Platelet Function and Therapeutic Applications in Dogs: Current Status and Future Prospects
Abstract
Simple Summary
Abstract
1. Introduction
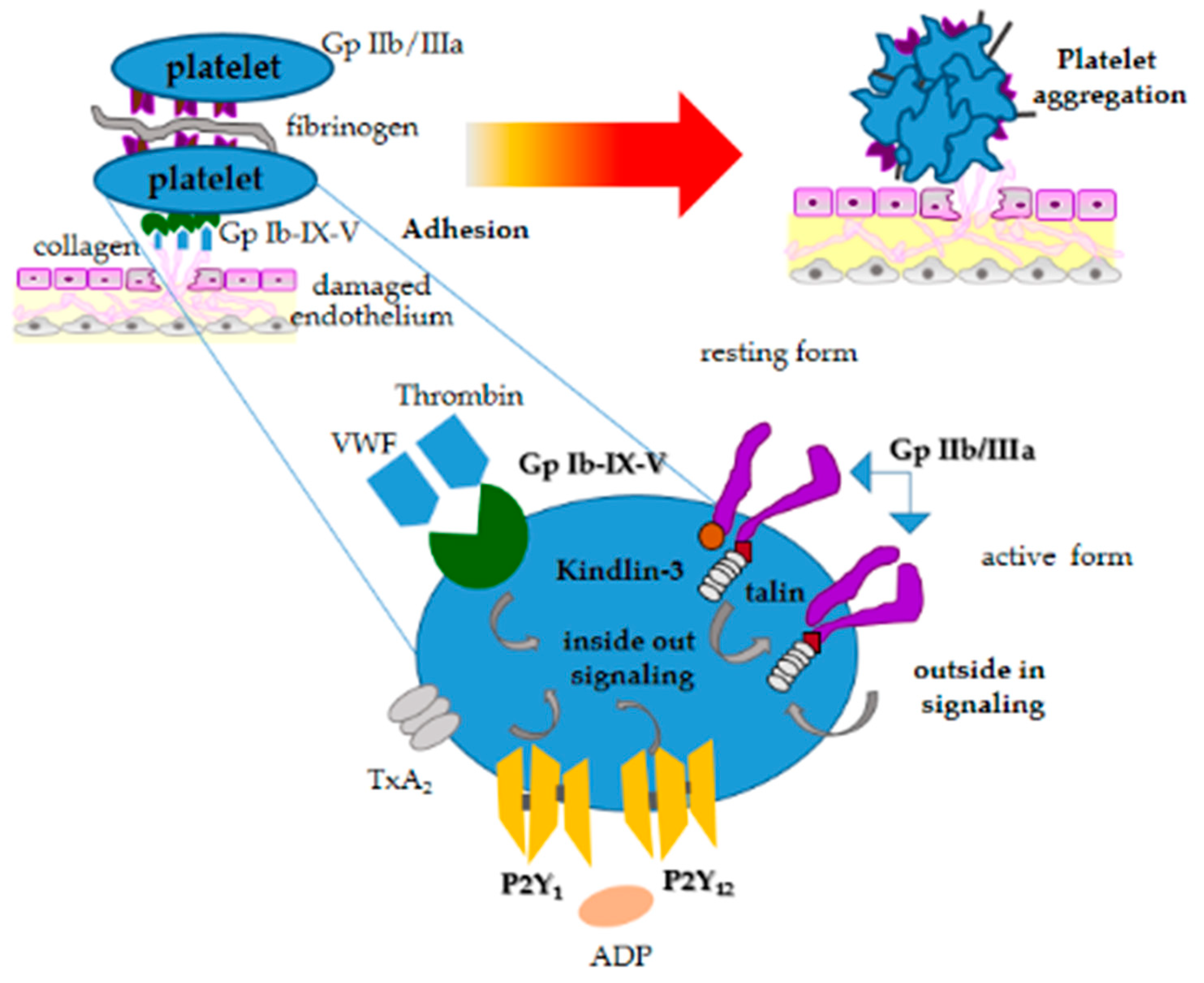
2. Structure and Function of Platelets in Dogs
3. Platelet Disorders in Dogs
3.1. Thrombocytopenias
3.2. Platelet Function Disorders
3.3. Thrombocytosis
4. Platelet Laboratory Testing in Dogs
5. New Therapies with Canine Platelets
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. 2008, 13, 3532–3548. [Google Scholar] [CrossRef]
- Yeaman, M.R. Platelets in defense against bacterial pathogens. Cell. Mol. Life Sci. 2010, 67, 525–544. [Google Scholar] [CrossRef]
- Leslie, M. Cell biology. Beyond clotting the powers of platelets. Science 2010, 328, 562–564. [Google Scholar]
- D’Souza, D.; Wu, K.K.; Hellum, J.D.; Phillips, M.D. Platelet activation and arterial thrombosis. Lancet 1994, 34, 991–995. [Google Scholar]
- Huang, H.S.; Chang, H.H. Platelets in inflammation and immune modulations: Functions beyond hemostasis. Arch. Immunol. Ther. Ex. 2012, 60, 443–451. [Google Scholar]
- Goubran, H.A.; Burnouf, T.; Radosevic, M.; El-Ekiaby, M. The platelet cancer loop. Eur. J. Intern. Med. 2013, 24, 393–400. [Google Scholar] [CrossRef]
- Badimon, L.; Suades, R.; Fuentes, E.; Palomo, I.; Padrò, T. Role of platelet-derived microvescicles as crosstalk mediators in atherothrombosis and future pharmacology targets: A link between inflammation, atherosclerosis and thrombosis. Front. Pharmacol. 2016, 7, 293. [Google Scholar] [CrossRef]
- Podda, G.; Femia, E.A.; Pugliano, M.; Cattaneo, M. Congenital defects of platelet function. Platelets 2012, 23, 552–563. [Google Scholar] [CrossRef]
- Lubkowska, A.; Dołęgowska, B.; Banfi, G. Growth factor content in PRP and their applicability in medicine. J. Biol. Regul. Homeost. Agents 2012, 26, 3S–22S. [Google Scholar]
- Masoudi, E.; Ribas, J.; Kaushik, G.; Leijten, J.; Khademhosseini, A. Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Curr. Stem Cell Rep. 2016, 2, 33–42. [Google Scholar] [CrossRef]
- Bogers, S.H. Cell-based therapies for joint disease in veterinary medicine: What we have learned and what we need to know. Front. Vet. Sci. 2018, 5, 70. [Google Scholar] [CrossRef]
- Bessis, M. Red cell shapes. An illustrated classification and its rationale. Nouv. Rev. Francaise D’Hématologie 1972, 12, 721–745. [Google Scholar]
- Halmay, D.; Sótonyi, P.; Vajdovich, P.; Gaál, T. Morphological evaluation of canine platelets on giemsa- and pas-stained blood smears. Acta Vet. Hung. 2005, 53, 337–350. [Google Scholar] [CrossRef]
- Tablin, F.; Jain, N.C.; Mandell, C.; Hopper, P.E.; Zinkl, J.G. Ultrastructural analysis of platelets and megakaryocytes from a dog with probable essential thrombocythemia. Vet. Pathol. 1989, 26, 289–293. [Google Scholar] [CrossRef]
- Hoffbrand, A.; Pettit, J.E. A Klinikai Haematologia Alapjai; Fundamentals of Clinical Hematology; Springer Hungarica: Budapest, Hungary, 1997; pp. 326–346. [Google Scholar]
- Day, M.; Mackin, A.; Littlewood, J. Manual of Canine and Feline Haematology and Transfusion Medicine; Iowa University Press: Ames, IA, USA, 2000. [Google Scholar]
- Stief, M.; Gottschalk, J.; Ionita, J.C.; Einspanier, A.; Oechtering, G.; Böttcher, P. Concentration of platelets and growth factors in canine autologous conditioned plasma. Vet. Comp. Orthop. Traumatol. 2011, 24, 122–125. [Google Scholar] [CrossRef]
- Silva, R.F.; Santana, G.C.; Leme, F.O.P.; Carmona, J.U.; Rezende, C.M.F. Release of transforming growth factor beta 1 and platelet derived growth factor type AB from canine platelet gels obtained by the tube method and activated with calcium salts. Arch. Med. Vet. 2013, 45, 159–165. [Google Scholar] [CrossRef][Green Version]
- Meyers, K.M.; Holmsen, H.; Seachord, C.L. Comparative study of platelet dense granule constituents. Am. J. Physiol. 1982, 243, R454–R461. [Google Scholar] [CrossRef]
- Curotto, S.; Lunsford, K.; Smith, W.; Thomason, J.; Bulla, C. Evidence of selective packaging and different a-granule subtypes in canine platelets. J. Comp. Pathol. 2012, 147, 499–502. [Google Scholar] [CrossRef]
- Kamykowski, J.; Carlton, P.; Sehgal, S.; Storrie, B. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet alpha-granules. Blood 2011, 118, 1370–1373. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet α–granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Callan, M.B.; Bennett, J.S.; Phillips, D.K.; Haskins, M.E.; Hayden, J.E.; Anderson, J.G.; Giger, U. Inherited platelet delta-storage pool disease in dogs causing severe bleeding: An animal model for a specific ADP deficiency. Thromb. Haemost. 1995, 74, 949–953. [Google Scholar]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef]
- Jackson, S.P. The growing complexity of platelet aggregation. Blood 2007, 109, 5087–5095. [Google Scholar] [CrossRef]
- Moser, M.; Bauer, M.; Schmid, S.; Ruppert, R.; Schmidt, S.; Sixt, M.; Wang, H.V.; Sperandio, M.; Fassler, R. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med. 2009, 15, 300–305. [Google Scholar] [CrossRef]
- Kim, S.; Kunapuli, S.P. P2Y12 receptor in platelet activation. Platelets 2011, 22, 56–60. [Google Scholar] [CrossRef]
- Wang, Y.X.; Vincelette, J.; da Cunha, V.; Martin-McNulty, B.; Mallari, C.; Fitch, R.M.; Alexander, S.; Islam, I.; Buckman, B.O.; Yuan, S.; et al. A novel P2Y(12) adenosine diphosphate receptor antagonist that inhibits platelet aggregation and thrombus formation in rat and dog models. Thromb. Haemost. 2007, 97, 847–855. [Google Scholar]
- Meyers, K.M.; Huston, L.Y.; Clemmons, R.M. Regulation of canine platelet function II. Catecholamines. Am. J. Physiol. 1983, 245, R100–R109. [Google Scholar] [CrossRef]
- Fullard, J.F. The role of the platelet glycoprotein IIb/IIIa in thrombosis and haemostasis. Curr. Pharm. Des. 2004, 10, 1567–1576. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Murphy, R.C.; Watson, S.P. Platelet lipidomics: Modern day perspective on lipid discovery and characterization in platelets. Circ. Res. 2014, 114, 1185–1203. [Google Scholar] [CrossRef]
