Movement Patterns and Diel Activity of Anguilla japonica in the Middle Part of a Large River in South Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
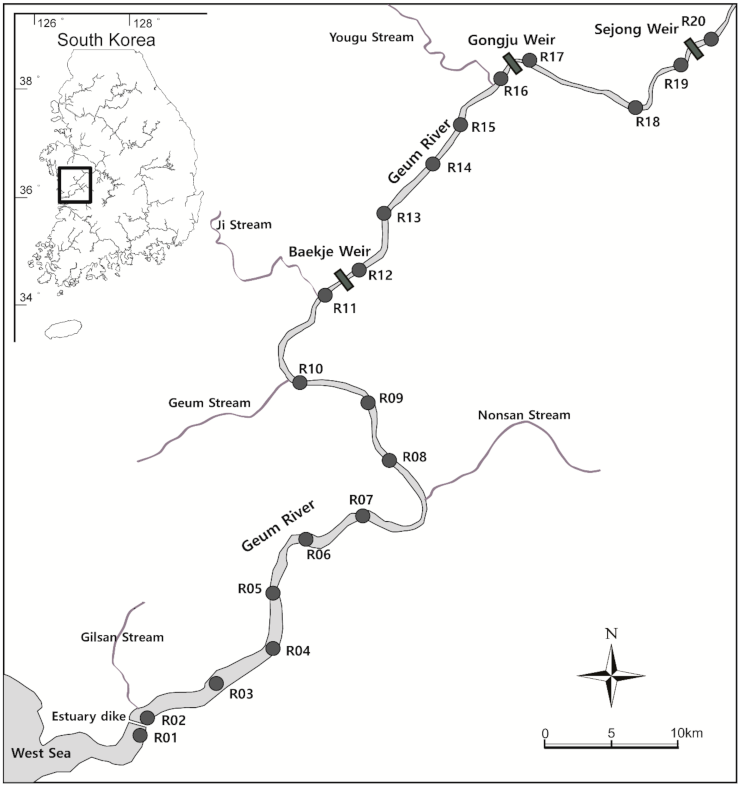
2.1. Study Site
2.2. Acoustic Tagging
2.3. Acoustic Monitoring
2.4. Data Analysis
3. Results
3.1. Longitudinal Movement
3.2. Diel Activity
3.3. Vertical Movement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tesch, F.-W. Homing of eels (Anguilla anguilla) in the southern North Sea. Mar. Biol. 1967, 1, 2–9. [Google Scholar] [CrossRef]
- Tesch, F.W. The Eel; Blackwell Publishing: Oxford, UK, 2003. [Google Scholar]
- Tsukamoto, K.; Chow, S.; Otake, T.; Kurogi, H.; Mochioka, N.; Miller, M.J.; Aoyama, J.; Kimura, S.; Watanabe, S.; Yoshinaga, T.; et al. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat. Commun. 2011, 2, 179. [Google Scholar] [CrossRef] [PubMed]
- Fishbase. Available online: http://www.fishbase.org (accessed on 1 November 2020).
- Ishikawa, S.; Aoyama, J.; Tsukamoto, K.; Nishida, M. Population structure of the Japanese eel Anguilla japonica as examined by mitochondrial DNA sequencing. Fish. Sci. 2001, 67, 246–253. [Google Scholar] [CrossRef]
- Han, Y.S.; Hung, C.L.; Liao, Y.F.; Tzeng, W.N. Population genetic structure of the Japanese eel Anguilla japonica: Panmixia at spatial and temporal scales. Mar. Ecol. Prog. Ser. 2010, 401, 221–232. [Google Scholar] [CrossRef]
- Minegishi, Y.; Henkel, C.V.; Dirks, R.P.; van den Thillart, G.E. Genomics in Eels—Towards Aquaculture and Biology. Mar. Biotechnol. 2012, 14, 583–590. [Google Scholar] [CrossRef][Green Version]
- FAO. Species Fact Sheets, Anguilla japonica (Temminck & Schlegel, 1847). 2013. Available online: http://www.fao.org/fishery/species/2988/en (accessed on 10 September 2020).
