Gradual Provision of Live Black Soldier Fly (Hermetia illucens) Larvae to Older Laying Hens: Effect on Production Performance, Egg Quality, Feather Condition and Behavior
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laying Hens and Housing Management
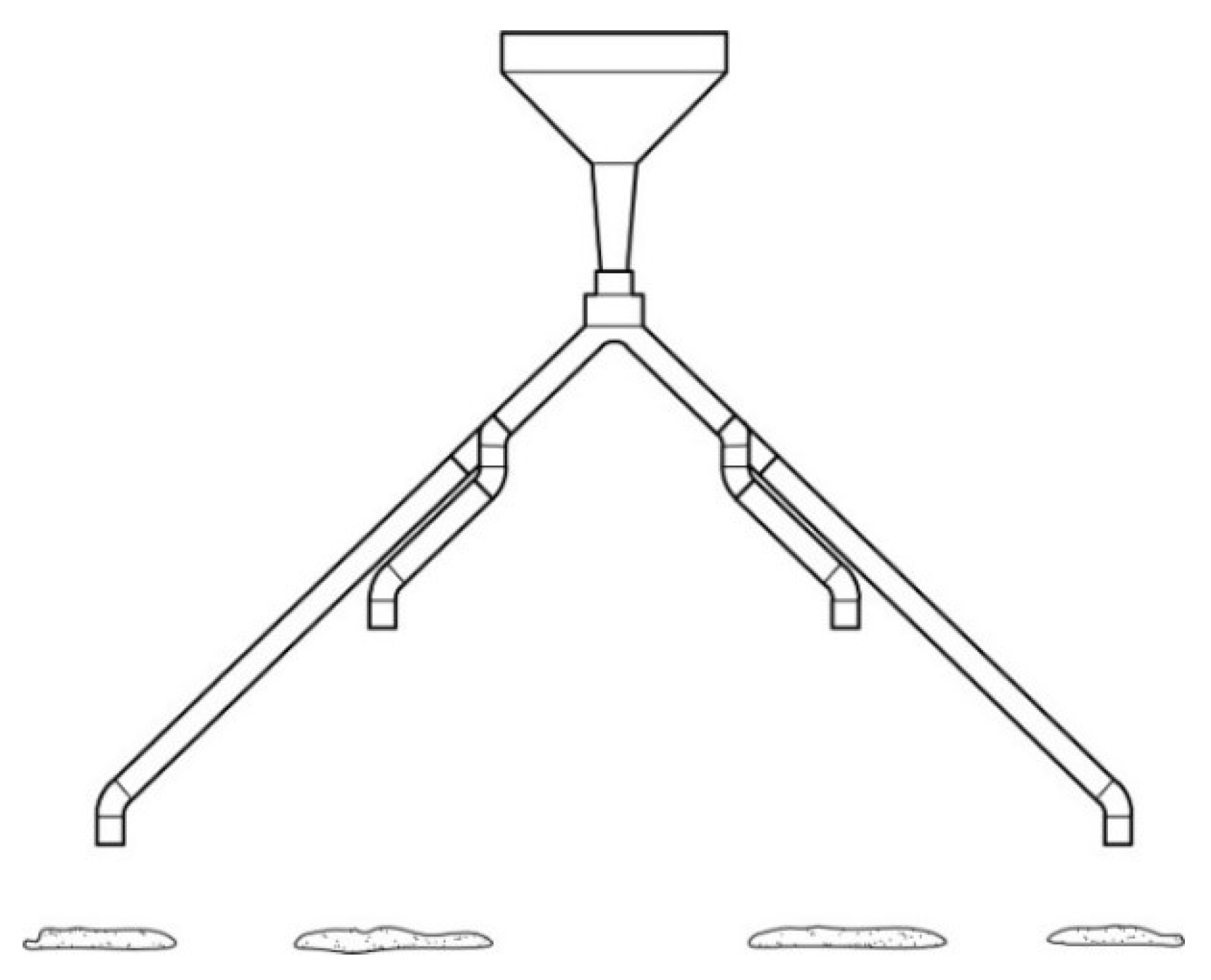
2.2. Black Soldier Fly Larvae and Larvae Dispenser
2.3. Experimental Diets
2.4. Study Design and Feeding Regime
2.5. Production Performance and Mortality
2.6. Egg Quality
2.7. Feather Condition
2.8. Bird Behavior
2.9. Data Exclusion Parameters and Statistical Analysis
2.10. Animal Ethics
3. Results
3.1. Production Performance
3.2. Egg Quality
3.3. Feather Condition
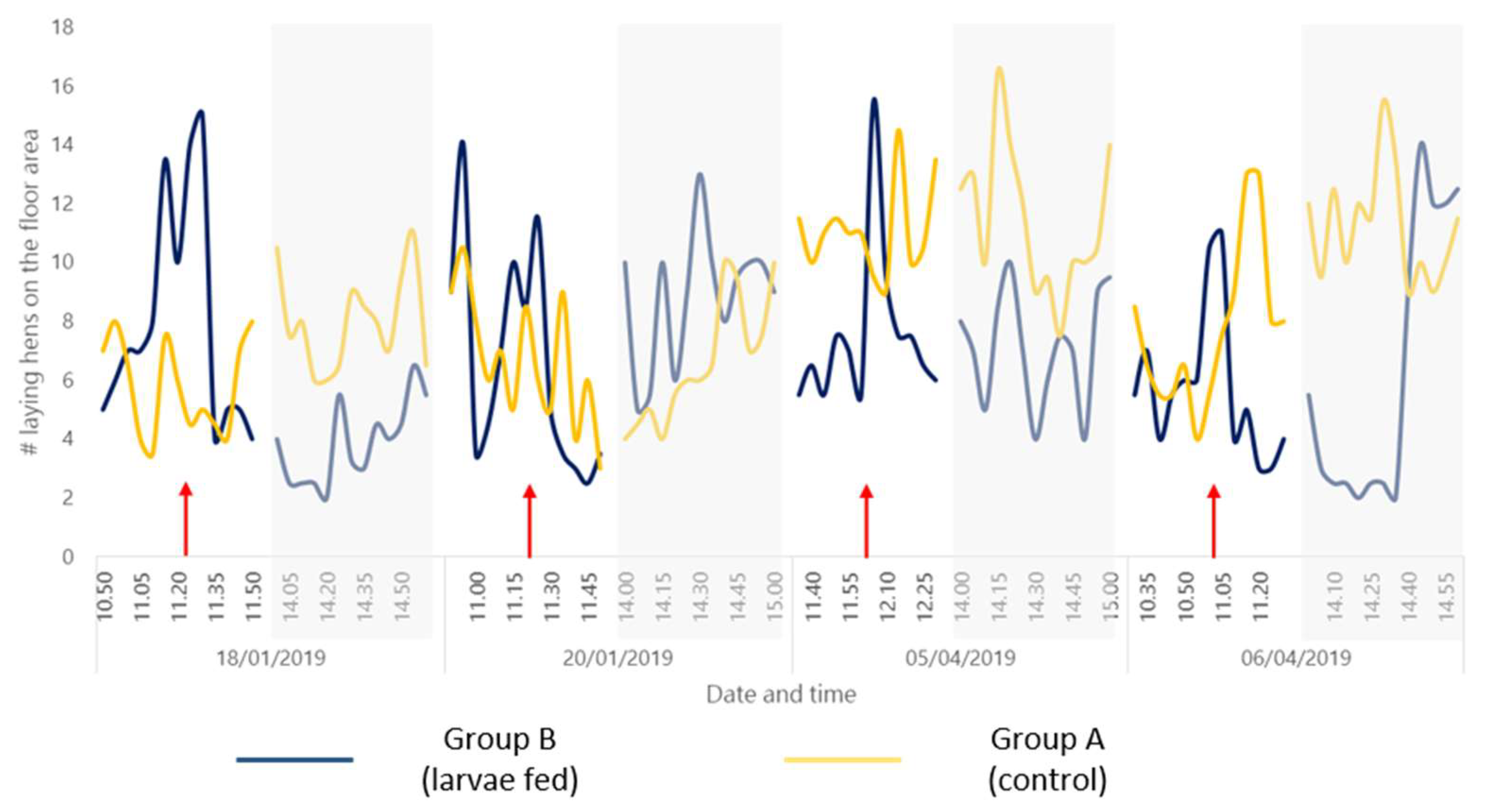
3.4. Bird Behavior
4. Discussion
4.1. Effect on Production Performance
4.2. Effect on Egg Quality
4.3. Effect on Feather Condition
4.4. Effect on Bird Behavior
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heerkens, J. Lifting Laying Hen Welfare in Aviaries to a Higher Level. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2016. [Google Scholar]
- Blokhuis, H.J.; Van Der Haar, J.W. Effects of floor type during rearing and of beak trimming on ground pecking and feather pecking in laying hens. Appl. Anim. Behav. Sci. 1989, 22, 359–369. [Google Scholar] [CrossRef]
- Grandin, T. Animal welfare and society concerns finding the missing link. Meat Sci. 2014, 98, 461–469. [Google Scholar] [CrossRef]