- Trichler, S.A.; Bulla, S.C.; Mahajan, N.; Lunsford, K.V.; Pendarvis, K.; Nanduri, B.; McCarthy, F.M.; Bulla, C. Identification of canine platelet proteins separated by differential detergent fractionation for nonelectrophoretic proteomics analyzed by Gene Ontology and pathways analysis. Veter. Med. Res. Rep. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Garcia, A. Platelet clinical proteomics: Facts, challenges, and future perspectives. Proteomics. Clin. Appl. 2016, 10, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Rabani, V. Lipid Rafts of Platelet Membrane as Therapeutic Target: Role of “Omics”. Human Health and Pathology. Ph.D. Thesis, Université Bourgogne Franche-Comté, Besançon, France, 2017. (In English). [Google Scholar]
- Rabani, V.; Davani, S. Translational approaches in cardiovascular diseases by omics. Curr. Issues Mol. Biol. 2017, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Geue, S.; Coman, C.; Münzer, P.; Kopczynski, D.; Has, C.; Hoffmann, N.; Manke, M.C.; Lang, F.; Sickmann, A.; et al. Identification of key lipids critical for platelet activation by comprehensive analysis of the platelet lipidome. Blood 2018, 132, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.D.; Haggstrom, J.; Olsen, L.H.; Christensen, K.; Selin, A.; Burmeister, M.L.; Larsen, H. Idiopathic asymptomatic thrombocytopenia in Cavalier King Charles Spaniels is an autosomal recessive trait. J. Vet. Int. Med. 2002, 16, 169–173. [Google Scholar] [CrossRef]
- Davis, E.G.; Wilkerson, M.J.; Rush, B.R. Flow cytometry: Clinical applications in equine medicine. J. Vet. Intern. Med. 2002, 16, 404–410. [Google Scholar] [CrossRef]
- Cowan, S.M.; Bartges, J.W.; Gompf, R.E.; Hayes, J.R.; Moyers, T.D.; Snider, C.C.; Gerard, D.A.; Craft, R.M.; Muenchen, R.A.; Carroll, R.C. Giant platelet disorder in the Cavalier King Charles spaniel. Exp. Hematol. 2004, 32, 344–350. [Google Scholar] [CrossRef]
- Singh, M.K.; Lamb, W.A. Idiopathic trombocytopenia in Cavalier King Charles Spaniels. Aust. Vet. J. 2005, 83, 700–703. [Google Scholar] [CrossRef]
- Gelain, M.E.; Bertazzolo, W.; Tutino, G.; Pogliani, E.; Cian, F.; Boudreaux, M.K. A novel point mutation in the β1-tubulin gene in asymptomatic macrothrombocytopenic Norfolk and Cairn Terriers. Vet. Clin. Pathol. 2014, 43, 317–321. [Google Scholar] [CrossRef]
- Sullivan, P.S.; Evans, H.L.; McDonald, T.P. Platelet concentration and hemoglobin function in greyhounds. J. Am. Vet. Med. Assoc. 1994, 205, 838–841. [Google Scholar]
- Steiss, J.E.; Brewer, W.G.; Welles, E.; Wright, J.C. Hematologic and serum biochemical reference values in retired Greyhounds. Comp. Cont. Educ. Pract. Vet. 2000, 22, 243–248. [Google Scholar]
- Zaldìvar-Lòpez, S.; Marìn, L.M.; Iazbik, M.C.; Westendorf-Stingle, N.; Hensley, S.; Couto, C.G. Clinical pathology of Greyhounds and other sighthounds. Vet. Clin. Pathol. 2011, 40, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Micuń, J.; Sobczak-Filipiak, M.; Winnicka, A.; Mieczkowska, J.; Zmudzka, M.; Garngarz, M.; Sokolowska, J.; Lechowski, R. Thrombocytopenia as a characteristic trait in the Polish ogar dog. Pol. J. Vet. Sci. 2009, 12, 523–525. [Google Scholar] [PubMed]
- Hayakawa, S.; Spangler, E.A.; Christopherson, P.W.; Boudreaux, M.K. A novel form of macrothrombocytopenia in Akita dogs. Vet. Clin. Pathol. 2016, 45, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.C.; Meyers, K.M. Canine idiopathic thrombocytopenic purpura. J. Vet. Int. Med. 1996, 10, 207–218. [Google Scholar] [CrossRef]
- Lewis, D.C.; Meyers, K.M. Studies of platelet-bound and serum platelet-bindable immunoglobulins in dogs with idiopathic thrombocytopenic purpura. Exp. Hematol. 1996, 24, 696–701. [Google Scholar]
- Lewis, D.C.; McVey, D.S.; Shuman, W.S.; Muller, W.B. Development and characterization of a flow cytometric assay for detection of platelet-bound immunoglobulin G in dogs. Am. J. Vet. Res. 1995, 56, 1555–1558. [Google Scholar]
- Neel, J.A.; Birkenheuer, A.J.; Grindem, C.B. Thrombocytopenia. In Kirk’s Current Veterinary Therapy XV.; Bonagura, J.D., Twedt, D.C., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2010; pp. 280–285. [Google Scholar]
- Nelson, R.W.; Couto, C.G. Small Animal Internal Medicine, 2nd ed.; Mosby: Maryland Heights, MO, USA, 1998. [Google Scholar]
- Grindem, C.B. Infectious and immune-mediated thrombocytopenia. In Kirk’s Current Veterinary Therapy XIII: Small Animal Practice, 13th ed.; Bonagura, J., Ed.; WB Saunders: Philadelphia, PA, USA, 2000; pp. 438–442. [Google Scholar]
- Grindem, C.B.; Breitschwerdt, E.B.; Corbett, W.T.; Jans, H.E. Epidemiologic survey of thrombocytopenia in dogs: A report on 987 cases. Vet. Clin. Pathol. 1991, 20, 38–43. [Google Scholar] [CrossRef]
- Gould, S.M.; McInnes, E.L. Immune-mediated thrombocytopenia associated with Angiostrongylus vasorum infection in a dog. J. Small Anim. Pract. 1999, 40, 227–232. [Google Scholar] [CrossRef]
- O’Neill, E.; Acke, E.; Tobin, E.; McCarthy, G. Immune-mediated thrombocytopenia associated with Angiostrongylus vasorum infection in a Jack Russel terrier. Irish Vet. J. 2010, 63, 434–440. [Google Scholar]
- Taboada, J. Babesiosis. In Infectious Diseases of the Dog and Cat, 2nd ed.; Greene, C.E., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1990; pp. 796–803. [Google Scholar]
- Wilkerson, M.J.; Shuman, W.; Swist, S.; Harkin, K.; Meinkoth, J.; Kocan, A.A. Platelet size, platelet surface-associated IgG, and reticulated platelets in dogs with immune-mediated thrombocytopenia. Vet. Clin. Pathol. 2001, 30, 141–149. [Google Scholar] [CrossRef]
- Grindem, C.B.; Breitschwerdt, E.B.; Perkins, P.C.; Cullins, L.D.; Thomas, T.J.; Hegarty, B.C. Platelet-associated immunoglobulin (antiplatelet antibody) in canine Rocky Mountain spotted fever and ehrlichiosis. J. Am. Anim. Hosp. Assoc. 1999, 35, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Troy, G.C.; Forrester, S.D. Ehrlichia canis, E equi and E risticii infections. In Infectious Diseases of the Dog and Cat, 2nd ed.; Greene, C.E., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1990; pp. 404–414. [Google Scholar]
- Harrus, S.; Waner, T.; Weiss, D.J.; Keysary, A.; Bark, H. Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet. Immunol. Immunopathol. 1996, 51, 13–20. [Google Scholar] [CrossRef]