- Tatsukawa, K. Eel resources in East Asia. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Janpan, 2003; pp. 293–298. [Google Scholar]
- Kimura, S.; Inoue, T.; Sugimoto, T. Fluctuation in the distribution of low-salinity water in the North Equatorial Current and its effect on the larval transport of the Japanese eel. Fish. Oceanogr. 2001, 10, 51–60. [Google Scholar] [CrossRef]
- Bonhommeau, S.; Chassot, E.; Rivot, E. Fluctuations in European eel (Anguilla anguilla) recruitment resulting from environmental changes in the Sargasso Sea. Fish. Oceanogr. 2008, 17, 32–44. [Google Scholar] [CrossRef]
- Miller, M.J.; Kimura, S.; Friedland, K.D.; Knights, B.; Kim, H.; Jellyman, D.J.; Tsukamoto, K. Review of ocean-atmospheric factors in the Atlantic and Pacific oceans influencing spawning and recruitment of anguillid eels. In Challenges for Diadromous Fishes in a Dynamic Global Environment; Haro, A., Smith, K.L., Rulifson, R.A., Moffitt, C.M., Klauda, R.J., Dadswell, M.J., Cunjak, R.A., Cooper, J.E., Beal, K.L., Avery, T.S., Eds.; Symposium 69; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 231–249. [Google Scholar]
- Tzeng, W.N.; Tseng, Y.H.; Han, Y.S.; Hsu, C.C.; Chang, C.W.; Di Lorenzo, E.; Hsieh, C.H. Evaluation of Multi-Scale Climate Effects on Annual Recruitment Levels of the Japanese Eel, Anguilla japonica, to Taiwan. PLoS ONE 2012, 7, e30805. [Google Scholar] [CrossRef][Green Version]
- IUCN. The IUCN Red List of Threatened Species. 2020. Available online: http://www.iucnredlist.org/ (accessed on 15 September 2020).
- Kimura, S.; Tsukamoto, K. The salinity front in the North Equatorial Current: A landmark for the spawning migration of the Japanese eel (Anguilla japonica) related to the stock recruitment. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 315–325. [Google Scholar] [CrossRef]
- Tsukamoto, K. Oceanic migration and spawning of anguillid eels. J. Fish Biol. 2009, 74, 1833–1852. [Google Scholar] [CrossRef]
- Kuroki, M.; Aoyama, J.; Miller, M.J.; Yoshinaga, T.; Shinoda, A.; Hagihara, S.; Tsukamoto, K. Sympatric spawning of Anguilla marmorata and Anguilla japonica in the western North Pacific Ocean. J. Fish Biol. 2009, 74, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, A.; Aoyama, J.; Miller, M.J.; Otake, T.; Mochioka, N.; Watanabe, S.; Minegishi, Y.; Kuroki, M.; Yoshinaga, T.; Yokouchi, K.; et al. Evaluation of the larval distribution and migration of the Japanese eel in the western North Pacific. Rev. Fish Biol. Fish. 2010, 21, 591–611. [Google Scholar] [CrossRef]
- Tzeng, W.N. Immigration timing and activity rhythms of the eel, Anguilla japonica, elvers in the estuary of northern Taiwan, with emphasis on environmental influences. Bull. Jpn. Soc. Fish. Oceanog. 1985, 47, 11–27. [Google Scholar]
- Kaifu, K.; Miyazaki, S.; Aoyama, J.; Kimura, S.; Tsukamoto, K. Diet of Japanese eels Anguilla japonica in the Kojima Bay-Asahi River system, Japan. Environ. Boil. Fishes 2012, 96, 439–446. [Google Scholar] [CrossRef]
