Effects of Different Concentrate Feed Proportions on Ruminal Ph Parameters, Duodenal Nutrient Flows and Efficiency of Microbial Crude Protein Synthesis in Dairy Cows During Early Lactation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
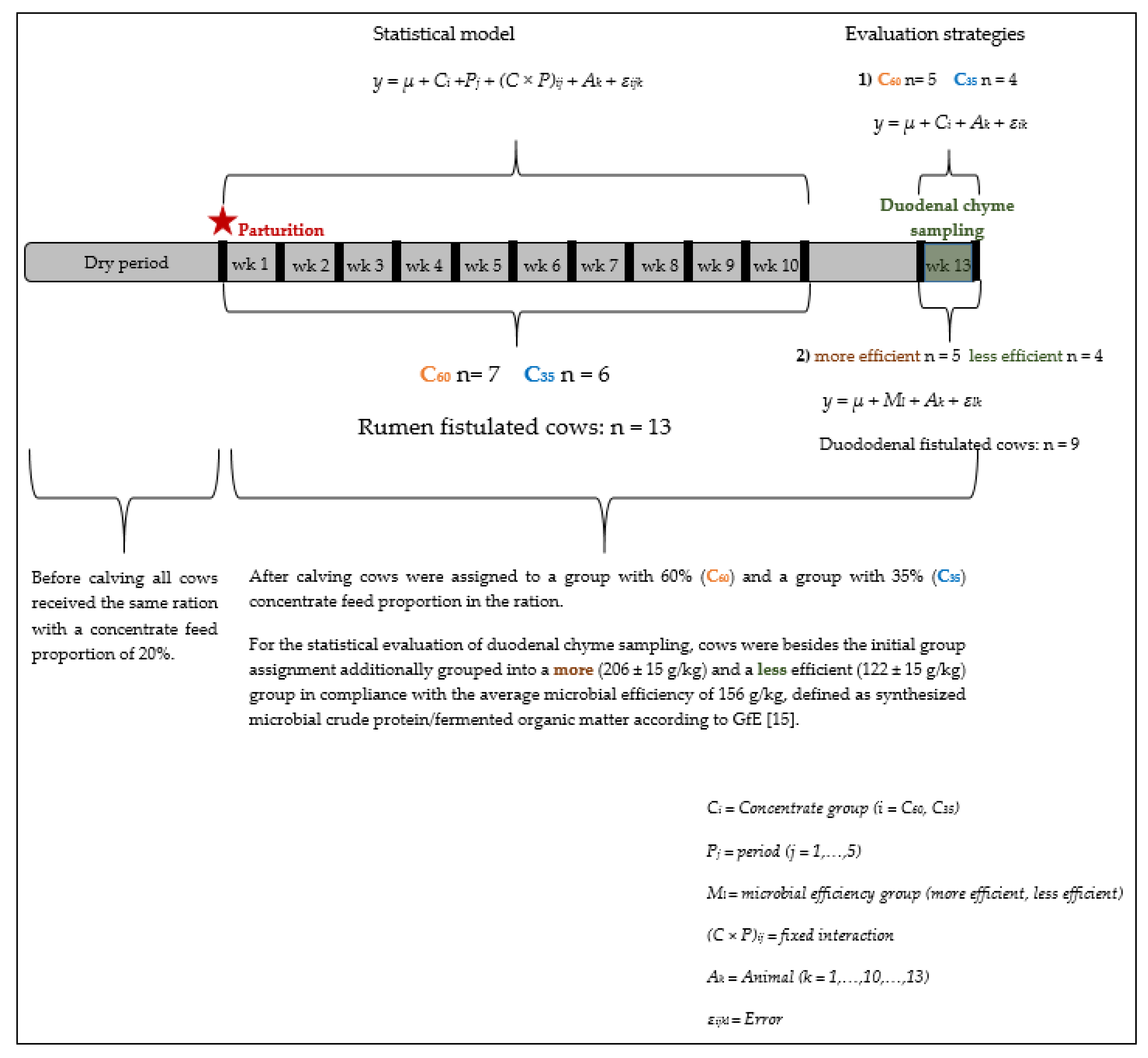
2.1. Experimental Design
2.2. Measurements and Sample Collections
2.2.1. Dry Matter Intake and Milk Yield
2.2.2. Feed and Milk Samples
2.2.3. Rumen Fluid Samples
2.2.4. Rumen pH and Rumination Behavior Measurements
2.2.5. Duodenal Chyme and Faeces Samples
2.3. Analyses
2.3.1. Feed and Milk Analyses
2.3.2. Rumen Fluid Analyses
2.3.3. Duodenal Chyme and Faeces Analyses
2.4. Calculations
(%))/100] × 6.25
(kg/day)]
the duodenum(kg/day))/aNDFom intake (kg/day)] × 100
2.5. Statistical Analysis
3. Results
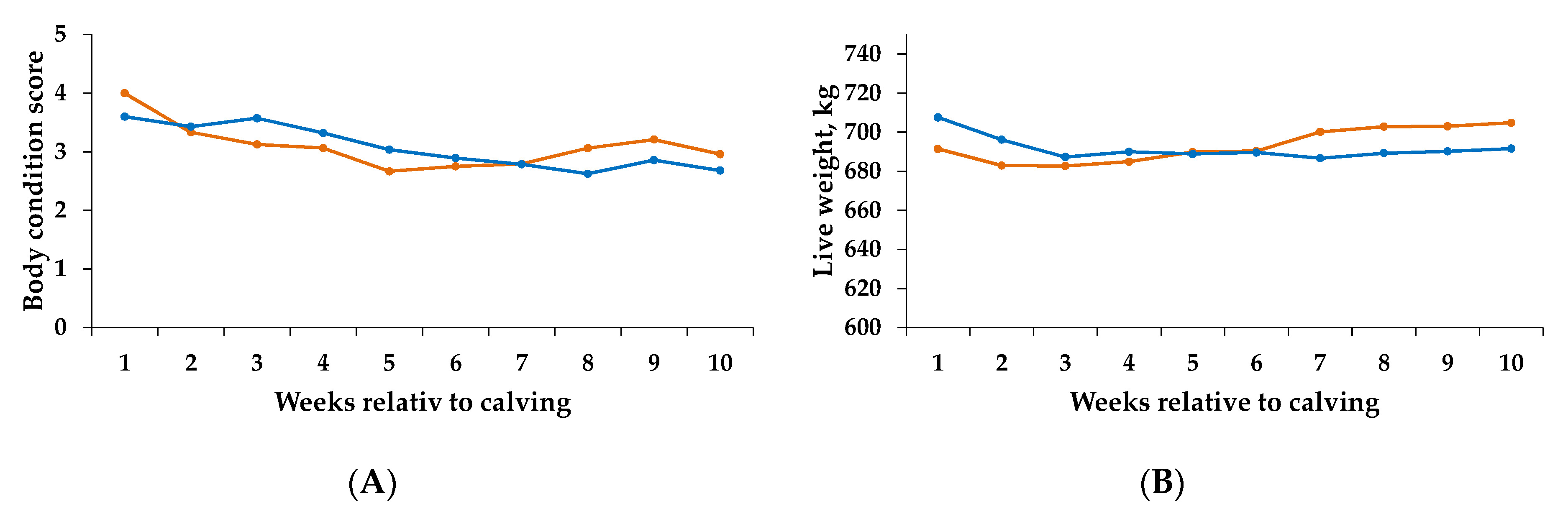
3.1. Performance Parameters
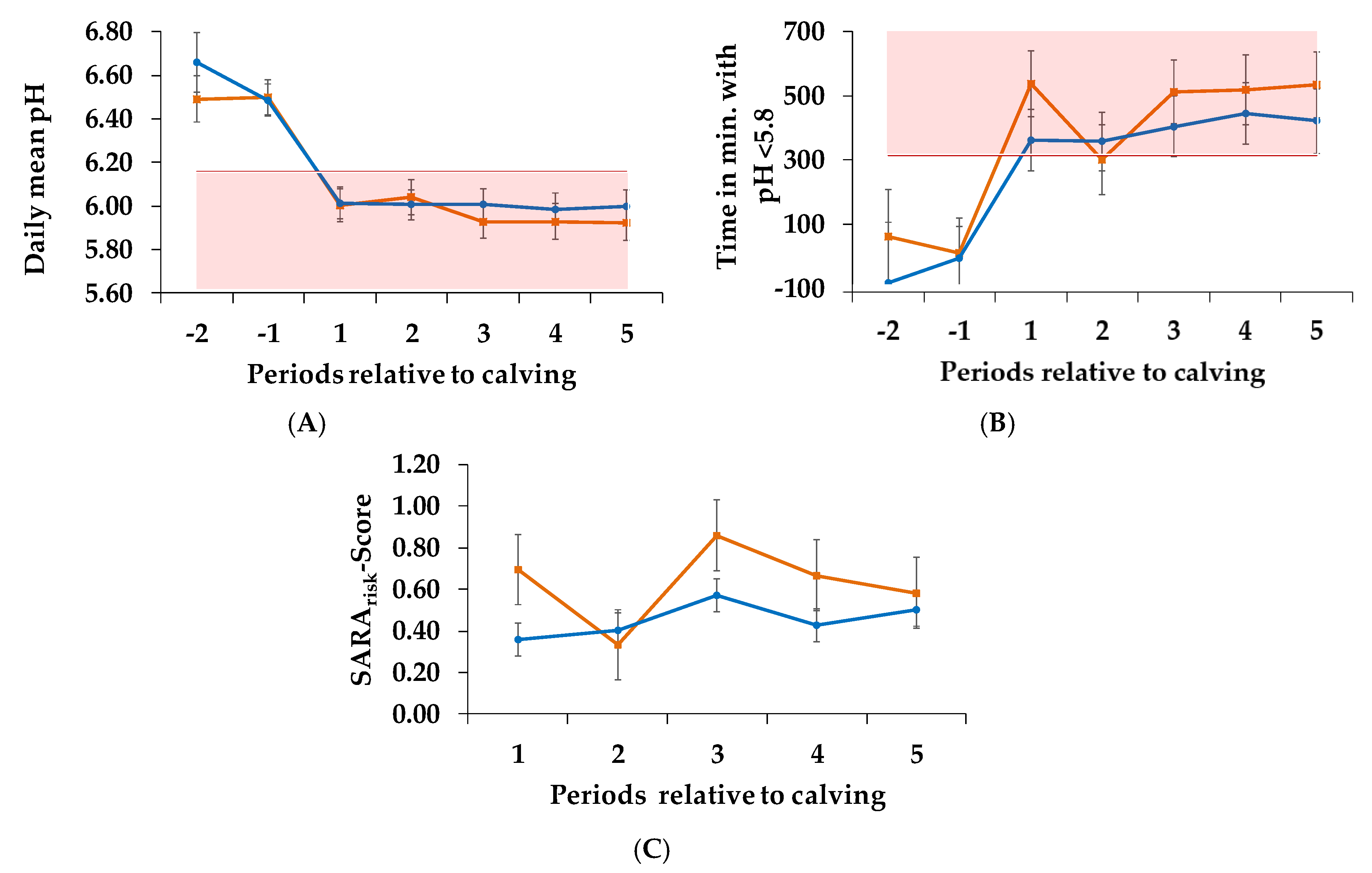
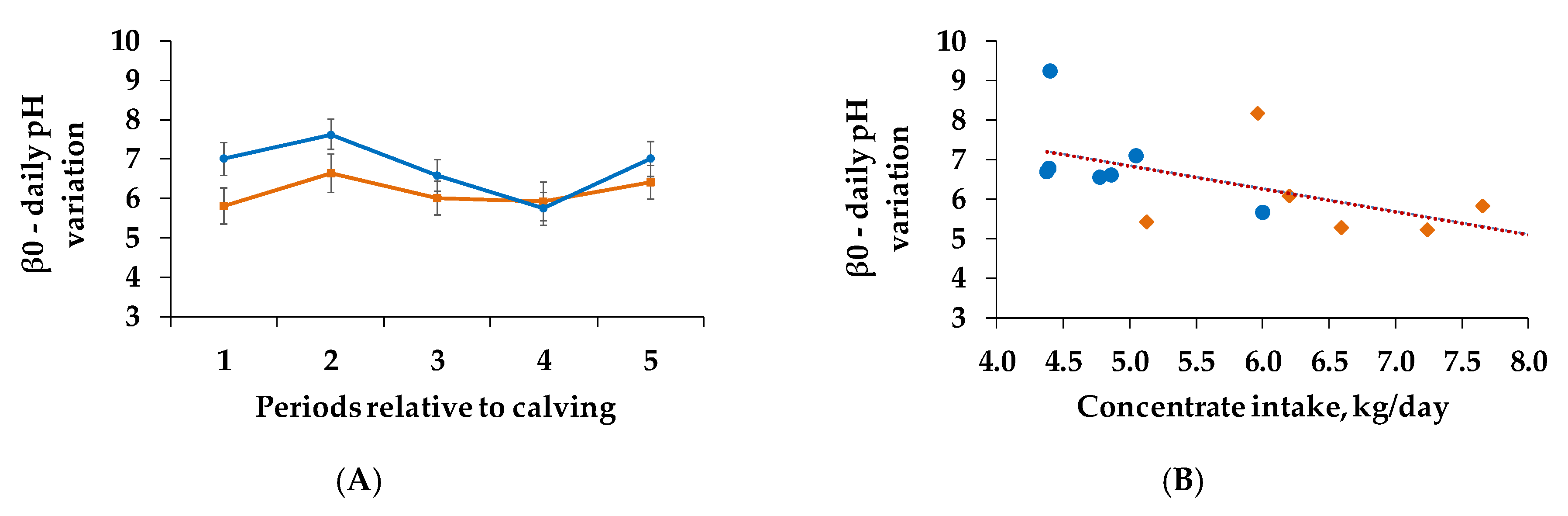
3.2. pH Parameters
3.3. Short Chain Fatty Acids
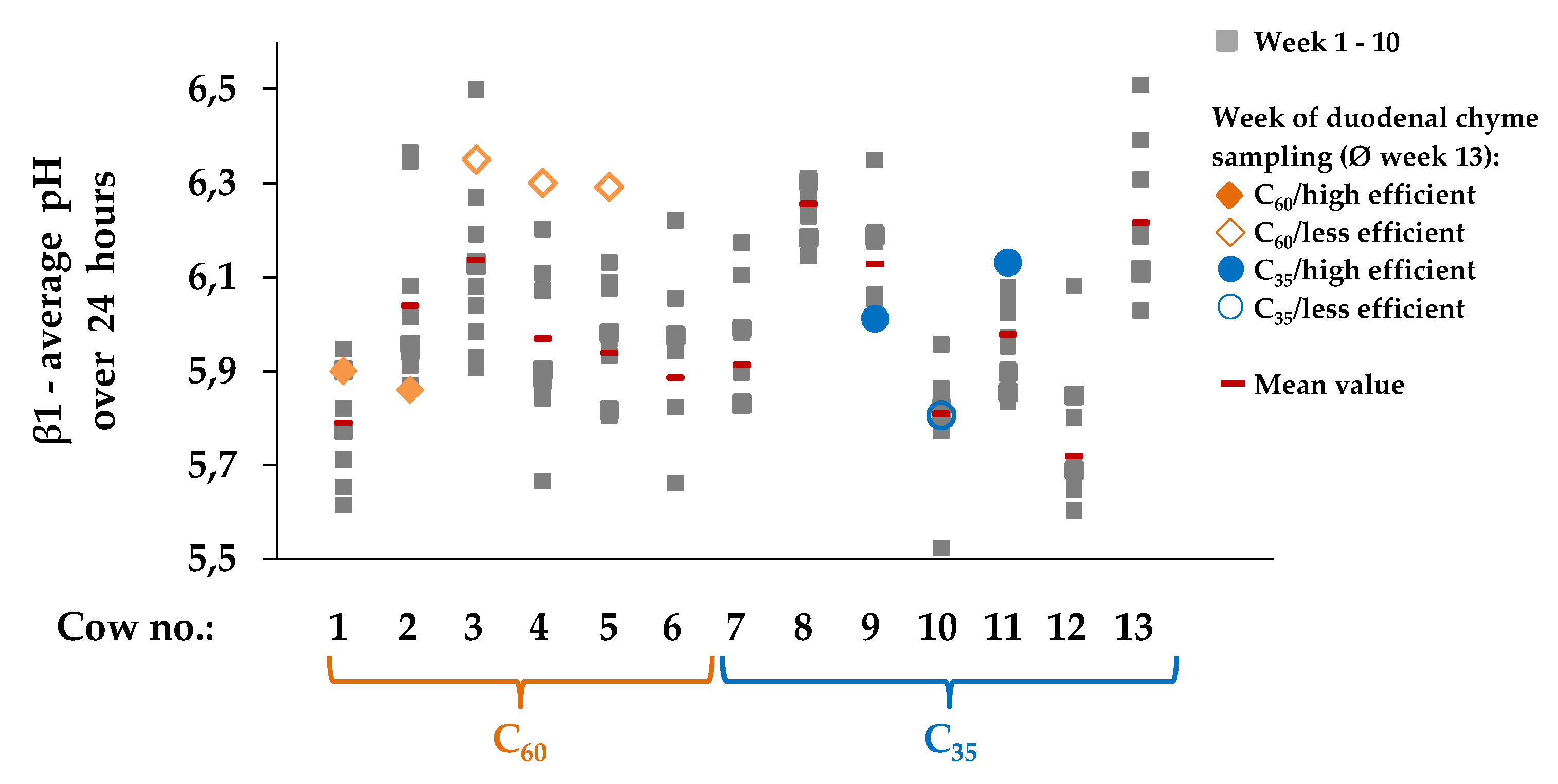
3.4. Evaluations of The Week of Duodenal Chyme Sampling by Different Statistical Evaluations
3.4.1. Microbial Efficiency, pH Parameters, Nutrient Flows and Digestibiliy at the Duodenum
3.4.2. pH Parameters
3.4.3. Digestibility
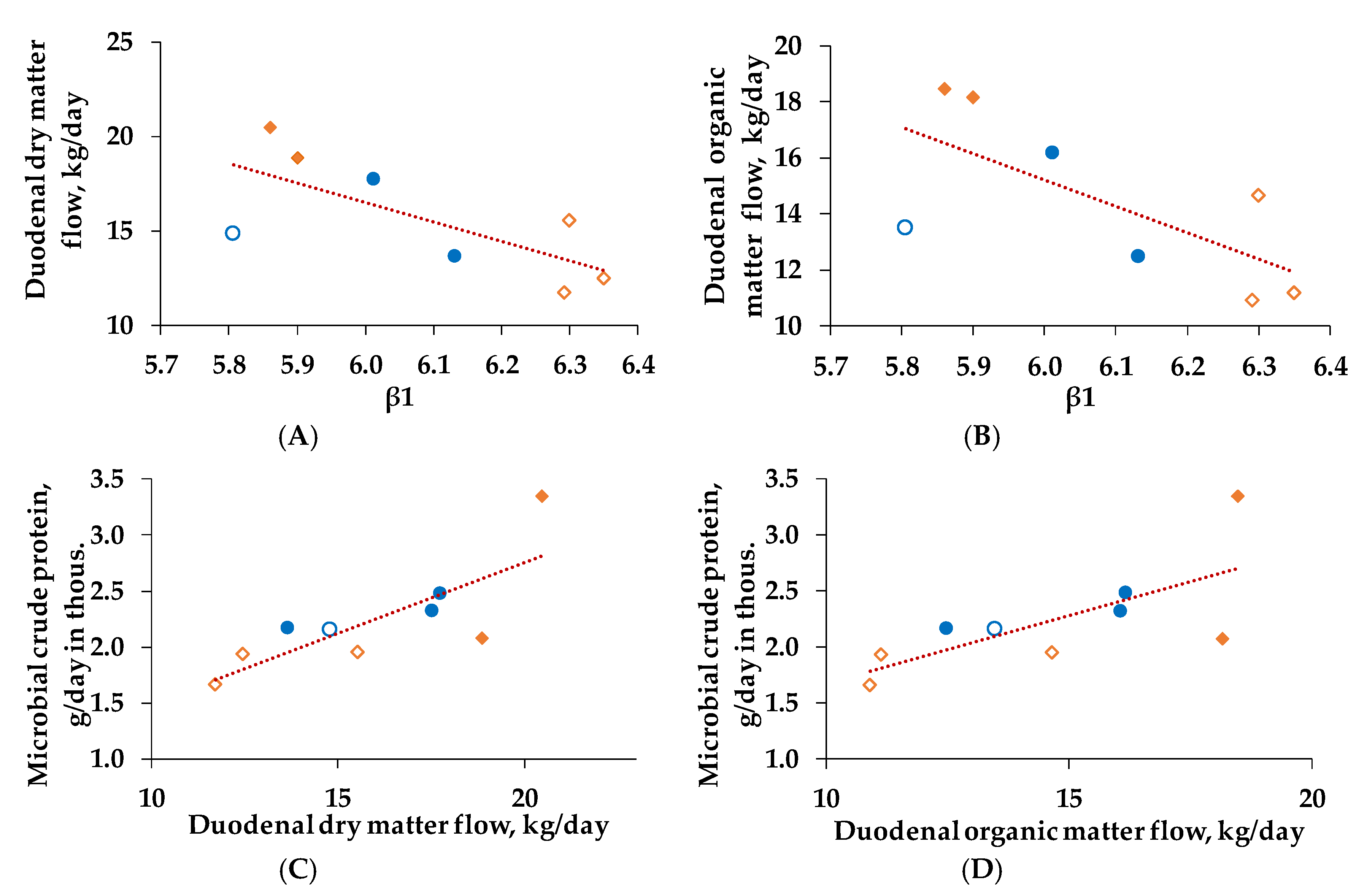
3.5. Correlations and Regression Analysis
Ruminal pH, Duodenal Nutrient Flows, and Microbial Crude Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Item | Grouping § | SEM # | p-Value | |||
|---|---|---|---|---|---|---|
| C60 n = 6 | C35 n = 7 | C | Period | C × Period | ||
| DMI, kg/day | ||||||
| Period 1 | 14.9 | 15.9 | 1.2 | 0.298 | <0.001 | 0.103 |
| Period 2 | 20.3 | 19.4 | ||||
| Period 3 | 23.3 | 21.0 | ||||
| Period 4 | 24.2 | 21.6 | ||||
| Period 5 | 24.5 | 22.7 | ||||
| NEI, MJ NEL/day | ||||||
| Period 1 | 106 c | 113 b | 8 | 0.133 | <0.001 | 0.016 |
| Period 2 | 146 b | 135 a | ||||
| Period 3 | 169 a | 146 a | ||||
| Period 4 | 176 a | 150 a | ||||
| Period 5 | 178 a | 158 a | ||||
| Starch intake, kg/day | ||||||
| Period 1 | 4.6 a | 4.5 a | 0.4 | 0.004 | <0.001 | <0.001 |
| Period 2 | 6.8 b | 5.7 b | ||||
| Period 3 | 8.3 Ac | 6.1 Bc | ||||
| Period 4 | 8.9 Ad | 6.2 Bd | ||||
| Period 5 | 8.8 Ae | 6.6 Be | ||||
| Milk yield, kg/day | ||||||
| Period 1 | 23.3 e | 29.0 b | 1.9 | 0.365 | <0.001 | <0.001 |