- Waner, T.; Harrus, S.; Weiss, D.J.; Bark, H.; Keysary, A. Demonstration of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet. Immunol. Immunopathol. 1995, 48, 177–182. [Google Scholar] [CrossRef]
- Waner, T.; Leykin, I.; Shinitsky, M.; Sharabani, E.; Buch, H.; Keysary, A.; Bark, H.; Harruset, S. Detection of platelet-bound antibodies in beagle dogs after artificial infection with Ehrlichia canis. Vet. Immunol. Immunopathol. 2000, 77, 145–150. [Google Scholar] [CrossRef]
- Chabanne, L.; Bonnefont, C.; Bernaud, J.; Rigal, D. Clinical applications of flow cytometry and cell immunophenotyping to companion animals (dog and cat). Methods Cell Sci. 2000, 22, 199–207. [Google Scholar] [CrossRef]
- Ciaramella, P.; Pelagalli, A.; Cortese, L.; Pero, M.E.; Corona, M.; Lombardi, P.; Avallone, L.; Persechino, A. Altered platelet aggregation and coagulation disorders related to clinical findings in 30 dogs naturally infected by Leishmania infantum. Vet. J. 2005, 169, 465–467. [Google Scholar] [CrossRef]
- Cortese, L.; Sica, M.; Piantedosi, D.; Ruggiero, G.; Pero, M.E.; Terrazzano, G.; Mastellone, V.; Ciaramella, P. Secondary immune-mediated thrombocytopenia in dogs naturally infected by Leishmania infantum. Vet. Rec. 2009, 164, 778–782. [Google Scholar] [CrossRef]
- Terrazzano, G.; Cortese, L.; Piantedosi, D.; Zappacosta, S.; Di Loria, A.; Santoro, D.; Ruggiero, G.; Ciaramella, P. Presence of anti-platelet IgM and IgG antibodies in dogs naturally infected by Leishmania infantum. Vet. Immunol. Immunopathol. 2006, 110, 331–337. [Google Scholar] [CrossRef]
- Cortese, L.; Terrazzano, G.; Piantedosi, D.; Sica, M.; Ruggiero, G.; Prisco, M.; Ciaramella, P. Prevalence of anti-platelet antibodies in dogs naturally co-infected by Leishmania infantum and Ehrlichia canis. Vet. J. 2011, 188, 118–121. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B. Infectious thrombocytopenia in dogs. Comp. Cont. Educ. Pract. 1988, 10, 1177–1191. [Google Scholar]
- Joshi, B.C.; Jain, N.C. Experimental immunologic thrombocytopenia in dogs: A study of thrombocytopenia and megakaryocytopoiesis. Res. Vet. Sci. 1977, 22, 11–17. [Google Scholar] [CrossRef]
- Johnson, G.S.; Turrentine, M.A.; Kraus, K.H. Canine von Willebrand’s disease. A heterogenous group of bleeding disorders. Vet. Clin. N. Am. Small 1988, 18, 195–229. [Google Scholar] [CrossRef]
- Brooks, M.; Raymond, S.; Catalfamo, J. Severe, recessive von Willebrand’s disease in German Wirehaired Pointers. J. Am. Vet. Med. Assoc. 1996, 209, 926–929. [Google Scholar] [PubMed]
- Boudreaux, M.K. Inherited platelet disorders. J. Vet. Emerg. Crit. Care 2012, 22, 30–41. [Google Scholar] [CrossRef]
- Kramer, J.W.; Venta, P.J.; Klein, S.R.; Cao, Y.; Schall, W.D.; Yuzbasiyan-Gurkan, V. A von Willebrand’s factor genomic nucleotide variant and polymerase chain reaction diagnostic test associated with inheritable type-2 von Willebrand’s disease in a line of German shorthaired pointer dogs. Vet. Pathol. 2004, 41, 221–228. [Google Scholar] [CrossRef]
- Zwall, R.F.A.; Comfurius, P.; Bevers, E.M. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim. Biophys. Acta 2004, 1636, 119–128. [Google Scholar] [CrossRef]
- Brooks, M.B.; Catalfamo, J.L.; Brown, H.A.; Ivanova, P.; Lovaglio, J. A hereditary bleeding disorder of dogs caused by a lack of platelet procoagulant activity. Blood 2002, 99, 2434–2441. [Google Scholar] [CrossRef]
- Brooks, M.B.; Catalfamo, J.L.; Friese, P.; Dale, G.L. Scott syndrome dogs have impaired coated—Platelet formation and calcein-release but normal mitochondrial depolarization. J. Thromb. Haemost. 2007, 5, 1972–1974. [Google Scholar] [CrossRef]
- Brooks, M.B.; Randolph, J.; Warner, K.; Center, S. Evaluation of platelet function screening tests to detect platelet procoagulant deficiency in dogs with Scott syndrome. Vet. Clin. Pathol. 2009, 38, 306–315. [Google Scholar] [CrossRef]
- Brooks, M.; Etter, K.; Catalfamo, J.; Brisbin, A.; Bustamante, C.; Mezey, J. A genome-wide linkage scan in German shepherd dogs localizes canine platelet procoagulant deficiency (Scott syndrome) to canine chromosome 27. Gene 2010, 450, 70–75. [Google Scholar] [CrossRef]
- Boudreaux, M.K. Inherited intrinsic platelet disorders. In Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley: Ames, IA, USA, 2010; pp. 619–625. [Google Scholar]
- Di Giacomo, R.F.; Hammond, W.P.; Kunz, L.L.; Cox, P.A. Clinical and pathologic features of cyclic hematopoiesis in grey collie dogs. Am. J. Pathol. 1983, 111, 224–233. [Google Scholar]
- Boudreaux, M.K.; Kvam, K.; Dillon, A.R.; Bourne, C.; Scott, M.; Schwartz, K.A.; Toivio-Kinnucan, M. Type I Glanzmann’s thrombasthenia in a Great Pyrenees dog. Vet. Pathol. 1996, 33, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, D.L.; Bourne, C.; Boudreaux, M.K. Two genetic defects in alphaIIb are associated with type I Glanzmann’s thrombasthenia in a Great Pyrenees dog: A 14-base insertion in exon 13 and a splicing defect of intron 13. Vet. Pathol. 2000, 37, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Boudreaux, M.K.; Catalfamo, J.L. Molecular and genetic basis for thrombasthenic thrombopathia in Otterhounds. Am. J. Vet. Res. 2001, 62, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Haysom, L.Z.; Kennerly, R.M.; Müller, R.D.; Smith-Carr, S.; Christopherson, P.W.; Boudreaux, M.K. Identification and characterization of Glanzmann thrombasthenia in 2 closely related mixed-breed dogs. J. Vet. Intern. Med. 2016, 30, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Boudreaux, M.K.; Martin, M. P2Y12 receptor gene mutation associated with postoperative hemorrhage in a Greater Swiss Mountain dog. Vet. Clin. Pathol. 2011, 40, 202–206. [Google Scholar] [CrossRef]
- Johnstone, I.B.; Lotz, F. An inherited platelet function defect in basset hounds. Can. Vet. J. 1979, 20, 211–215. [Google Scholar]
- Boudreaux, M.K.; Crager, C.; Dillon, A.R.; Stanz, K.; Toivio-Kinnucan, M. Identification of an intrinsic platelet function defect in Spitz dogs. J. Vet. Intern. Med. 1994, 8, 93–98. [Google Scholar] [CrossRef]
