Expression of Transient Receptor Potential Ankyrin 1 and Transient Receptor Potential Vanilloid 1 in the Gut of the Peri-Weaning Pig Is Strongly Dependent on Age and Intestinal Site
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling Procedures
2.2. RNA Isolation and Reverse-Transcription Quantitative Real-Time PCR
2.3. Immunohistochemistry
2.3.1. TRPA1 and TRPV1 in Enteroendocrine Cells
2.3.2. TRPA1 and TRPV1 Colocalization with GLP-1 Positive Cells
2.4. Statistics
3. Results
3.1. TRPA1 and TRPV1 Expression in the Gut
3.2. Immunohistochemistry
3.2.1. TRPA1 and TRPV1 on Enteroendocrine Cells
3.2.2. TRPA1 and TRPV1 on GLP-1 Immunoreactive Cells
4. Discussion
4.1. TRPV1 Expression Is Age and Location-Dependent and Colocalizes with GLP-1-IR Cells
4.2. TRPA1 Exhibits Peak Expression in Pylorus around Weaning and Highly Colocalizes with Enteroendocrine Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gribble, F.M.; Reimann, F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef]
- Roura, E.; Humphrey, B.; Tedó, G.; Ipharraguerre, I. Unfolding the codes of short-term feed appetence in farm and companion animals. A comparative oronasal nutrient sensing biology review. Can. J. Anim. Sci. 2008, 88, 535–558. [Google Scholar] [CrossRef]
- Roura, E.; Fu, M. Taste, nutrient sensing and feed intake in pigs (130 years of research: Then, now and future). Anim. Feed Sci. Technol. 2017, 233, 3–12. [Google Scholar] [CrossRef]
- Pingle, S.; Matta, J.; Ahern, G. Capsaicin receptor: TRPV1 a promiscuous TRP channel. In Transient Receptor Potential (TRP) Channels; Springer: Berlin/Heidelberg, Germany, 2007; pp. 155–171. [Google Scholar]
- Gunthorpe, M.J.; Benham, C.D.; Randall, A.; Davis, J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002, 23, 183–191. [Google Scholar] [CrossRef]
- Bandell, M.; Macpherson, L.J.; Patapoutian, A. From chills to chilis: Mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr. Opin. Neurobiol. 2007, 17, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef] [Green Version]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [Green Version]
- Owsianik, G.; Talavera, K.; Voets, T.; Nilius, B. Permeation and selectivity of TRP channels. Annu. Rev. Physiol. 2006, 68, 685–717. [Google Scholar] [CrossRef]
- Chen, J.; Hackos, D.H. TRPA1 as a drug target—Promise and challenges. Naunyn Schmiedeberg’s Arch. Pharmacol. 2015, 388, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Andrei, S.R.; Sinharoy, P.; Bratz, I.N.; Damron, D.S. TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: Co-localization at z-discs, costameres and intercalated discs. Channels 2016, 10, 395–409. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, S.; Michlig, S.; De Senarclens-Bezençon, C.; Meylan, J.; Meystre, J.; Pezzoli, M.; Markram, H.; Le Coutre, J. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, E.C.; Diakogiannaki, E.; Gentry, C.; Psichas, A.; Habib, A.M.; Bevan, S.; Fischer, M.J.M.; Reimann, F.; Gribble, F.M. Stimulation of GLP-1 secretion downstream of the ligand-gated ion channel TRPA1. Diabetes 2015, 64, 1202–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Yan, Z.; Zhong, J.; Chen, J.; Ni, Y.; Li, L.; Ma, L.; Zhao, Z.; Liu, D.; Zhu, Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 2012, 61, 2155–2165. [Google Scholar] [CrossRef] [Green Version]