- Wakiya, R.; Mochioka, N. Contrasting diets of the Japanese eel, Anguilla japonica, in the upper and lower areas of Tsuchikawa-gawa River, Kagoshima, Japan. Ichthyol. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Hong, Y.K.; Song, M.Y.; Yi, S.E.; Lee, W.O. Change in morphological and biological characteristics by maturation of Japanese eel, Anguilla japonica, collected in Korea waters. Korean J. Ichthyol. 2015, 27, 263–274. [Google Scholar]
- Piper, A.T.; Manes, C.; Siniscalchi, F.; Marion, A.; Wright, R.M.; Kemp, P.S. Response of seaward-migrating European eel (Anguilla anguilla) to manipulated flow fields. Proc. R. Soc. B Boil. Sci. 2015, 282, 20151098. [Google Scholar] [CrossRef]
- Piper, A.T.; Wright, R.M.; Walker, A.M.; Kemp, P.S. Escapement, route choice, barrier passage and entrainment of seaward migrating European eel, Anguilla anguilla, within a highly regulated lowland river. Ecol. Eng. 2013, 57, 88–96. [Google Scholar] [CrossRef]
- Verhelst, P.; Buysse, D.; Reubens, J.; Pauwels, I.; Aelterman, B.; Van Hoey, S.; Goethals, P.; Coeck, J.; Moens, T.; Mouton, A. Downstream migration of European eel (Anguilla anguilla L.) in an anthropogenically regulated freshwater system: Implications for management. Fish. Res. 2018, 199, 252–262. [Google Scholar] [CrossRef]
- Verhelst, P.; Reubens, J.; Pauwels, I.; Buysse, D.; Aelterman, B.; Van Hoey, S.; Goethals, P.; Moens, T.; Coeck, J.; Mouton, A. Movement behaviour of large female yellow European eel (Anguilla anguilla L.) in a freshwater polder area. Ecol. Freshw. Fish 2018, 27, 471–480. [Google Scholar] [CrossRef]
- Trancart, T.; Carpentier, A.; Acou, A.; Danet, V.; Elliott, S.; Feunteun, E. Behaviour of endangered European eels in proximity to a dam during downstream migration: Novel insights using high accuracy 3D acoustic telemetry. Ecol. Freshw. Fish 2020, 29, 266–279. [Google Scholar] [CrossRef]
- Béguer-Pon, M.; Castonguay, M.; Benchetrit, J.; Hatin, D.; Legault, M.; Verreault, G.; Mailhot, Y.; Tremblay, V.; Dodson, J.J. Large-scale, seasonal habitat use and movements of yellow American eels in the St. Lawrence River revealed by acoustic telemetry. Ecol. Freshw. Fish 2015, 24, 99–111. [Google Scholar] [CrossRef]
- Kwak, T.J.; Engman, A.C.; Lilyestrom, C.G. Ecology and conservation of the American eel in the Caribbean region. Fish. Manag. Ecol. 2019, 26, 42–52. [Google Scholar] [CrossRef]
- Okamura, A.; Yamada, Y.; Yokouchi, K.; Horie, N.; Mikawa, N.; Utoh, T.; Tanaka, S.; Tsukamoto, K. A silvering index for the Japanese eel Anguilla japonica. Environ. Biol. Fishes 2007, 80, 77–89. [Google Scholar] [CrossRef]
- Parker, S.J. Homing ability and home range of yellow-phase American eels in a tidally dominated estuary. J. Mar. Biol. Assoc. United Kingd. 1995, 75, 127–140. [Google Scholar] [CrossRef]
- Baras, E.; Jeandrain, D.; Serouge, B.; Philippart, J.C. Seasonal variations of time and space utilisation by radiotagged yellow eels Anguilla anguilla (L.) in a small stream. Hydrobiologia 1998, 371/372, 187–198. [Google Scholar] [CrossRef]