- Boudreaux, M.K.; Catalfamo, J.L.; Klok, M. Calcium-diacylglycerol guanine nucleotide exchange factor I gene mutations associated with loss of function in canine platelets. Transl. Res. 2007, 150, 81–92. [Google Scholar] [CrossRef][Green Version]
- Boudreaux, M.K.; Wardrop, K.J.; Kiklevich, V.; Felsburg, P.; Snekvik, K. A mutation in the canine Kindlin-3 gene associated with increased bleeding risk and susceptibility to infections. Thromb. Haemost. 2010, 103, 475–477. [Google Scholar]
- Gant, P.; McBride, D.; Humm, K. Abnormal platelet activity in dogs and cats—Impact and measurement. J. Small Anim. Pract. 2020, 61, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Lothrop, C.D.; Candler, R.V.; Pratt, H.L.; Jones, J.B.; Carroll, R.C. Characterization of platelet function in cyclic hematopoietic dogs. Exp. Hematol. 1991, 19, 916–922. [Google Scholar] [PubMed]
- Christopherson, P.W.; Hill, A.; Brooks, M.B.; Scofield, M.; King, K.B.; Boudreaux, M.K. Identification of a single base deletion in the glycoprotein IIB gene causing Glanzmann thrombasthenia in a golden retriever. 2017. Available online: https://cdn.ymaws.com/www.acvp.org/resource/resmgr/Meetings_&_Events/2017_Annual_Meeting/Abstracts/2017-10-13_Abstracts-revised.pdf (accessed on 20 November 2019).
- Flores, R.S.; Boudreaux, M.K.; Vasquez, B.; Bristow, P.; Aronson, L.R.; Santoro-Beer, K.; Callan, M.B. Heterozygosity for P2Y12 receptor gene mutation associated with postoperative hemorrhage in a Greater Swiss Mountain dog. Vet. Clin. Pathol. 2017, 46, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Callan, M.B.; Walton, R.; Jezyk, P.F.; Giger, U. Thrombopathies causing bleeding in a boxer and mixed-breed dog. J. Am. Anim. Hospit. Assoc. 2001, 37, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.B.; Catalfamo, J.; MacNguyen, R.; Tim, D.; Fancher, S.; McCardle, J.A. A TMEM16F point mutation causes an absence of canine platelet TMEM16F and ineffective activation and death-induced phospholipid scrambling. J. Thromb. Haemost. 2015, 13, 2240–2252. [Google Scholar] [CrossRef]
- Dunn, J.; Heath, M.; Jefferies, A.; Blackwood, L.; McKay, J.S.; Nicholls, P.K. Diagnostic and hematologic features of probable essential thrombocythemia in two dogs. Vet. Clin. Pathol. 1999, 28, 131–138. [Google Scholar] [CrossRef]
- Topper, M.J.; Welles, E.G. Hemostasis. In Duncan & Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 4th ed.; Latimer, K.S., Mahaffey, E.A., Prasse, K.W., Eds.; Iowa State Press: Ames, IA, USA, 2003; pp. 99–135. [Google Scholar]
- Stockham, S.L.; Scott, M.A. Hemostasis. In Fundamentals of Veterinary Clinical Pathology, 2nd ed.; Stockham, S.L., Scott, M.A., Eds.; Blackwell: Ames, IA, USA, 2008; pp. 259–321. [Google Scholar]
- Federici, A.B.; Rand, J.H.; Bucciarelli, P.; Budde, U.; Van Gendern, P.J.; Mohri, H.; Meyer, D.; Rodeghiero, F.; Sadler, J.E. Acquired von Willebrand syndrome: Data from an international registry. Thromb. Haemost. 2000, 84, 345–349. [Google Scholar]
- Dodds, W.J. Von Willebrand’s disease in dogs. Mod. Vet. Pract. 1984, 65, 681–686. [Google Scholar]
- Avgeris, S.; Lothrop, C.D.; McDonald, T.P. Plasma von Willebrand factor concentration and thyroid function in dogs. JAVMA 1990, 196, 921. [Google Scholar]
- Whitley, N.T.; Corzo-Menendez, N.; Carmichael, N.G.; Mc Garry, J.W. Cerebral and conjunctival haemorrhages associated with von Willebrand factor deficiency and canine angiostrongylosis. J. Small Anim. Pract. 2005, 46, 75–78. [Google Scholar] [CrossRef]
- Hausmann, L.; Pack, A.; Hausmann, S.; Neiger, R. Acquired von-Willebrand factor and factor-VIII deficiencies caused by angiostrongylosis in a dog. Tierärztliche Praxis (K) 2016, 3, 189–193. [Google Scholar]
- Boudreaux, M.K.; Dillon, A.R.; Spano, J.S. Enhanced platelet reactivity in heartworm-infected dogs. Am. J. Vet. Res. 1989, 50, 1544–1547. [Google Scholar] [PubMed]
- Maruyama, H.; Kaneko, M.; Otake, T.; Kano, R.; Yamaya, Y.; Watari, T.; Hasegawa, A.; Kamata, H. Evaluation of a disintegrin-like and metalloprotease with thrombospondin type 1 repeat motifs 13 (ADAMTS13) activity enzyme-linked immunosorbent assay for measuring plasma ADAMTS13 activity in dogs. J. Vet. Diagn. Invest. 2014, 26, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.L.; Andelman, N.C.; Moore, F.M.; King, N.W., Jr. Idiopathic cutaneous and renal glomerular vasculopathy of greyhounds. Vet. Pathol. 1988, 25, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.P.; Hawkins, I.; Robin, C.; Newton, R.J.; Jepson, R.; Stanzani, G.; McMahon, L.A.; Pesavento, P.; Carr, T.; Cogan, T.; et al. Cutaneous and renal glomerular vasculopathy as a cause of acute kidney injury in dogs. in the UK. Vet. Rec. 2015, 176, 384. [Google Scholar] [CrossRef] [PubMed]
- Grindem, C.; Corbett, W.; Levy, M.; Davidson, M.G.; Breitschwerdt, E.B. Platelet aggregation in dogs experimentally infected with Rickettsia rickettsii. Vet. Clin. Pathol. 1990, 19, 25–28. [Google Scholar] [CrossRef]
- Pelagalli, A.; Ciaramella, P.; Lombardi, P.; Pero, M.E.; Cortese, L.; Corona, M.; Oliva, G.; Avallone, L. Evaluation of adenosine 5’-diphosphate (ADP)- and collagen-induced platelet aggregation in canine leishmaniasis. J. Comp. Pathol. 2004, 130, 124–129. [Google Scholar] [CrossRef]
- Cortese, L.; Pelagalli, A.; Piantedosi, D.; Mastellone, V.; Manco, A.; Lombardi, P.; Ciaramella, P.; Avallone, L. Platelet aggregation and haemostatic response in dogs naturally co-infected by Leishmania infantum and Ehrlichia canis. J. Vet. Med. Ser. A 2006, 53, 546–548. [Google Scholar] [CrossRef]
- Cortese, L.; Pelagalli, A.; Piantedosi, D.; Cestaro, A.; Di Loria, A.; Lombardi, P.; Avallone, L.; Ciaramella, P. Effects of therapy on haemostasis in dogs infected with Leishmania infantum, Ehrlichia canis, or both combined. Vet. Rec. 2009, 164, 433–434. [Google Scholar] [CrossRef]
- Cortese, L.; Pelagalli, A.; Piantedosi, D.; Mastellone, V.; Di Loria, A.; Lombardi, P.; Ciaramella, P.; Avallone, L. The effects of prednisone on haemostasis in leishmaniotic dogs treated with meglumine antimoniate and allopurinol. Vet. J. 2008, 177, 405–410. [Google Scholar] [CrossRef]