| Period 2 | 32.8 d | 36.9 a | ||||
| Period 3 | 36.8 cd | 40.0 a | ||||
| Period 4 | 40.8 b | 40.0 a | ||||
| Period 5 | 41.0 a | 40.2 a | ||||
| Milk fat content, % | ||||||
| Period 1 | 4.88 a | 4.82 a | 0.37 | 0.233 | <0.001 | <0.001 |
| Period 2 | 3.93 b | 4.35 ab | ||||
| Period 3 | 3.32 bc | 4.07 b | ||||
| Period 4 | 2.78 c | 3.82 b | ||||
| Period 5 | 2.81 c | 3.79 b | ||||
| Milk fat yield, kg/day | ||||||
| Period 1 | 1.19 c | 1.28 c | 0.12 | 0.148 | 0.372 | 0.049 |
| Period 2 | 1.30 bc | 1.35 bc | ||||
| Period 3 | 1.22 ab | 1.51 ab | ||||
| Period 4 | 1.14 a | 1.53 a | ||||
| Period 5 | 1.17 a | 1.50 a | ||||
| Milk protein content, % | ||||||
| Period 1 | 3.90 | 3.52 | 0.17 | 0.297 | <0.001 | 0.363 |
| Period 2 | 3.34 | 2.96 | ||||
| Period 3 | 3.26 | 3.04 | ||||
| Period 4 | 3.23 | 3.16 | ||||
| Period 5 | 3.29 | 3.18 | ||||
| Milk protein yield, kg/day | ||||||
| Period 1 | 0.94 | 1.04 | 0.08 | 0.944 | <0.001 | 0.162 |
| Period 2 | 1.11 | 1.09 | ||||
| Period 3 | 1.20 | 1.22 | ||||
| Period 4 | 1.33 | 1.27 | ||||
| Period 5 | 1.36 | 1.27 | ||||
| Milk lactose content, % | ||||||
| Period 1 | 4.49 | 4.26 | 0.21 | 0.560 | 0.002 | 0.312 |
| Period 2 | 4.74 | 4.31 | ||||
| Period 3 | 4.76 | 4.61 | ||||
| Period 4 | 4.79 | 4.80 | ||||
| Period 5 | 4.80 | 4.85 | ||||
| Milk lactose yield, kg/day | ||||||
| Period 1 | 1.10 c | 1.23 b | 0.17 | 0.762 | <0.001 | <0.001 |
| Period 2 | 1.57 b | 1.59 a | ||||
| Period 3 | 1.75 b | 1.71 a | ||||
| Period 4 | 1.97 a | 1.72 a | ||||
| Period 5 | 1.97 a | 1.73 a | ||||
| Milk fat:protein ratio | ||||||
| Period 1 | 1.26 a | 1.31 a | 0.12 | 0.175 | <0.001 | 0.002 |
| Period 2 | 1.18 ab | 1.34 a | ||||
| Period 3 | 1.03 bc | 1.29 a | ||||
| Period 4 | 0.86 c | 1.21 a | ||||
| Period 5 | 0.86 c | 1.19 a | ||||
| ECM, kg/day | ||||||
| Period 1 | 27.69 | 34.61 | 2.17 | 0.093 | <0.001 | 0.210 |
| Period 2 | 32.85 | 38.77 | ||||
| Period 3 | 33.62 | 39.80 | ||||
| Period 4 | 34.87 | 38.93 | ||||
| Period 5 | 35.36 | 38.64 | ||||
| Cow Number | Group Allocation | Individual Microbial Efficiency |
|---|---|---|
| 1 | More efficient | 191.86 |
| 2 | More efficient | 215.75 |
| 3 | Less efficient | 114.65 |
| 4 | More efficient | 198.03 |
| 5 | More efficient | 193.02 |
| 6 | Less efficient | 122.20 |
| 7 | More efficient | 231.77 |