- Kaukonen, E.; Valros, A. Feather Pecking and Cannibalism in Non-Beak-Trimmed Laying Hen Flocks—Farmers’ Perspectives. Animals 2019, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Lambton, S.L.; Knowles, T.G.; Yorke, C.; Nicol, C.J. The risk factors affecting the development of gentle and severe feather pecking in loose housed laying hens. Appl. Anim. Behav. Sci. 2010, 123, 32–42. [Google Scholar] [CrossRef]
- Tahamtani, F.M.; Brantsæter, M.; Nordgreen, J.; Sandberg, E.; Hansen, T.B.; Nødtvedt, A.; Rodenburg, T.B.; Moe, R.O.; Janczak, A.M. Effects of litter provision during early rearing and environmental enrichment during the production phase on feather pecking and feather damage in laying hens. Poult. Sci. 2016, 95, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Cronin, G.M.; Hopcroft, R.L.; Groves, P.J.; Hall, E.J.S.; Phalen, D.N.; Hemsworth, P.H. Why did severe feather pecking and cannibalism outbreaks occur? An unintended case study while investigating the effects of forage and stress on pullets during rearing. Poult. Sci. 2018, 97, 1484–1502. [Google Scholar] [CrossRef]
- Bright, A. Plumage colour and feather pecking in laying hens, a chicken perspective? Br. Poult. Sci. 2007, 48, 253–263. [Google Scholar] [CrossRef]
- Giersberg, M.F.; Spindler, B.; Kemper, N. Assessment of Plumage and Integument Condition in Dual-Purpose Breeds and Conventional Layers. Animals 2017, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Blokhuis, H.J.; Wiepkema, P.R. Studies of feather pecking in poultry. Vet. Q. 1998, 20, 6–9. [Google Scholar] [CrossRef]
- Carr, N. Domestic Animals and Leisure; Springer: Berlin, Germany, 2016; ISBN 978-1-137-41554-7. [Google Scholar]