- Abrams, C.S.; Shattil, S.J.; Bennett, J.S. Acquired qualitative platelet disorders. In Williams Hematology, 7th ed.; Lichtman, M.A., Beutler, E., Kipps, T.J., Kaushansky, K., Seligsohn, U., Prchal, J., Eds.; McGraw-Hill: New York, NY, USA, 2006; pp. 833–1855. [Google Scholar]
- Kristensen, A.; Weiss, D.; Klausner, J. Platelet dysfunction associated with immune-mediated thrombocytopenia in dogs. J. Vet. Intern. Med. 1994, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S. Semeiotica di laboratorio. In Malattie Renali del Cane e del Gatto. Manuale di Diagnostica e Terapia; Zatelli, A., Ed.; Edra LSWR S.p.A: Milano, Italy, 2015; p. 31. [Google Scholar]
- Shinya, H.; Matsuo, N.; Takeyama, N.; Tanaka, T. Hyperammonemia inhibits platelet aggregation in rats. Thromb. Res. 1996, 81, 195–201. [Google Scholar] [CrossRef]
- Willis, S.; Jackson, M.; Meric, S.; Rousseaux, C.G. Whole blood platelet aggregation in dogs with liver disease. Am. J. Vet. Res. 1989, 50, 1893–1897. [Google Scholar] [PubMed]
- Stokol, T. Disorders of haemostasis. In BSAVA Manual of Canine and Feline Clinical Pathology, 3rd ed.; Villiers, E., Ristic, J., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 114–115. [Google Scholar]
- Schwartz, D.; Sharkey, L.; Armstrong, P.J.; Knudson, C.; Kelley, J. Platelet volume and plateletcrit in dogs with presumed primary immune-mediated thrombocytopenia. J. Vet. Intern. Med. 2014, 28, 1575–1579. [Google Scholar] [CrossRef]
- Kelley, J.; Sharkey, L.C.; Christopherson, P.W.; Rendahl, A. Platelet count and plateletcrit in Cavalier King Charles Spaniels and Greyhounds using the Advia 120 and 2120. Vet. Clin. Pathol. 2014, 43, 622. [Google Scholar] [CrossRef]
- Davis, B.; Toivio-Kinnucan, M.; Schuller, S.; Boudreaux, M.K. Mutation in beta-tubulin correlates with macrothrombocytopenia in Cavalier King Charles Spaniels. J. Vet. Intern. Med. 2008, 22, 540–545. [Google Scholar] [CrossRef]
- Tvedten, H.; Lilliehookk, I.; Hillstrom, A.; Haggstrom, J. PCT is superior to platelet count for assessing platelet status in Cavalier King Charles Spaniels. Vet. Clin. Pathol. 2008, 37, 266–271. [Google Scholar] [CrossRef]
- Christopherson, P.W.; Spangler, E.A.; Boudreaux, M.K. Evaluation and clinical application of platelet function testing in small animal practice. Vet. Clin. N. Am. Small 2012, 42, 173–188. [Google Scholar] [CrossRef]
- Burgess, H.J.; Woods, J.P.; Abrams-Ogg, A.C.G.; Wood, R.D. Evaluation of laboratory methods to improve characterization of dogs with von Willebrand disease. Can. J. Vet. Res. 2009, 73, 252–259. [Google Scholar]
- Pelagalli, A.; Lombardi, P.; d’Angelo, D.; Della Morte, R.; Avallone, L.; Staiano, N. Species variability in platelet aggregation response to different agonists. J. Comp. Pathol. 2002, 127, 126–132. [Google Scholar] [CrossRef]
- Catalfamo, J.L.; Raymond, S.L.; White, J.G.; Dodds, W.J. Defective platelet-fibrinogen interaction in hereditary canine thrombopathia. Blood 1986, 67, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Callan, M.B.; Shofer, F.S.; Catalfamo, J.L. Effects of anticoagulant on pH, ionized calcium concentration, and agonist-induced platelet aggregation in canine platelet-rich plasma. Am. J. Vet. Res. 2009, 70, 472–477. [Google Scholar] [CrossRef]
- Lombardi, P.; Pelagalli, A.; Avallone, L.; d’Angelo, D.; Belisario, M.A.; d’Angelo, A.; Staiano, N. Species-dependent specificity of platelet aggregation inhibitors from snake venom. J. Comp. Pathol. 1999, 121, 185–190. [Google Scholar] [CrossRef]
- Soloviev, M.V.; Okazaki, Y.; Harasaki, H. Whole blood platelet aggregation in humans and animals: A comparative study. J. Surg. Res. 1999, 82, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.S. Comparison of platelet aggregation and adenosine triphosphate secretion in whole blood and platelet-rich plasma from normal dogs. Comp. Haematol. Int. 1996, 6, 70–76. [Google Scholar] [CrossRef]
- Saati, S.; Abrams-Ogg, A.C.G.; Blois, S.L.; Wood, R.D. Comparison of multiplate, platelet function analyzer-200, and platelet works in healthy dogs treated with aspirin and clopidogrel. J. Vet. Intern. Med. 2018, 32, 111–118. [Google Scholar] [CrossRef]
- Pelagalli, A.; Pero, M.E.; Mastellone, V.; Cestaro, A.; Signoriello, S.; Lombardi, P.; Avallone, L. Characterization of canine platelet adhesion to extracellular matrix proteins. Vet. J. 2011, 189, 115–117. [Google Scholar] [CrossRef]
- Ferkau, A.; Ecklebe, S.; Jahn, K.; Calmer, S.; Theilmeier, G.; Mischke, R. A dynamic flow-chamber-based adhesion assay to assess canine platelet-matrix interactions in vitro. Vet. Clin. Pathol. 2013, 42, 150–156. [Google Scholar] [CrossRef]
- Spiess, B.; McCarthy, R.; Ivankovich, A. Primary fibrinolysis or D.I.C. differentiated by different viscoelastic tests. Anesthesiol. 1989, 71, A415. [Google Scholar] [CrossRef]
- Tuman, K.; Naylor, B.; Spiess, B.; McCarthy, R.; Ivankovich, A. Effects of hematocrit on thromboelastography and sonoclot analysis. Anesthesiol. 1989, 71, A414. [Google Scholar] [CrossRef]
- Tuman, K.J.; Mccarthy, R.J.; Patel, R.B.; Ivankovich, A.D. Quantification of aprotinin reversal of severe fibrinolysis in dogs using thromboelastography. Anesth. Analg. 1993, 76, S439. [Google Scholar]
- Mousa, S. Synergistic interactioons between GPIIb/IIIa antagonists and low molecular weight heparin in inhibiting platelet-fibrin clot dynamics in human blood and in canine model using thromboelastography. Blood 2002, 100, 3986. [Google Scholar]
- Kol, A.; Nelson, R.W.; Gosselin, R.C.; Borjesson, D.L. Characterization of thrombelastography over time in dogs with hyperadrenocorticism. Vet. J. 2013, 197, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; McMichael, M.A.; Gilor, S.; Galligan, A.J.; Hoh, C.M. Correlation of hematocrit, platelet concentration, and plasma coagulation factors with results of thromboelastometry in canine whole blood samples. Am. J. Vet. Res. 2012, 73, 789–798. [Google Scholar] [CrossRef]
- Goggs, R.; Borrelli, A.; Brainard, B.M.; Chan, D.L.; de Laforcade, A.; Goy-Thollot, I.; Jandrey, K.E.; Kristensen, A.T.; Kutter, A.; Marschner, C.B.; et al. Multicenter in vitro thromboelastography and thromboelastometry standardization. J. Vet. Emerg. Crit. Car. 2018, 28, 201–212. [Google Scholar] [CrossRef]