- Lamothe, P.J.; Gallagher, M.; Chivers, D.P.; Moring, J.R. Homing and movement of yellow-phase American eels in freshwater ponds. Environ. Biol. Fishes 2000, 58, 393–399. [Google Scholar] [CrossRef]
- Ford, T.E.; Mercer, E. Density, size distribution and home range of American eels, Anguilla rostrata, in a Massachusetts salt marsh. Environ. Biol. Fishes 1986, 17, 309–314. [Google Scholar] [CrossRef]
- McGovern, P.; McCarthy, T.K. Local movements of freshwater eels (Anguilla anguilla L.) in western Ireland. In Wildlife Telemetry: Remote Sensing and Monitoring of Animals; Priede, I.G., Swift, S.M., Eds.; Ellis Horwood: Chichester, UK, 1992; pp. 319–327. [Google Scholar]
- Walker, A.M.; Godard, M.J.; Davison, P. The home range and behaviour of yellow-stage European eel Anguilla anguilla in an estuarine environment. Aquat. Conserv. 2014, 24, 155–165. [Google Scholar] [CrossRef]
- Itakura, H.; Miyake, Y.; Kitagawa, T.; Kimura, S. Site fidelity, diel and seasonal activities of yellow-phase Japanese eels (Anguilla japonica) in a freshwater habitat as inferred from acoustic telemetry. Ecol. Freshw. Fish 2018, 27, 737–751. [Google Scholar] [CrossRef]
- Chae, B.S.; Song, H.B.; Park, J.Y.; Cho, K.H. A Field Guide to the Freshwater Fishes of Korea; LG Evergreen Foundation: Seoul, Korea, 2019. [Google Scholar]
- White, E.M.; Knights, B. Dynamics of upstream migration of the European eel, Anguilla anguilla (L.), in the Rivers Severn and Avon, England, with special reference to the effects of man-made barriers. Fish. Manag. Ecol. 1997, 4, 311–324. [Google Scholar] [CrossRef]
- Feunteun, E. Management and restoration of European eel population (Anguilla anguilla): An impossible bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Verreault, G.; Dumont, P.; Mailhot, Y. Habitat losses and anthropogenic barriers as a cause of population decline for American Eel (Anguilla rostrata) in the St. Lawrence watershed, Canada. In Proceedings of the 2004 ICES Annual Science Conference (ICES CM 2004/S: 04), Vigo, Spain, 22–25 September 2004; p. 12. [Google Scholar]
- Hitt, N.P.; Eyler, S.; Wofford, J.E.B. Dam removal increases American eel abundance in distant headwater streams. Trans. Am. Fish. Soc. 2012, 141, 1171–1179. [Google Scholar] [CrossRef]
- Matsushige, K.; Hibino, Y.; Yasutake, Y.; Mochioka, N. Japanese eels, Anguilla japonica, can surmount a 46-m-high natural waterfall of the Amikake River system of Kyushu Island, Japan. Ichthyol. Res. 2020. [Google Scholar] [CrossRef]
- Kim, H.S. A Study on Eco-Convergence Type Fish-Way for Korean River. Master’s Thesis, Kumoh National Institute of Technology, Gumi, Korea, 2014. [Google Scholar]
- Jellyman, D.J.; Sykes, J.R.E. Diel and seasonal movements of radio-tagged freshwater eels, Anguilla spp., in two New Zealand streams. Environ. Biol. Fishes 2003, 66, 143–154. [Google Scholar] [CrossRef]