- Widowski, T.M.; Duncan, I.J.M. Working for a dustbath: Are hens increasing pleasure rather than reducing suffering? Appl. Anim. Behav. Sci. 2000, 68, 39–53. [Google Scholar] [CrossRef]
- Despins, J.L.; Axtell, R.C. Feeding behavior and growth of broiler chicks fed larvae of the darkling beetle, Alphitobius diaperinus. Poult. Sci. 1995, 74, 331–336. [Google Scholar] [CrossRef]
- de Vries, H. Observations on behaviour and feed intake of chicken kept on free range in Muy Muy, Nicaragua. In Proceedings of the World Poultry Science Congress, Montreal, Canada, 20–24 August 2000. [Google Scholar]
- Paul, A.; Frederich, M.; Uyttenbroeck, R.; Hatt, S.; Malik, P.; Lebecque, S.; Hamaidia, M.; Miazek, K.; Goffin, D.; Willems, L.; et al. Grasshoppers as a food source? A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 337–352. [Google Scholar]
- Paul, A.; Frederich, M.; Uyttenbroeck, R.; Malik, P.; Filocco, S.; Richel, A.; Heuskin, S.; Alabi, T.; Megido, R.C.; Franck, T.; et al. Nutritional composition and rearing potential of the meadow grasshopper (Chorthippus parallelus Zetterstedt). J. Asia-Pac. Entomol. 2016, 19, 1111–1116. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia-Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Clara, E.; Regolin, L.; Vallortigara, G.; Rogers, L.J. Chicks prefer to peck at insect-like elongated stimuli moving in a direction orthogonal to their longer axis. Anim. Cogn. 2009, 12, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of Feed Materials. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32013R0068 (accessed on 16 September 2019).