- Shropshire, S.B.; Olver, C.S.; Lappin, M.R. Variability of tissue factor-activated thromboelastography and whole blood impedance platelet aggregometry in healthy dogs. J. Vet. Emerg. Crit. Car. 2018, 28, 334–339. [Google Scholar] [CrossRef]
- Brooks, A.C.; Guillaumin, J.; Cooper, E.S.; Couto, C.G. Effects of hematocrit and red blood cell-independent viscosity on canine thromboelastographic tracings. Transfusion 2014, 54, 727–734. [Google Scholar] [CrossRef]
- Wiinberg, B.; Jensen, A.L.; Rozanski, E.; Johansson, P.I.; Kjelgaard-Hansen, M.; Tranholm, M.; Kristensen, A.T. Tissue factor activated thromboelastography correlates to clinical signs of bleeding in dogs. Vet. J. 2009, 179, 121–129. [Google Scholar] [CrossRef]
- Bucknoff, M.C.; Hanel, R.M.; Marks, S.L.; Motsinger-Reif, A.A.; Suter, S.E. Evaluation of thromboelastography for prediction of clinical bleeding in thrombocytopenic dogs after total body irradiation and hematopoietic cell transplantation. Am. J. Vet. Res. 2014, 75, 425–432. [Google Scholar] [CrossRef]
- Rubanick, J.V.; Pashmakova, M.B.; Bishop, M.A.; Barr, J.W. Correlation between thromboelastography and traditional coagulation test parameters in hospitalized dogs. Veter. Med. Res. Rep. 2017, 8, 21–26. [Google Scholar] [CrossRef][Green Version]
- Yeo, E.L.; Gemmell, C.H.; Sutherland, D.R.; Sefton, M.V. Characterization of canine platelet P-selectin (CD 62) and its utility in flow cytometry platelet studies. Comp. Biochem. Physiol. B 1993, 105, 625–636. [Google Scholar] [CrossRef]
- Weiss, D.J. Application of flow cytometric techniques to veterinary clinical hematology. Vet. Clin. Pathol. 2002, 31, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A.; Walcheck, B.K.; Weiss, D.J. Flow cytometric detection of activated platelets in the dog. Vet. Clin. Pathol. 2003, 32, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Żmigrodzka, M.; Guzera, M.; Winnicka, A. Flow cytometric assessment of activation of peripheral blood platelets in dogs with normal platelet count and asymptomatic thrombocytopenia. Pol. J. Vet. Sci. 2016, 19, 407–414. [Google Scholar] [CrossRef]
- Kristensen, A.T.; Weiss, D.J.; Klausner, J.S.; Laber, J.; Christie, D.J. Comparison of microscopic and flow cytometric detection of platelet antibody in dogs suspected of having immune-mediated thrombocytopenia. Am. J. Vet. Res. 1994, 55, 1111–1114. [Google Scholar]
- Oellers, D.E.; Bauer, N.; Ginder, M.; Johannes, S.; Pernecker, I.; Moritz, A. Optimized gating and reference ranges of reticulated platelets in dogs for the Sysmex XT-2000iV. BMC Vet. Res. 2016, 22, 148. [Google Scholar] [CrossRef][Green Version]
- Cremer, S.E.; Krogh, A.K.H.; Hedström, M.E.K.; Christiansen, L.B.; Tarnow, I.; Kristensen, A.T. Analytical validation of a flow cytometric protocol for quantification of platelet microparticles in dogs. Vet. Clin. Pathol. 2018, 47, 186–196. [Google Scholar] [CrossRef]
- Marcondes, N.A.; Terra, S.R.; Lasta, C.S.; Hlavac, N.R.C.; Dalmolin, M.L.; Lacerda, L.A.; Faulhaber, G.A.M.; González, F.H.D. Comparison of JC-1 and MitoTracker probes for mitochondrial viability assessment in stored canine platelet concentrates: A flow cytometry study. Cytometry A 2018, 95, 214–218. [Google Scholar] [CrossRef]
- Shropshire, S.; Dow, S.; Lappin, M. Validation of a clinically applicable flow cytometric assay for the detection of immunoglobulin associated platelets in dogs. Vet. Immunol. Immunopathol. 2018, 202, 109–114. [Google Scholar] [CrossRef]
- Tarrant, J.M. The role of flow cytometry in companion animal diagnostic medicine. Vet. J. 2005, 170, 278–288. [Google Scholar] [CrossRef]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy 2012, 28, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.H.; Malhotra, A.; Brighton, T.; Walsh, W.R.; Underman, R. Platelet function and constituents of platelet rich plasma. Int. J. Sports Med. 2013, 34, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.K.; Mishra, A.; Rodeo, S.R.; Fu, F.; Terry, M.A.; Randelli, P.; Canale, S.T.; Kelly, F.B. Platelet-rich plasma in orthopaedic applications: Evidence-based recommendations for treatment. J. Am. Acad. Orthop. Sur. 2013, 21, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.J.; Canapp, S.O.; Mason, D.R.; Cox, C.; Hess, T. Canine platelet-rich plasma systems: A prospective analysis. Front. Vet. Sci. 2016, 2, 73. [Google Scholar] [CrossRef]
- Mc Carrel, T.M.; Minas, T.; Fortier, L.A. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J. Bone Joint Surg. 2012, 94, e143. [Google Scholar] [CrossRef]
- Hoareau, G.L.; Jandrey, K.E.; Burges, J.; Bremer, D.; Tablin, F. Comparison of the platelet-rich plasma and buffy coat protocols for preparation of canine platelet concentrates. Vet. Clin. Pathol. 2014, 43, 513–518. [Google Scholar] [CrossRef]
- Hlavac, N.; Lasta, C.S.; Dalmolin, M.L.; Lacerda, L.A.; de Korte, D.; Marcondes, N.A.; Terra, S.R.; Fernandes, F.B.; González, F.H.D. In vitro properties of concentrated canine platelets stored in two additive solutions: A comparative study. BMC Vet. Res. 2017, 13, 334. [Google Scholar] [CrossRef]
- Parra, E.; Vergara, A.; Silva, R.F. Autologous platelet concentrates as treatment for avascular necrosis of femoral head in a dog. Top. Companion Anim. M 2017, 32, 31–35. [Google Scholar] [CrossRef]
- Bansal, H.; Comella, K.; Leon, J.; Verma, P.; Agrawal, D.; Koka, P.; Ichim, T. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J. Transl. Med. 2017, 15, 141. [Google Scholar] [CrossRef]
- Fahie, M.A.; Ortolano, G.A.; Guercio, V.; Schaffer, J.A.; Johnston, G.; Au, J.; Hettlich, B.A.; Phillips, T.; Allen, M.J.; Bertone, A.L. A randomized controlled trial of the efficacy of autologous platelet therapy for the treatment of osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2013, 243, 1291–1297. [Google Scholar] [CrossRef]
