L.E.; Ajami, N.J.; MacDonald, A.S.; Hsieh, M.H.; Brindley, P.J.; Cantacessi, C.; Rinaldi, G. Schistosoma mansoni infection is associated with quantitative and qualitative modifications of the mammalian intestinal microbiota. Sci. Rep. 2018, 8, 12072. [Google Scholar] [CrossRef]
- Holzscheiter, M.; Layland, L.E.; Loffredo-Verde, E.; Mair, K.; Vogelmann, R.; Langer, R.; Wagner, H.; Prazeres da Costa, C. Lack of host gut microbiota alters immune responses and intestinal granuloma formation during schistosomiasis. Clin. Exp. Immunol. 2014, 175, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Schoster, A.; Weese, J.S.; Guardabassi, L. Probiotic use in horses—What is the evidence for their clinical efficacy? J. Vet. Intern. Med. 2014, 28, 1640–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoster, A. Probiotic Use in Equine Gastrointestinal Disease. Vet. Clin. N. Am. Equine Pract. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoster, A.; Arroyo, L.G.; Staempfli, H.R.; Weese, J.S. Comparison of microbial populations in the small intestine, large intestine and feces of healthy horses using terminal restriction fragment length polymorphism. BMC Res. Notes 2013, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougal, K.; Harris, P.A.; Edwards, A.; Pachebat, J.A.; Blackmore, T.M.; Worgan, H.J.; Newbold, C.J. A comparison of the microbiome and the metabolome of different regions of the equine hindgut. FEMS Microbiol. Ecol. 2012, 82, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.C.; Silva, G.; Ramos, R.V.; Staempfli, H.R.; Arroyo, L.G.; Kim, P.; Weese, J.S. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments in horses. Vet. J. 2015, 205, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Anderson, M.E.; Lowe, A.; Monteith, G.J. Preliminary investigation of the probiotic potential of Lactobacillus rhamnosus strain GG in horses: Fecal recovery following oral administration and safety. Can. Vet. J. 2003, 44, 299–302. [Google Scholar]
- Desrochers, A.M.; Dolente, B.A.; Roy, M.F.; Boston, R.; Carlisle, S. Efficacy of Saccharomyces boulardii for treatment of horses with acute enterocolitis. J. Am. Vet. Med. Assoc. 2005, 227, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Respondek, F.; Goachet, A.G.; Julliand, V. Effects of dietary short-chain fructooligosaccharides on the intestinal microflora of horses subjected to a sudden change in diet. J. Anim. Sci. 2008, 86, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castano-Rodriguez, N. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohns. Colitis. 2017, 11, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- McKinney, C.A.; Oliveira, B.C.M.; Bedenice, D.; Paradis, M.R.; Mazan, M.; Sage, S.; Sanchez, A.; Widmer, G. The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea. PLoS ONE 2020, 15, e0230148. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.P.M.; Sousa, S.S.; Molezini, F.A.; Ferreira, H.S.D.; Campos, R.D. Efficacy of faecal microbiota transplantation for treating acute colitis in horses undergoing colic surgery. Pesqui. Vet. Bras. 2018, 38, 1564–1569. [Google Scholar] [CrossRef]
- Mullen, K.R.; Yasuda, K.; Divers, T.J.; Weese, J.S. Equine faecal microbiota transplant: Current knowledge, proposed guidelines and future directions. Equine Vet. Educ. 2018, 30, 151–160. [Google Scholar] [CrossRef]
- Petkevicius, S.; Bach Knudsen, K.E.; Murrell, K.D.; Wachmann, H. The effect of inulin and sugar beet fibre on oesophagostomum dentatum infection in pigs. Parasitology 2003, 127 Pt 1, 61–68. [Google Scholar] [CrossRef]
- Thomsen, L.E.; Petkevicius, S.; Bach Knudsen, K.E.; Roepstorff, A. The influence of dietary carbohydrates on experimental infection with Trichuris suis in pigs. Parasitology 2005, 131 Pt 6, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Petkevicius, S.; Murrell, K.D.; Bach Knudsen, K.E.; Jorgensen, H.; Roepstorff, A.; Laue, A.; Wachmann, H. Effects of short-chain fatty acids and lactic acids on survival of Oesophagostomum dentatum in pigs. Vet. Parasitol. 2004, 122, 293–301. [Google Scholar] [CrossRef]
- White, E.C.; Houlden, A.; Bancroft, A.J.; Hayes, K.S.; Goldrick, M.; Grencis, R.K.; Roberts, I.S. Manipulation of host and parasite microbiotas: Survival strategies during chronic nematode infection. Sci. Adv. 2018, 4, eaap7399. [Google Scholar] [CrossRef] [Green Version]
- Tinker, M.K.; White, N.A.; Lessard, P.; Thatcher, C.D.; Pelzer, K.D.; Davis, B.; Carmel, D.K. Prospective study of equine colic risk factors. Equine Vet. J. 1997, 29, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Bull, K.; Davies, G.; Jenkins, T.; Meier, S.; Peachey, L. The composition of equine gut microbiota undergoes a stepwise change in response to increasing levels of domestication. Unpubl. Data 2020. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walshe, N.; Mulcahy, G.; Hodgkinson, J.; Peachey, L. No Worm Is an Island; The Influence of Commensal Gut Microbiota on Cyathostomin Infections. Animals 2020, 10, 2309. https://doi.org/10.3390/ani10122309
Walshe N, Mulcahy G, Hodgkinson J, Peachey L. No Worm Is an Island; The Influence of Commensal Gut Microbiota on Cyathostomin Infections. Animals. 2020; 10(12):2309. https://doi.org/10.3390/ani10122309
Chicago/Turabian StyleWalshe, Nicola, Grace Mulcahy, Jane Hodgkinson, and Laura Peachey. 2020. "No Worm Is an Island; The Influence of Commensal Gut Microbiota on Cyathostomin Infections" Animals 10, no. 12: 2309. https://doi.org/10.3390/ani10122309
APA StyleWalshe, N., Mulcahy, G., Hodgkinson, J., & Peachey, L. (2020). No Worm Is an Island; The Influence of Commensal Gut Microbiota on Cyathostomin Infections. Animals, 10(12), 2309. https://doi.org/10.3390/ani10122309