- Schmitt, E.; Belghit, I.; Johansen, J.; Leushuis, R.; Lock, E.-J.; Melsen, D.; Kathirampatti Ramasamy Shanmugam, R.; Van Loon, J.; Paul, A. Growth and Safety Assessment of Feed Streams for Black Soldier Fly Larvae: A Case Study with Aquaculture Sludge. Animals 2019, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, K.; Hashimoto, Y.; Hori, A.; Kawasaki, T.; Hirayasu, H.; Iwase, S.; Hashizume, A.; Ido, A.; Miura, C.; Miura, T.; et al. Evaluation of Black Soldier Fly (Hermetia illucens) Larvae and Pre-Pupae Raised on Household Organic Waste, as Potential Ingredients for Poultry Feed. Animals 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals Fed Insect-Based Diets: State-of-the-Art on Digestibility, Performance and Product Quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Bilcík, B.; Keeling, L.J. Changes in feather condition in relation to feather pecking and aggressive behaviour in laying hens. Br. Poult. Sci. 1999, 40, 444–451. [Google Scholar] [CrossRef]
- Uitdehaag, K.; Komen, H.; Rodenburg, T.B.; Kemp, B.; van Arendonk, J. The novel object test as predictor of feather damage in cage-housed Rhode Island Red and White Leghorn laying hens. Appl. Anim. Behav. Sci. 2008, 109, 292–305. [Google Scholar] [CrossRef]
- Boer, H.C.d.; Krimpen, M.M.v.; Blonk, H.; Tyszler, M. Replacement of Soybean Meal in Compound Feed by European Protein Sources: Effects on Carbon Footprint; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2014. [Google Scholar]
- Gregory, M.; Polsterer, N. Agriculture and deforestation- The EU Common Agricultural Policy, soy and forest destruction (Proposal for Reform); Fern in-house: Brussels, Belgium, 2017; pp. 1–20. [Google Scholar]
- van Gelder, J.W.; Dros, J.M. Corporate Actors in the South American Soy Production Chain; World Wide Fund for Nature: Switzerland, The Netherlands, 2002; pp. 1–90. [Google Scholar]
- Oliveira, G.d.L.T.; Hecht, S.B. Soy, Globalization, and Environmental Politics in South America; Routledge: Abingdon, UK, 2017; ISBN 978-1-351-58374-9. [Google Scholar]
- Boerema, A.; Peeters, A.; Swolfs, S.; Vandevenne, F.; Jacobs, S.; Staes, J.; Meire, P. Soybean Trade: Balancing Environmental and Socio-Economic Impacts of an Intercontinental Market. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Mwaniki, Z.; Neijat, M.; Kiarie, E. Egg production and quality responses of adding up to 7.5% defatted black soldier fly larvae meal in a corn–soybean meal diet fed to Shaver White Leghorns from wk 19 to 27 of age. Poult. Sci. 2018, 97, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Mancini, S.; Iaconisi, V.; Gasco, L.; Basto, A.; Parisi, G. Can the inclusion of black soldier fly (Hermetia illucens) in diet affect the flesh quality/nutritional traits of rainbow trout (Oncorhynchus mykiss) after freezing and cooking? Int. J. Food Sci. Nutr. 2018, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, T.; de Jong, I.; van Krimpen, M.; Reuvekamp, B.; van Tuijl, O.; Bestman, M. Emergency Measures Against Feather Pecking; Wageningen Livestock Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- van Hierden, Y.M. Behavioural Neurobiology of Feather Pecking. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2003. [Google Scholar]
- Leenstra, F.R.; Maurer, V.; Galea, F.; Bestman, M.W.P.; Amsler, Z.; Visscher, J.; Vermeij, I.; Krimpen, M.M. van Laying hen performance in different production systems; why do they differ and how to close the gap? Results of discussions with groups of farmers in The Netherlands, Switzerland and France, benchmarking and model calculations. Eur. Poult. Sci. 2014, 78, 1–10. [Google Scholar]
- The Life of Laying Hens; Farm Animal Welfare Compendium; Compassion in World Farming: Surrey, UK, 2012; pp. 1–6.