- Purhonen, A.K.; Louhivuori, L.M.; Kiehne, K.; Åkerman, K.E.O.; Herzig, K.H. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008, 582, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Aihara, E.; Nakamura, A.; Xin, H.; Matsui, H.; Kohama, K.; Takeuchi, K. Expression of vanilloid receptors in rat gastric epithelial cells: Role in cellular protection. Biochem. Pharmacol. 2003, 66, 1115–1121. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.-S.; Taddei, A.; Bizzoco, E.; Lazzeri, M.; Vannucchi, M.G.; Bechi, P. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem. Cell Biol. 2005, 124, 61–68. [Google Scholar] [CrossRef]
- Buckinx, R.; van Nassauw, L.; Avula, L.R.; Alpaerts, K.; Adriaensen, D.; Timmermans, J.P. Transient receptor potential vanilloid type 1 channel (TRPV1) immunolocalization in the murine enteric nervous system is affected by the targeted C-terminal epitope of the applied antibody. J. Histochem. Cytochem. 2013, 61, 421–432. [Google Scholar] [CrossRef]
- Watanabe, T.; Terada, Y. Food compounds activating thermosensitive TRP channels in Asian herbal and medicinal foods. J. Nutr. Sci. Vitaminol. 2015, 61, S86–S88. [Google Scholar] [CrossRef] [PubMed]
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Indian J. Med Res. 2004, 119, 167–179. [Google Scholar] [PubMed]
- Kim, M.J.; Son, H.J.; Song, S.H.; Jung, M.; Kim, Y.; Rhyu, M.R. The TRPA1 Agonist, Methyl Syringate Suppresses Food Intake and Gastric Emptying. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boesmans, W.; Owsianik, G.; Tack, J.; Voets, T.; Vanden Berghe, P. TRP channels in neurogastroenterology: Opportunities for therapeutic intervention. Br. J. Pharmacol. 2011, 162, 18–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef]
- De Lange, C.; Pluske, J.; Gong, J.; Nyachoti, C. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Van Hoorick, A.; Ovyn, A.; Fremaut, D.; De Smet, S.; Dierick, N. Effects of dose and formulation of carvacrol and thymol on bacteria and some functional traits of the gut in piglets after weaning. Arch. Anim. Nutr. 2010, 64, 136–154. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Nabuurs, M.; Hoogendoorn, A.; Van der Molen, E.; Van Osta, A. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in the Netherlands. Res. Vet. Sci. 1993, 55, 78–84. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, X.; Wang, X.; Tan, B.; Li, T.; Yin, Y. Effects of weaning on intestinal upper villus epithelial cells of piglets. PLoS ONE 2016, 11, e0150216. [Google Scholar] [CrossRef] [Green Version]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Dierick, N.; Decuypere, J.; Molly, K.; Vanderbeke, E. Microbial protease addition to a soybean meal diet for weaned piglets: Effects on performance, digestion, gut flora and gut function. Publ. Eur. Assoc. Anim. Prod. 2004, 110, 229–234. [Google Scholar]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Feeding a diet with a decreased protein content reduces both nitrogen content in the gastrointestinal tract and post-weaning diarrhoea, but does not affect apparent nitrogen digestibility in weaner pigs challenged with an enterotoxigenic strain of Escheri. Anim. Feed Sci. Technol. 2010, 160, 148–159. [Google Scholar] [CrossRef]
- Gilbert, M.S.; Ijssennagger, N.; Kies, A.K.; van Mil, S.W.C. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs and poultry. Am. J. Physiol. Gastrointest Liver Physiol. 2018, 315, G159–G170. [Google Scholar] [CrossRef]