- Riley, W.D.; Walker, A.M.; Bendall, B.; Ives, M.J. Movements of the European eel (Anguilla anguilla) in a chalk stream. Ecol. Freshw. Fish 2011, 20, 628–635. [Google Scholar] [CrossRef]
- Itakura, H.; Kaino, T.; Miyake, Y.; Kitagawa, T.; Kimura, S. Feeding, condition, and abundance of Japanese eels from natural and revetment habitats in the Tone River, Japan. Environ. Biol. Fishes 2015, 98, 1871–1888. [Google Scholar] [CrossRef]
- Barry, J.; Newton, M.; Dodd, J.A.; Hooker, O.E.; Boylan, P.; Lucas, M.C.; Adams, C.E. Foraging specialisms influence space use and movement patterns of the European eel Anguilla anguilla. Hydrobiologia 2015, 766, 333–348. [Google Scholar] [CrossRef]
- Bouchereau, J.; Marques, C.; Pereira, P.; Guelorget, O. Feeding behaviour of Anguilla anguilla and trophic resources in the Ingril Lagoon (Mediterranean, France). Cah. Biol. Mar. 2009, 50, 319–332. [Google Scholar]
- Barbin, G.P. The role of olfaction in homing and estuarine migratory behavior of yellow-phase American eels. Can. J. Fish. Aquat. Sci. 1998, 55, 564–575. [Google Scholar] [CrossRef]
- Dou, S.-Z.; Tsukamoto, K. Observations on the Nocturnal Activity and Feeding Behavior of Anguilla japonica Glass Eels Under Laboratory Conditions. Environ. Boil. Fishes 2003, 67, 389–395. [Google Scholar] [CrossRef]
- Shimatani, Y.; Oguri, S.; Kayaba, Y.I. Impact of Stream Modification on Habitat Component and Fish in Tagawa River. Proc. Hydraul. Eng. 1994, 38, 337–344. [Google Scholar] [CrossRef][Green Version]
- Itakura, H.; Kitagawa, T.; Miller, M.J.; Kimura, S. Declines in catches of Japanese eels in rivers and lakes across Japan: Have river and lake modifications reduced fishery catches? Landsc. Ecol. Eng. 2015, 11, 147–160. [Google Scholar] [CrossRef]
- Morrison, W.E.; Secor, D.H. Abundance of Yellow-Phase American Eels in the Hudson River Estuary. Trans. Am. Fish. Soc. 2004, 133, 896–910. [Google Scholar] [CrossRef]
- Machut, L.S.; Limburg†, K.E.; Schmidt, R.E.; Dittman, D.E. Anthropogenic impacts on American eel demographics in Hudson river tributaries, New York. Trans. Am. Fish. Soc. 2007, 136, 1699–1713. [Google Scholar] [CrossRef]





| ID | Total Length (mm) | Body Weight (g) | Release Site | Release Date (yy-mm-dd) | Last Detected Date (yy-mm-dd) | Last Detected Site | Total Number of Detection | Number of Receivers Detected on |
|---|---|---|---|---|---|---|---|---|
| AJ1 | 550 | 221.6 | R11 | 2015-05-30 | 2015-06-01 | R11 | 19 | 1 |
| AJ2 | 510 | 154.4 | R11 | 2015-05-30 | 2015-06-09 | R11 | 379 | 1 |
| AJ3 | 510 | 147.7 | R11 | 2015-05-30 | 2015-09-09 | R11 | 782 | 1 |
| AJ4 * | 493 | 141 | R11 | 2015-10-15 | 2015-11-05 | R11 | 110 | 1 |
| AJ5 * | 478 | 128 | R11 | 2015-10-15 | 2015-10-19 | R11 | 270 | 1 |
| AJ6 * | 470 | 110 | R11 | 2015-10-15 | 2015-10-21 | R11 | 237 | 1 |
| AJ7 * | 465 | 102 | R11 | 2015-10-15 | 2015-11-08 | R11 | 32 | 1 |
| AJ8 * | 410 | 81 | R11 | 2015-10-17 | 2015-11-16 | R16 | 174 | 2 |