| 8 | Less efficient | 145.98 |
| 9 | Less efficient | 106.92 |
| Item + | Grouping § | SD # | p-Value | |
|---|---|---|---|---|
| More Efficient n = 5 | Less Efficient n = 4 | |||
| DMI, kg/day | 23.9 | 25.3 | 2.7 | 0.515 |
| Milk yield, kg/day | 41.2 | 40.3 | 4.7 | 0.783 |
| Milk fat content, % | 3.32 | 3.16 | 0.51 | 0.731 |
| Milk fat yield, kg/day | 1.35 | 1.30 | 0.21 | 0.781 |
| Milk protein content, % | 3.31 | 3.26 | 0.20 | 0.711 |
| Milk protein yield, kg/day | 1.36 | 1.35 | 0.18 | 0.923 |
| Milk lactose content, % | 4.77 | 4.88 | 0.08 | 0.100 |
| Milk fat:protein ratio | 0.99 | 0.97 | 0.15 | 0.864 |
| ECM, kg/day | 37.4 | 37.1 | 4.4 | 0.919 |
References
- Beauchemin, K.; Penner, G. New developments in understanding ruminal acidosis in dairy cows. In Proceedings of the Tri-State Dairy Nutrition Conference, Grand Wayne Convention Center, Fort Wayne, IN, USA, 21–22 April 2009; pp. 1–12. [Google Scholar]
- Veerkamp, R.; Beerda, B.; Van der Lende, T. Effects of genetic selection for milk yield on energy balance, levels of hormones, and metabolites in lactating cattle, and possible links to reduced fertility. Livest. Sci. 2003, 83, 257–275. [Google Scholar] [CrossRef]
- Bannink, A.; Gerrits, W.; France, J.; Dijkstra, J. Variation in rumen fermentation and the rumen wall during the transition period in dairy cows. Anim. Feed. Sci. Technol. 2012, 172, 80–94. [Google Scholar] [CrossRef]
- Sutton, J.D.; Knight, R.; McAllan, A.B.; Smith, R.H. Digestion and synthesis in the rumen of sheep given diets supplemented with free and protected oils. Br. J. Nutr. 1983, 49, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Ferlay, A.; Chabrot, J.; Loor, J.; Chilliard, Y.; Doreau, M. Effect of linseed oil supplementation on ruminal digestion in dairy cows fed diets with different forage: Concentrate ratios. J. Dairy Sci. 2003, 86, 3999–4007. [Google Scholar] [CrossRef] [Green Version]
- Dirksen, G.; Liebich, H.; Brosi, G.; Hagemeister, H.; Mayer, E. Morphology of the rumen mucosa and fatty acid absorption in cattle—Important factors for health and production. Transbound. Emerg. Dis. 1984, 31, 414–430. [Google Scholar]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Oetzel, G.R. Subacute ruminal acidosis in dairy herds: Physiology, pathophysiology, milk fat responses, and nutritional management. In Dairy Herd Problem Investigation Strategies: Lameness, Cow Comfort, and Ruminal Acidosis. 40th Annual Conference; American Association of Bovine Practitioners: Vancouver, BC, Canada, 17 September 2007; pp. 89–119. [Google Scholar]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef] [Green Version]