- Pieper, R.; Villodre Tudela, C.; Taciak, M.; Bindelle, J.; Pérez, J.F.; Zentek, J. Health relevance of intestinal protein fermentation in young pigs. Anim. Health Res. Rev. 2016, 17, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Bikker, P.; Dirkzwager, A.; Fledderus, J.; Trevisi, P.; le Huerou-Luron, I.; Lalles, J.; Awati, A. The effect of dietary protein and fermentable carbohydrates levels on growth performance and intestinal characteristics in newly weaned piglets. J. Anim. Sci. 2006, 84, 3337–3345. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Saito, S.; Mori, Y.; Itoh, S.G.; Okumura, H.; Tominaga, M. Structural basis of TRPA1 inhibition by HC-030031 utilizing species-specific differences. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vercelli, C.; Barbero, R.; Re, G. Transient Receptor Potential Vanilloid 1 in animal tissues: An overview to highlight similarities and differences with human species. Recept. Clin. Investig. 2015, 2, 1–9. [Google Scholar]
- Kunert-Keil, C.; Bisping, F.; Krüger, J.; Brinkmeier, H.; Montell, C.; Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.; Bruford, E.; et al. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genom. 2006, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Dubin, A.E.; Bursulaya, B.; Viswanath, V.; Jegla, T.J.; Patapoutian, A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J. Neurosci. 2008, 28, 9640–9651. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Beaulieu, J.-F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Degroote, J.; Van Ginneken, C.; Van Poucke, M.; Vergauwen, H.; Dam, T.M.T.; Vanrompay, D.; Peelman, L.J.; De Smet, S.; Michiels, J. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J. 2016, 30, 863–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Nygard, A.-B.; Jørgensen, C.B.; Cirera, S.; Fredholm, M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol. 2007, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Callaghan, B.; Bron, R.; Bravo, D.M.; Furness, J.B. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 2014, 356, 77–82. [Google Scholar] [CrossRef]
- Hatzimanikatis, V.; Choe, L.H.; Lee, K.H. Proteomics: Theoretical and experimental considerations. Biotechnol. Prog. 1999, 15, 312–318. [Google Scholar] [CrossRef]
- Van Ginneken, C.; Verlinden, K.; Van Meir, F.; Sys, S.; Weyns, A. A stereologic evaluation of glucagon-like peptide-1 (GLP-1) mucosal cells in the small intestine of the developing pig. Anat. Embryol. 2002, 205, 153–157. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90. [Google Scholar] [CrossRef]
- Tolhurst, G.; Reimann, F.; Gribble, F.M. Nutritional regulation of glucagon-like peptide-1 secretion. J. Physiol. 2009, 587, 27–32. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D.J.N. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 is activated by both acidic and basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawardene, A.R.; Corfe, B.M.; Staton, C.A. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 2011, 92, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Priori, D.; Colombo, M.; Clavenzani, P.; Jansman, A.J.M.; Lallès, J.P.; Trevisi, P.; Bosi, P. The olfactory receptor OR51E1 is present along the gastrointestinal tract of pigs, co-localizes with enteroendocrine cells and is modulated by intestinal microbiota. PLoS ONE 2015, 10, e0129501. [Google Scholar] [CrossRef]