- Canapp, S.O., Jr.; Canapp, D.A.; Ibrahim, V.; Carr, B.J.; Cox, C.; Barrett, J.G. The use of adipose-derived progenitor cells and platelet-rich plasma combination for the treatment of supraspinatus tendinopathy in 55 dogs: A retrospective study. Front. Vet. Sci. 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, D.; Shams Asenjan, K.; Dehdilani, N.; Parsa, H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: Macroscopic and histological assessments. Bone Joint Res. 2017, 6, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, C.; Park, H.M. Curative effect of autologous platelet-rich plasma on a large cutaneous lesion in a dog. Vet. Dermatol. 2009, 20, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.M.; Baghaban Eslaminejad, M.; Motallebizadeh, N.; Ashrafi Halan, J.; Tagiyar, L.; Soroori, S.; Nikmahzar, A.; Pedram, M.; Shahverdi, A.; Kazemi Mehrjerdi, H.; et al. Transplantation of autologous bone marrow mesenchymal stem cells with platelet-rich plasma accelerate distraction osteogenesis in a canine model. Cell J. 2015, 17, 243–252. [Google Scholar]
- López, S.; Vilar, J.M.; Sopena, J.J.; Damià, E.; Chicharro, D.; Carrillo, J.M.; Cuervo, B.; Rubio, A.M. Assessment of the Efficacy of Platelet-Rich Plasma in the Treatment of Traumatic Canine Fractures. Int. J. Mol. Sci. 2019, 1, 1075. [Google Scholar] [CrossRef]
- Filgueira, F.G.F.; Watanabe Minto, B.; Granato Chung, D.; Carmagni Prada, T.; Rosa-Ballaben, N.M.; Nogueira Campos, M.G. Platelet-rich plasma, bone marrow and chitosan in minimally invasive plate osteosynthesis of canine tibia fractures—A randomized study. Veter. Med. 2019, 64, 309–316. [Google Scholar] [CrossRef]
- Keskiner, I.; Alkan, A.; Acikgoz, G.; Arpak, N.; Kaplan, S.; Arslan, H. Platelet-rich plasma and autogenous bone graft combined with guided tissue regeneration in periodontal fenestration defects in dogs. Int. J. Periodont. Rest. 2014, 34, e112–e120. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Psalla, D.; Kazakos, G.; Loukopoulos, P.; Giannakas, N.; Savvas, I.; Kritsepi-Konstantinou, M.; Chantes, A.; Papazoglou, L.G. Effect of locally injected autologous platelet-rich plasma on second intention wound healing of acute full-thickness skin defects in dogs. Vet. Comp. Orthop. Traumatol. 2015, 28, 172–178. [Google Scholar]
- Jee, C.H.; Eom, N.Y.; Jang, H.M.; Jung, H.W.; Choi, E.S.; Won, J.H.; Hong, I.H.; Kang, B.T.; Jeong, D.W.; Jung, D.I. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J. Vet. Sci. 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Bozynski, C.C.; Stannard, J.P.; Smith, P.; Hanypsiak, B.T.; Kuroki, K.; Stoker, A.; Cook, C.; Cook, J.L. Acute management of anterior cruciate ligament injuries using novel canine models. J. Knee Surg. 2016, 29, 594–603. [Google Scholar] [CrossRef]
- Cook, J.L.; Smith, P.A.; Bozynski, C.C.; Kuroki, K.; Cook, C.R.; Stoker, A.M.; Pfeiffer, F.M. Multiple injections of leukoreduced platelet rich plasma reduce pain and functional impairment in a canine model of ACL and meniscal deficiency. J. Orthop. Res. 2016, 34, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.I.; Kim, J.H.; Kwak, H.H.; Woo, H.M.; Han, J.H.; Yayon, A.; Jung, Y.C.; Cho, J.M.; Kang, B.J. A placebo-controlled study comparing the efficacy of intra-articular injections of hyaluronic acid and a novel hyaluronic acid-platelet-rich plasma conjugate in a canine model of osteoarthritis. J. Orthop. Surg. Res. 2019, 18, 314. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, D.; Fakhrjou, A. Leukocyte and platelet rich plasma (l-PRP) versus leukocyte and platelet rich fibrin (l-PRF) for articular cartilage repair of the knee: A comparative evaluation in an animal model. Iran. Red. Crescent. Med. 2015, 17, e19594. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, D.; Dizaji, V.M.; Alishahi, M.K. Effect of autologous platelet rich fibrin on the healing of experimental articular cartilage defects of the knee in an animal model. Biomed Res. Int. 2014, 2014, 486436. [Google Scholar] [CrossRef] [PubMed]
- Abouelnasr, K.; Hamed, M.; Lashen, S.; El-Adl, M.; Eltaysh, R.; Tagawa, M. Enhancement of abdominal wall defect repair using allogenic platelet-rich plasma with commercial polyester/cotton fabric (Damour) in a canine model. J. Vet. Med. Sci. 2017, 79, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Drago, V.; Izzo, M.; Lamagna, B.; Avallone, L.; Lamagna, F. Platelet gel preparation technique and its use in tissue repair processes in dogs: A pilot study. Comp. Med. 2011, 61, 558–559. [Google Scholar]
- Everts, P.A.M.; Knape, J.T.A.; Weibrich, G.; Schönberger, J.P.A.M.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.M.; van Zundert, A. Platelet-rich plasma and platelet gel: A review. J. Extra Corpor. Technol. 2006, 38, 174–187. [Google Scholar]
- Carter, A.C.; Jolly, D.G.; Worden, C.E.; Hendren, D.G.; Kane, C.J.M. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp. Mol. Pathol. 2003, 74, 244–255. [Google Scholar] [CrossRef]
- Rahimzadeh, P.; Imani, F.; Reza Faiz, S.H.; Reza Entezary, S.; Zamanabadi, M.N.; Reza Alebouyeh, M. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin. Interv. Aging 2018, 13, 73–79. [Google Scholar] [CrossRef]
- Allahverdi, A.; Sharifi, D.; Takhtfooladi, M.A.; Hesaraki, S.; Khansari, M.; Dorbeh, S.S. Evaluation of low-level laser therapy, platelet-rich plasma, and their combination on the healing of Achilles tendon in rabbits. Laser Med. Sci. 2015, 30, 1305–1313. [Google Scholar] [CrossRef]
- Min, S.; Yoon, J.Y.; Park, S.Y.; Moon, J.; Kwon, H.H.; Suh, D.H. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg. Med. 2018, 50, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Rabago, D.; Nourani, B. Prolotherapy for osteoarthritis and tendinopathy: A descriptive review. Curr. Rheumatol. Rep. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Gladstein, B. A Case for Prolotherapy and Its Place in Veterinary Medicine. J. Prolother. 2012, 4, e870–e885. [Google Scholar]


| Granule Type | Content | Reference |
|---|---|---|
| alpha granules | Fibrinogen Von Willebrand factor growth factors: (1) Insulin-like growth factor-1 (IGF-1) (2) Epidermal growth factor (EGF) (3) Vascular endothelial growth factor (VEGF) (4) Platelet derived growth factor BB (5) Transforming growth factor β1 | [17] [17] [17] [17] [18] |
| dense-granules | Adenosine diphosphate (ADP) Adenosine triphosphate (ATP) Serotonin (5-HT) Ca2+ Mg2+ | [19] [19] [19] [19] [19] |
| Canine Congenital Platelet Disorders Associated with Bleeding | Alteration Type | Breed | References |