- Nordlund, K.V.; Garrett, E.F.; Oetzel, G.R. Herd-based rumenocentesis-a clinical approach to the diagnosis of sub acute rumen acidosis. Transbound. Emerg. Dis 1995, 17, 48–56. [Google Scholar]
- Schmitz, R.; Schnabel, K.; von Soosten, D.; Meyer, U.; Hüther, L.; Spiekers, H.; Rehage, J.; Dänicke, S. Changes of ruminal pH, rumination activity and feeding behaviour during early lactation as affected by different energy and fibre concentrations of roughage in pluriparous dairy cows. Arch. Anim. Nutr. 2018, 72, 458–477. [Google Scholar] [CrossRef]
- Yang, W.; Beauchemin, K.; Rode, L. Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion by dairy cows. J. Dairy Sci. 2001, 84, 2203–2216. [Google Scholar] [CrossRef]
- Ørskov, E.; Fraser, C. The effects of processing of barley-based supplements on rumen pH, rate of digestion and voluntary intake of dried grass in sheep. Br. J. Nutr. 1975, 34, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, J.B.; Wilson, D.B. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 1996, 79, 1503–1509. [Google Scholar] [CrossRef]
- GfE. Gesellschaft für Ernährungsphysiologie. Empfehlungen zur Energie-und Nährstoffversorgung der Milchkühe und Aufzuchtrinder; DLG-Verlags-GmbH: Frankfurt am Main, Germany, 2001. [Google Scholar]
- Strobel, H.J.; Russell, J.B. Effect of pH and energy spilling on bacterial protein synthesis by carbohydrate-limited cultures of mixed rumen bacteria. J. Dairy Sci. 1986, 69, 2941–2947. [Google Scholar] [CrossRef]
- Clark, J.; Klusmeyer, T.; Cameron, M. Microbial protein synthesis and flows of nitrogen fractions to the duodenum of dairy cows. J. Dairy Sci. 1992, 75, 2304–2323. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M. Effects of diet fermentability on efficiency of microbial nitrogen production in lactating dairy cows. J. Dairy Sci. 2003, 86, 195–207. [Google Scholar] [CrossRef] [Green Version]