- Dixon, L.M. Feather Pecking Behaviour and associated Welfare issues in Laying Hens. Avian Biol. Res. 2008, 1, 73–87. [Google Scholar] [CrossRef]
- van Krimpen, M. Impact of Nutritional Factors on Eating Behavior and Feather Damage of Laying Hens. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2008. [Google Scholar]
- Council for Agriculture Science and Technology Task Force. Impact of Free-Ramge Poultry Production Systems on Animal Health, Human Health, Productivity, Environment, Food Safety and Animal Welfare Issues; Council for Agriculture Science and Technology: Ames, IA, USA, 2018; pp. 1–20. [Google Scholar]
- Veldkamp, T.; van Niekerk, T. Live black soldier fly larvae (Hermetia illucens) for turkey poults. J. Insects Food Feed 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Rodenburg, T.B. Feather Pecking and Related Behavioural Characterstics in Laying Hens. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2003. [Google Scholar]
- Hocking, P.M.; Hughes, B.O.; Keer-Keer, S. Comparison of food intake, rate of consumption, pecking activity and behaviour in layer and broiler breeder males. Br. Poult. Sci. 1997, 38, 237–240. [Google Scholar] [CrossRef]
- Chen, D.H.; Bao, J. General Behaviors and Perching Behaviors of Laying Hens in Cages with Different Colored Perches. Asian-Australas J. Anim. Sci. 2012, 25, 717–724. [Google Scholar] [CrossRef]
- van Niekerk, T.G.C.M.; Veldkamp, T. Insects for Turkeys; Wageningen Livestock Research, WUR: Wageningen, The Netherlands, 2017; p. 41. [Google Scholar]
| Nutrients | Live Larvae |
|---|---|
| Moisture (g/kg) | 700.0 |
| Crude protein (g/kg) | 135.0 |
| Crude fat (g/kg) | 105.0 |
| % Ingredients | Commercial Mash Diet | Soy-Free Mash Diet |
|---|---|---|
| Maize | 30.00 | 30.00 |
| Wheat | 34.61 | 35.52 |
| Soybean meal > 48% CP | 9.70 | 0.00 |
| Sunflower seed meal 38% CP < 1% CF | 6.00 | 6.00 |
| Rapeseed meal (SE) | 2.50 | 7.42 |
| Poultry fat | 2.36 | 2.07 |
| Maize gluten meal > 60% CP | 2.22 | 5.16 |
| Alfalfa 16–19% CP | 1.02 | 0.00 |
| Potato protein | 0.00 | 1.89 |
| Limestone | 8.88 | 8.90 |
| Monocalcium phosphate | 0.23 | 0.23 |
| Sodium bicarbonate | 0.25 | 0.27 |
| Potassium bicarbonate | 0.03 | 0.32 |
| Salt | 0.02 | 0.00 |
| Premix 1 | 1.00 | 1.00 |
| Lysine-HCl (L 79%) | 0.13 | 0.21 |
| Methionine (DL 99%) | 0.03 | 0.00 |
| Premix red | 0.50 | 0.50 |
| Phytase | 0.26 | 0.27 |
| NSP-enzyme | 0.25 | 0.25 |
| Nutrients | Commercial Mash Diet | Soy-Free Mash Diet |
|---|---|---|
| Energy (kcal/kg) | 2800.00 | 2800.00 |
| Moisture (g/kg) | 112.00 | 111.00 |
| Ash (g/kg) | 124.00 | 121.00 |
| Crude protein (g/kg) | 158.00 | 160.00 |
| Crude fat (g/kg) | 50.00 | 48.30 |
| Crude fibre (g/kg) | 32.00 | 32.70 |
| Starch (g/kg) | 387.00 | 396.00 |
| Ca (g/kg) | 38.00 | 38.00 |
| P (g/kg) | 3.93 | 4.01 |
| Na (g/kg) | 1.50 | 1.50 |
| Cl (g/kg) | 1.80 | 1.80 |
| K (g/kg) | 6.47 | 6.48 |
| Lysine (g/kg) 1 | 6.40 | 6.40 |
| Methionine + cysteine (g/kg) 1 | 6.08 | 6.34 |
| Threonine (g/kg) 1 | 4.54 | 4.80 |
| Tryptophan (g/kg) 1 | 1.54 | 1.41 |
| Period | Corresponding Observation Day | Morning | Afternoon |
|---|---|---|---|
| Beginning of trial | First day (18/01/2019) | 11.00 a.m. to 12.00 p.m. 1 | 2:00 p.m. to 3:00 p.m. 2 |