|---|---|---|---|
| Extrinsic platelet disorders | |||
| Von Willebrand Disease (VWF) | Defects or deficiencies of VWF (three forms are described) leading to reduced/absent platelet adhesion | Type I: purebreds, mixed breed dogs; Type II: German Shorthaired Pointer and German Wirehaired Pointer; Type III: Dutch Kooiker, Scottish terrier, Shetland sheepdog (familial trait), many sporadic cases in Border collie, Chesapeake Bay retriever, Cocker spaniel, Eskimo dog, Labrador retriever, Maltese, Pitbull and in mixed breed | [70,71,72,73] |
| Intrinsic Platelet Disorders | |||
| Procoagulant expression Scott Syndrome | Lack of phosphatidylserine (PS) expression, membrane microvesciculation failure upon activation with calcium ionophore | German shepherd | [74,75,76,77,78] |
| Storage pool disorders Cyclic hematopoiesis | Platelet dense granules absence | Grey Collie | [79,80] |
| Dense Granule Defects | Platelet dense granule defects | American Cocker Spaniel | [23] |
| Receptors disorders Glanzmann thrombasthenia (GT) | Absence/marked reduction of the glycoprotein receptor IIb-IIIa (GPIIb-IIIa) | Great Pyrenees and Otterhound Mixed-Breed, Golden Retriever | [72,81,82,83,84] |
| P2Y12 | Altered function of the P2Y12 (ADP) receptor on platelet membranes | Greater Swiss Mountain dog | [85] |
| Signal Transduction Disorders | |||
| CalDAG-GEFI platelet disorders | Decreased fibrinogen receptor activation and platelet aggregation to multiple agonists | Basset Hound, Spitz, Landseer dog | [79,86,87,88] |
| Kindlin-3 | Causes decreased/absent activation of beta integrins on leukocytes and platelets | German Shepherd | [89] |
| Methods of Testing Platelet Function | Sample | Pros | Cons and Limitations |
|---|---|---|---|
| Light transmission platelet aggregometry | PRP | flexible, sensitive to antiplatelet therapy | manual sample processing individual variability |
| Whole blood aggregometry | WB | easy and time sparing, centrifugation not required, small sample required, maintenance of platelets in their natural milieu | consideration of possible interaction between blood cells |
| Impedance aggregometry: Multiplate | platelet function under more physiological conditions, good reproducibly to assess platelet aggregation in dogs | Limited hematocrit and platelet count range, Hirudin as anticoagulant to define the optimal concentrations at which various agonists should be used | |
| Aperture closure instruments. Platelet function analyzer (PFA-100, PFA-200) | easy and sensitive to severe platelet defect | rigid closed system, not sensitive to platelet secretion defects and anemia, possible influence by citrate concentration and time from blood collection | |
| Platelet aggregation and ATP secretion | WB | simultaneous response regarding aggregation and ATP content | need to allow the whole blood sample to stand 60 min at room temperature after blood collection |
| Thromboelastography | WB | higher versatility than traditional coagulation tests | reduced reproducibility, difficult interpretation in subjects with alteration of Hct, platelets, possible request of specialist staff to perform the test |
| Flow cytometry | WB, PRP, WP | minimal sample required possibility to evaluate resting as well as activated state of the platelets | evaluation of thrombopoiesis, diagnosis of platelet function disorders, and monitoring antiplatelet therapy complexity of the test procedure; need for standardization, and quality control |
| Organ and Tissue Recipients | Platelet Product | Possible Adjuvants Associated | Examined Cases (N) | Reference | Advantages |
|---|---|---|---|---|---|
| Bone | |||||
| Tibia | PRP | BM − MSCs | 10 | [169] | Reduction in the time for bone consolidation |
| ---- | 65 | [170] | Acceleration of bone healing and fracture consolidation | ||
| BM + CHI | 30 | [171] | Reduction in the time for bone consolidation | ||
| Teeth | PRP | Autologous bone | 6 | [172] | Improvement in bone and cementum formation |
| Skin | PRP | ---- | 6 | [173] | Increase of tissue perfusion and organized collagen bundles |
| 3 | [174] | Increase in angiogenesis, collagen deposition, and epithelization | |||
| Tendon | PRP | Adipose tissue derived MSCs | 55 | [166] | Increase in chondrogenic cells recruitment, cell proliferation, and synthesis of cartilage matrix |
| Ligament | PRP | ---- | 27 | [175] | Reduction of lameness, pain, and effusion |
| Leukocyte reduced | 12 | [176] | Reduction of pain and increase of limb function | ||
| HA | 20 | [177] | Limb function improvement | ||
| Cartilage | PRF | MSCs | 12 | [167] | Improvement in cartilage regeneration. Increase of proliferation and differentiation of BM-MSCs into chondrocytes |
| PRP + Leukocyte | PRF | 18 | [178] | Improvement in cartilage tissue repair by promoting increased cellular proliferation, extracellular matrix synthesis, and gene expression of chondrocytes | |
| PRF | ---- | 12 | [179] | Improvement in both articular cartilage repair and regeneration | |
| Others | PRP | ---- | 24 | [180] | Increase of collagen deposition, improvement in new vessel formation, and overexpression of angiogenic and myofibroblastic genes (COL1α1, COL3α1, VEGF and TGFβ1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortese, L.; Christopherson, P.W.; Pelagalli, A. Platelet Function and Therapeutic Applications in Dogs: Current Status and Future Prospects. Animals 2020, 10, 201. https://doi.org/10.3390/ani10020201
Cortese L, Christopherson PW, Pelagalli A. Platelet Function and Therapeutic Applications in Dogs: Current Status and Future Prospects. Animals. 2020; 10(2):201. https://doi.org/10.3390/ani10020201
Chicago/Turabian StyleCortese, Laura, Pete W. Christopherson, and Alessandra Pelagalli. 2020. "Platelet Function and Therapeutic Applications in Dogs: Current Status and Future Prospects" Animals 10, no. 2: 201. https://doi.org/10.3390/ani10020201
APA StyleCortese, L., Christopherson, P. W., & Pelagalli, A. (2020). Platelet Function and Therapeutic Applications in Dogs: Current Status and Future Prospects. Animals, 10(2), 201. https://doi.org/10.3390/ani10020201