- De Veth, M.; Kolver, E. Diurnal variation in pH reduces digestion and synthesis of microbial protein when pasture is fermented in continuous culture. J. Dairy Sci. 2001, 84, 2066–2072. [Google Scholar] [CrossRef]
- Russell, J.B.; O’Connor, J.; Fox, D.; Van Soest, P.; Sniffen, C. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef]
- Thompson, F.; Lamming, G. The flow of digesta, dry matter and starch to the duodenum in sheep given rations containing straw of varying particle size. Br. J. Nutr. 1972, 28, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Firkins, J.; Yu, Z.; Morrison, M. Ruminal nitrogen metabolism: Perspectives for integration of microbiology and nutrition for dairy. J. Dairy Sci. 2007, 90. [Google Scholar] [CrossRef]
- Wells, J.E.; Russell, J.B. Why do many ruminal bacteria die and lyse so quickly? J. Dairy Sci. 1996, 79, 1487–1495. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88. [Google Scholar] [CrossRef] [Green Version]
- Hoover, W.H. Chemical factors involved in ruminal fiber digestion. J. Dairy Sci. 1986, 69, 2755–2766. [Google Scholar] [CrossRef]
- Schäfers, S.; Meyer, U.; von Soosten, D.; Krey, B.; Hüther, L.; Tröscher, A.; Pelletier, W.; Kienberger, H.; Rychlik, M.; Dänicke, S. Influence of vitamin E on organic matter fermentation, ruminal protein and fatty acid metabolism, protozoa concentrations and transfer of fatty acids. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1111–1119. [Google Scholar] [CrossRef]
- Geissler, C.; Hoffmann, M.; Hiokel, B. Ein Beitrag zur gaschromatographischen Bestimmung flüchtiger Fettsäuren. Arch. Anim. Nutr. 1976, 26, 123–129. [Google Scholar] [CrossRef]
- Anonymous. DIN 38406-E5-2. In Deutsche Einheitsverfahren zur Wasser-, Abwasser-und Schlammuntersuchung. 40. Lieferung; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- VDLUFA. Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten. Handbuch der landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III: Die chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2006. [Google Scholar]
- Williams, C.; David, D.J.; Iismaa, O. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J. Agric. Sci. 1962, 59, 381–385. [Google Scholar] [CrossRef]
- Lebzien, P.; Paul, C. Use of near-infrared reflectance spectroscopy for the estimation of the microbial portion of non-ammonia-nitrogen in the duodenum of dairy cows. Anim. Feed Sci. Technol. 1997, 68, 225–233. [Google Scholar] [CrossRef]