| Beginning of trial | Second day (20/01/2019) | ||
| Termination of trial | Second last day (05/04/2019) | ||
| Termination of trial | Last day (06/04/2019) |
| Treatment | Feed Intake 2 (g/h/d) | Laying Rate (%) | Egg Weight (g) | Egg Mass (g/d) | Mortality (%) | Feed Conversion Ratio (g/g) |
|---|---|---|---|---|---|---|
| Group A | 133 a | 83.3 | 63.11 | 52.58 | 2.8 | 2.534 |
| Group B | 123 b | 81.9 | 63.32 | 51.79 | 1.1 | 2.391 |
| SEM 1 | 2.538 | 1.893 | 0.153 | 1.193 | 0.845 | 0.0238 |
| p-value | 0.029 | 0.601 | 0.353 | 0.657 | 0.197 | 0.004 |
| Parameters | Group A | Group B |
|---|---|---|
| Nutrient composition of diets 1 | ||
| Crude protein (g/kg) | 158.0 | 160.0 |
| Crude fat (g/kg) | 50.0 | 48.3 |
| Energy (kcal/kg) | 2800.0 | 2800.0 |
| Nutrient composition of larvae 2 | ||
| Crude protein (g/kg) | - | 135.0 |
| Crude fat (g/kg) | - | 105.0 |
| Total feed and nutrient intake | ||
| Feed intake (g/h/d) | 133.1 | 123.3 |
| Larvae intake (g/h/d) | 0 | 12.0 |
| Crude protein intake (g/d) | 21.0 | 21.3 |
| Crude fat intake (g/d) | 6.66 | 7.21 |
| Treatment | 67 Weeks (g) | 78 Weeks (g) |
|---|---|---|
| Group A | 1669 | 1660 |
| Group B | 1664 | 1675 |
| SEM 1 | 11.1 | 16.2 |
| p-value | 0.752 | 0.529 |
| Treatment | Egg Weight 2 (g) | Breaking Strenght (N) | Elasticity (N/S) | Haugh Unit | ||||
|---|---|---|---|---|---|---|---|---|
| 67 Weeks | 78 Weeks | 67 Weeks | 78 Weeks | 67 Weeks | 78 Weeks | 67 Weeks | 78 Weeks | |
| Group A | 63.54 | 62.86 | 37.7 | 38.9 | 536 | 623 | 79.3 | 75.6 |
| Group B | 63.80 | 63.07 | 39.5 | 38.7 | 541 | 595 | 79.5 | 77.0 |
| SEM 1 | 0.607 | 0.825 | 0.871 | 1.038 | 9.20 | 19.43 | 0.776 | 1.875 |
| p-value | 0.768 | 0.856 | 0.181 | 0.886 | 0.710 | 0.347 | 0.836 | 0.619 |
| Treatment | Feather Score 2 | |
|---|---|---|
| 67 Weeks (g) | 78 Weeks (g) | |
| Group A | 3.4 | 2.9 a |
| Group B | 3.6 | 2.2 b |
| SEM 1 | 0.077 | 0.107 |
| p-value | 0.060 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Star, L.; Arsiwalla, T.; Molist, F.; Leushuis, R.; Dalim, M.; Paul, A. Gradual Provision of Live Black Soldier Fly (Hermetia illucens) Larvae to Older Laying Hens: Effect on Production Performance, Egg Quality, Feather Condition and Behavior. Animals 2020, 10, 216. https://doi.org/10.3390/ani10020216
Star L, Arsiwalla T, Molist F, Leushuis R, Dalim M, Paul A. Gradual Provision of Live Black Soldier Fly (Hermetia illucens) Larvae to Older Laying Hens: Effect on Production Performance, Egg Quality, Feather Condition and Behavior. Animals. 2020; 10(2):216. https://doi.org/10.3390/ani10020216
Chicago/Turabian StyleStar, Laura, Tarique Arsiwalla, Francesc Molist, Raymond Leushuis, Monika Dalim, and Aman Paul. 2020. "Gradual Provision of Live Black Soldier Fly (Hermetia illucens) Larvae to Older Laying Hens: Effect on Production Performance, Egg Quality, Feather Condition and Behavior" Animals 10, no. 2: 216. https://doi.org/10.3390/ani10020216
APA StyleStar, L., Arsiwalla, T., Molist, F., Leushuis, R., Dalim, M., & Paul, A. (2020). Gradual Provision of Live Black Soldier Fly (Hermetia illucens) Larvae to Older Laying Hens: Effect on Production Performance, Egg Quality, Feather Condition and Behavior. Animals, 10(2), 216. https://doi.org/10.3390/ani10020216