- Asakawa, A.; Inui, A.; Kaga, T.; Yuzuriha, H.; Nagata, T.; Fujimiya, M.; Katsuura, G.; Makino, S.; Fujino, M.A.; Kasuga, M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 2001, 74, 143–147. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S. Significance of TRP channels in oxidative stress. Eur. J. Pharmacol. 2016, 793, 109–111. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.W. Emerging roles of TRPA1 in sensation of oxidative stress and its implications in defense and danger. Arch. Pharmacal Res. 2013, 36, 783–791. [Google Scholar] [CrossRef]
- Beumer, J.; Artegiani, B.; Post, Y.; Reimann, F.; Gribble, F.; Nguyen, T.N.; Zeng, H.; Van den Born, M.; Van Es, J.H.; Clevers, H. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat. Cell Biol. 2018, 20, 909. [Google Scholar] [CrossRef]
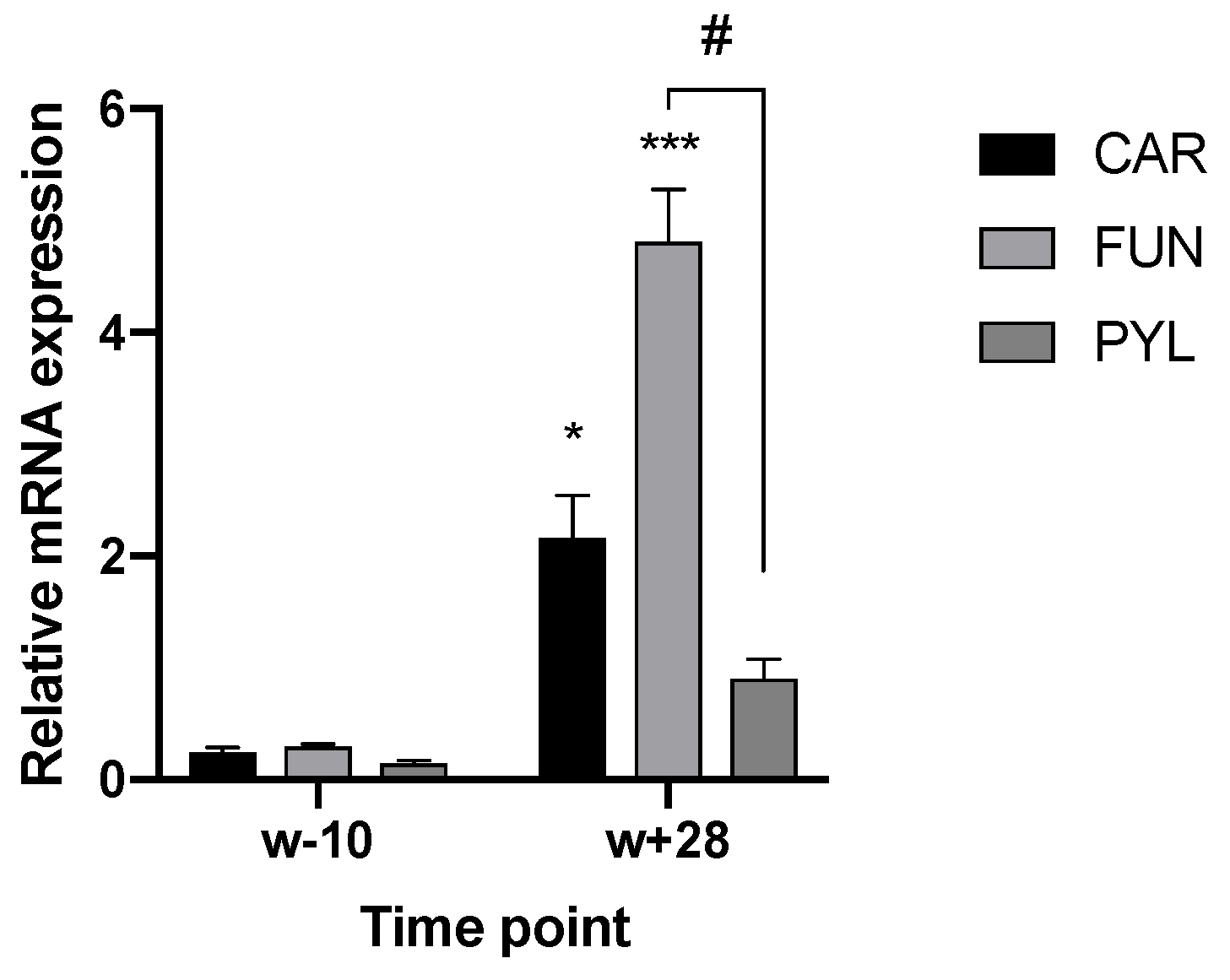








| Gene Symbol | Accession Number | Nucleotide Sequence of Primers, 5′-3′ | Ta (°C) | Product Length (bp) |
|---|---|---|---|---|
| HPRT1 | DQ178126 | Forward: CCGAGGATTTGGAAAAGGT Reverse: CTATTTCTGTTCAGTGCTTTGATGT | 60 | 181 |
| YWHAZ | DQ178130 | Forward: ATGCAACCAACACATCCTATC Reverse: GCATTATTAGCGTGCTGTCTT | 60 | 178 |
| RPL4 | XM_005659862 | Forward: CAAGAGTAACTACAACCTTC Reverse: GAACTCTACGATGAATCTTC | 58 | 122 |
| TRPA1 | XM_021089237.1 | Forward: GAATTTACTCATTGGTTTGGCAGTTGGTG Reverse: CGGTGATGGATTTCTGATCGACCTTG | 58 | 155 |
| TRPV1 | XM_013981216.2 | Forward: GGACAGCGAGTTCAAAGACC Reverse: CCGTTTTCCACCAGAAGTGT | 63 | 240 |
| Target | Species Raised in; Clonality | Dilution | Research Resource Identifier | |
|---|---|---|---|---|
| Primary antibodies | TRPA1 | Rabbit, polyclonal | 1:100 | Cat#NB110-40763, Novus Bio |
| TRPV1 | Rabbit, polyclonal | 1:200 | Cat#orb13755, Biorbyt | |
| Chromogranin A | Mouse, polyclonal | 1:200 | Cat#M0869, Dako | |
| GLP-1 | Mouse, monocloal | 1:5000 | Cat#A6104-1, Immun Diagnostik | |
| Secondary antibodies | Anti Rabbit IgG | Goat, polyclonal | 1:200 | Cat#31823, Invitrogen |
| Envision + System-HRP labeled polymer | Mouse | 1:1 | Cat#K4001, Dako |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Liefferinge, E.; Van Noten, N.; Degroote, J.; Vrolix, G.; Van Poucke, M.; Peelman, L.; Van Ginneken, C.; Roura, E.; Michiels, J. Expression of Transient Receptor Potential Ankyrin 1 and Transient Receptor Potential Vanilloid 1 in the Gut of the Peri-Weaning Pig Is Strongly Dependent on Age and Intestinal Site. Animals 2020, 10, 2417. https://doi.org/10.3390/ani10122417
Van Liefferinge E, Van Noten N, Degroote J, Vrolix G, Van Poucke M, Peelman L, Van Ginneken C, Roura E, Michiels J. Expression of Transient Receptor Potential Ankyrin 1 and Transient Receptor Potential Vanilloid 1 in the Gut of the Peri-Weaning Pig Is Strongly Dependent on Age and Intestinal Site. Animals. 2020; 10(12):2417. https://doi.org/10.3390/ani10122417
Chicago/Turabian StyleVan Liefferinge, Elout, Noémie Van Noten, Jeroen Degroote, Gunther Vrolix, Mario Van Poucke, Luc Peelman, Chris Van Ginneken, Eugeni Roura, and Joris Michiels. 2020. "Expression of Transient Receptor Potential Ankyrin 1 and Transient Receptor Potential Vanilloid 1 in the Gut of the Peri-Weaning Pig Is Strongly Dependent on Age and Intestinal Site" Animals 10, no. 12: 2417. https://doi.org/10.3390/ani10122417
APA StyleVan Liefferinge, E., Van Noten, N., Degroote, J., Vrolix, G., Van Poucke, M., Peelman, L., Van Ginneken, C., Roura, E., & Michiels, J. (2020). Expression of Transient Receptor Potential Ankyrin 1 and Transient Receptor Potential Vanilloid 1 in the Gut of the Peri-Weaning Pig Is Strongly Dependent on Age and Intestinal Site. Animals, 10(12), 2417. https://doi.org/10.3390/ani10122417