- Pappritz, J.; Lebzien, P.; Meyer, U.; Jahreis, G.; Kramer, R.; Flachowsky, G.; Dänicke, S. Duodenal availability of conjugated linoleic acids after supplementation to dairy cow diets. Eur. J. Lipid Sci. Technol. 2011, 113, 1443–1455. [Google Scholar] [CrossRef]
- Simon, O. Verdauung und Resorption. In Ernährung landwirtschaftlicher Nutztiere: Ernährungsphysiologie, Futtermittelkunde, Fütterung; Jeroch, H., Drochner, W., Simon, O., Eds.; Eugen Ulmer KG: Stuttgard, Germany, 2008; Volume 130, pp. 109–132. [Google Scholar]
- McGinn, S.; Beauchemin, K.; Coates, T.; Colombatto, D. Methane emissions from beef cattle: Effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J. Anim. Sci. 2004, 82, 3346–3356. [Google Scholar] [CrossRef]
- Littell, R.; Henry, P.; Ammerman, C. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [Green Version]
- AlZahal, O.; Kebreab, E.; France, J.; McBride, B. A mathematical approach to predicting biological values from ruminal pH measurements. J. Dairy Sci. 2007, 90, 3777–3785. [Google Scholar] [CrossRef]
- Colman, E.; Tas, B.; Waegeman, W.; De Baets, B.; Fievez, V. The logistic curve as a tool to describe the daily ruminal pH pattern and its link with milk fatty acids. J. Dairy Sci. 2012, 95, 5845–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schären, M.; Seyfang, G.; Steingass, H.; Dieho, K.; Dijkstra, J.; Hüther, L.; Frahm, J.; Beineke, A.; von Soosten, D.; Meyer, U.; et al. The effects of a ration change from a total mixed ration to pasture on rumen fermentation, volatile fatty acid absorption characteristics, and morphology of dairy cows. J. Dairy Sci. 2016, 99, 3549–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dänicke, S.; Meyer, U.; Kersten, S.; Frahm, J. Animal models to study the impact of nutrition on the immune system of the transition cow. Res. Vet. Sci. 2018, 116, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Combs, D.K.; Beauchemin, K.A. Effects of forage particle size and grain fermentability in midlactation cows. II. Ruminal pH and chewing activity. J. Dairy Sci. 2002, 85, 1947–1957. [Google Scholar] [CrossRef] [Green Version]
- Agle, M.; Hristov, A.N.; Zaman, S.; Schneider, C.; Ndegwa, P.M.; Vaddella, V.K. Effect of dietary concentrate on rumen fermentation, digestibility, and nitrogen losses in dairy cows. J. Dairy Sci. 2010, 93, 4211–4222. [Google Scholar] [CrossRef]