| AJ9 * | 450 | 117 | R11 | 2015-10-17 | 2015-10-30 | R11 | 2328 | 1 |
| AJ10 * | 475 | 109 | R11 | 2015-10-17 | 2015-10-21 | R11 | 1067 | 1 |
| AJ11 * | 444 | 97 | R11 | 2015-10-17 | 2015-10-21 | R11 | 158 | 1 |
| AJ12 * | 422 | 95 | R11 | 2015-10-17 | 2015-10-19 | R11 | 192 | 1 |
| AJ13 | 550 | 203.1 | R16 | 2015-05-30 | 2015-06-01 | R16 | 145 | 1 |
| AJ14 | 511 | 159.3 | R16 | 2015-05-30 | 2015-06-13 | R15 | 48 | 2 |
| AJ15 * | 480 | 126 | R16 | 2015-10-15 | 2015-11-17 | R16 | 1 | 1 |
| AJ16 | 513 | 153.3 | R19 | 2015-05-30 | 2015-06-15 | R19 | 87 | 1 |
| AJ17 | 515 | 175.6 | R19 | 2015-05-30 | 2015-06-13 | R19 | 84 | 1 |
| AJ18 * | 620 | 305 | R19 | 2015-10-15 | 2015-11-11 | R18 | 134 | 2 |
| AJ19 * | 475 | 119 | R19 | 2015-10-15 | 2015-10-30 | R18 | 81 | 3 |
| ID | Detection Period (Day) | Longitudinal Movement (km) | Vertical Movement (m) | ||||
|---|---|---|---|---|---|---|---|
| Movement Boundary | Total Distance | Nighttime | Daytime | ||||
| Total | Average of One Day (SD) | Total | Average of One Day (SD) | ||||
| AJ1 | 2 | 0 | 0 | - | - | - | - |
| AJ2 | 10 | 0 | 0 | - | - | - | - |
| AJ3 | 102 | 0 | 0 | - | - | - | - |
| AJ4 * | 21 | 0 | 0 | 0 | 0 | 0.4 | 0.4 (0) |
| AJ5 * | 4 | 0 | 0 | 69.4 | 34.7 (11.7) | 35.6 | 8.9 (6.8) |
| AJ6 * | 6 | 0 | 0 | 138.8 | 34.7 (60.5) | 1.0 | 1 (0) |
| AJ7 * | 24 | 0 | 0 | 7.6 | 7.6 (0) | 0 | 0 |
| AJ8 * | 30 | 24.2 | 72.6 | 59.2 | 29.6 (13.9) | 0 | 0 |
| AJ9 * | 13 | 0 | 0 | 107.8 | 33.9 (14.9) | 0 | 0 |
| AJ10 * | 4 | 0 | 0 | 198.8 | 49.7 (31.6) | 66 | 22 (7.4) |
| AJ11 * | 4 | 0 | 0 | 3.6 | 1.8 (1.4) | 1.2 | 0.4 (0.2) |
| AJ12 * | 2 | 0 | 0 | 13.2 | 4.4 (3.0) | 9.2 | 4.6 (4.8) |
| AJ13 | 2 | 0 | 0 | - | - | - | - |
| AJ14 | 14 | 5.6 | 5.6 | - | - | - | - |
| AJ15 * | 33 | 0 | 0 | 0 | 0 | 0 | 0 |
| AJ16 | 16 | 0 | 0 | - | - | - | - |
| AJ17 | 14 | 0 | 0 | - | - | - | - |
| AJ18 * | 27 | 6.5 | 6.5 | 77.4 | 19.4 (15.8) | 0.8 | 0.8 (0) |
| AJ19 * | 15 | 43 | 79.5 | 47 | 15.7 (14.4) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Park, S.-H.; Baek, S.-H.; Jang, M.-H.; Yoon, J.-D. Movement Patterns and Diel Activity of Anguilla japonica in the Middle Part of a Large River in South Korea. Animals 2020, 10, 2424. https://doi.org/10.3390/ani10122424
Kim J-H, Park S-H, Baek S-H, Jang M-H, Yoon J-D. Movement Patterns and Diel Activity of Anguilla japonica in the Middle Part of a Large River in South Korea. Animals. 2020; 10(12):2424. https://doi.org/10.3390/ani10122424
Chicago/Turabian StyleKim, Jeong-Hui, Sang-Hyeon Park, Seung-Ho Baek, Min-Ho Jang, and Ju-Duk Yoon. 2020. "Movement Patterns and Diel Activity of Anguilla japonica in the Middle Part of a Large River in South Korea" Animals 10, no. 12: 2424. https://doi.org/10.3390/ani10122424
APA StyleKim, J.-H., Park, S.-H., Baek, S.-H., Jang, M.-H., & Yoon, J.-D. (2020). Movement Patterns and Diel Activity of Anguilla japonica in the Middle Part of a Large River in South Korea. Animals, 10(12), 2424. https://doi.org/10.3390/ani10122424