- Gao, X.; Oba, M. Relationship of severity of subacute ruminal acidosis to rumen fermentation, chewing activities, sorting behavior, and milk production in lactating dairy cows fed a high-grain diet. J. Dairy Sci. 2014, 97, 3006–3016. [Google Scholar] [CrossRef] [Green Version]
- Rustomo, B.; AlZahal, O.; Cant, J.; Fan, M.; Duffield, T.; Odongo, N.; McBride, B. Acidogenic value of feeds II. Effects of rumen acid load from feeds on dry matter intake, ruminal pH, fibre degradability and milk production in the lactating dairy cow. Can. J. Anim. Sci. 2006, 86, 119–127. [Google Scholar]
- Nagaraja, T.; Titgemeyer, E. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90. [Google Scholar] [CrossRef] [Green Version]
- Slyter, L.; Oltjen, R.; Kern, D.; Blank, F. Influence of type and level of grain and diethylstilbestrol on the rumen microbial populations of steers fed all-concentrate diets. J. Anim. Sci. 1970, 31, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Mould, F.; Ørskov, E. Manipulation of rumen fluid pH and its influence on cellulolysis in sacco, dry matter degradation and the rumen microflora of sheep offered either hay or concentrate. Anim. Feed Sci. Technol. 1983, 10, 1–14. [Google Scholar] [CrossRef]
- Hiltner, P.; Dehority, B. Effect of soluble carbohydrates on digestion of cellulose by pure cultures of rumen bacteria. Appl. Environ. Microbiol. 1983, 46, 642–648. [Google Scholar] [CrossRef] [Green Version]
- De Veth, M.; Kolver, E. Digestion of ryegrass pasture in response to change in pH in continuous culture. J. Dairy Sci. 2001, 84, 1449–1457. [Google Scholar] [CrossRef]
- Weimer, P.J. Manipulating ruminal fermentation: A microbial ecological perspective. J. Anim. Sci. 1998, 76, 3114–3122. [Google Scholar] [CrossRef]
- Wallace, R.J.; McPherson, C.A. Factors affecting the rate of breakdown of bacterial protein in rumen fluid. Br. J. Nutr. 1987, 58, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, J.; Kebreab, E.; Bannink, A.; France, J.; Lopez, S. Application of the gas production technique to feed evaluation systems for ruminants. Anim. Feed Sci. Technol. 2005, 123, 561–578. [Google Scholar] [CrossRef]
- Rode, L.; Weakley, D.; Satter, L. Effect of forage amount and particle size in diets of lactating dairy cows on site of digestion and microbial protein synthesis. Can. J. Anim. Sci. 1985, 65, 101–111. [Google Scholar] [CrossRef]
- Faichney, G.; Gordon, G.; Welch, R.; Rintoul, A. Effect of dietary free lipid on anaerobic fungi and digestion in the rumen of sheep. Aust. J. Agr. Res. 2002, 53, 519–527. [Google Scholar] [CrossRef]
- Satter, L.; Slyter, L. Effect of ammonia concentration on rumen microbial protein production in vitro. Br. J. Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef]

| Components, g/kg of Fresh Matter | Concentrates | ||
|---|---|---|---|
| Dry Period | Lactating Period | ||
| C35 | C60 | ||
| Soybean meal | 115 | ||
| Rapeseed meal | 150 | 400 | 200 |
| Wheat | 330 | 150 | 213 |
| Barley | 144 | 213 | |
| Maize | 200 | 290 | |
| Dried sugar beet pulp | 296 | 50 | 50 |
| Urea | 30 | 8 | |
| Calcium carbonate | 24 | 13 | 12 |
| Soybean oil | 15 | 10 | 10 |
| Vitamin-mineral premix + | 40 | ||
| Vitamin-mineral premix # | 25 | 12 | |
| Chemical Composition | Dry Period Diet | C35-Ration | C60-Ration |
|---|---|---|---|
| Dry matter, g/kg | 239 | 251 | 198 |
| Nutrients, g/kg DM § | |||
| Crude ash | 63 | 96 | 59 |
| Crude protein | 131 | 144 | 153 |
| Ether extract | 34 | 38 | 42 |
| a † Neutral detergent fiberom $ | 327 | 353 | 294 |
| Acid detergent fiberom $ | 225 | 204 | 169 |
| Starch content | 247 | 285 | 353 |
| Energy ‡, MJ/kg of DM | |||
| ME | 10.9 | 11.3 | 11.8 |
| NEL | 6.6 | 6.9 | 7.3 |
| p-Values | |||
|---|---|---|---|
| C | Period § | C × Period | |
| (A) Daily mean pH | 0.700 | 0.506 | 0.724 |
| (A) Time in min. with pH < 5.8 | 0.557 | 0.205 | 0.545 |
| p-Values | |||
|---|---|---|---|
| C | Period § | C × Period | |
| (A) β0 $ | 0.099 | 0.032 | 0.517 |
| Item | Grouping § | SEM # | p-Value | |||
|---|---|---|---|---|---|---|
| C60 n = 6 | C35 n = 7 | C | Period | C × Period | ||
| β1 | ||||||
| Period 1 | 5.93 | 6.02 | 0.08 | 0.654 | 0.306 | 0.668 |
| Period 2 | 6.07 | 6.02 | ||||
| Period 3 | 5.96 | 6.01 | ||||
| Period 4 | 5.92 | 5.98 | ||||
| Period 5 | 5.94 | 5.99 | ||||
| Item | Grouping § | SEM # | p-Value | |||
|---|---|---|---|---|---|---|
| C60 n = 6 | C35 n = 7 | C | Period | C × period | ||
| Total short chain fatty acids, mmol/L | ||||||
| Period 1 | 76.1 | 71.1 | 5.7 | 0.883 | 0.020 | 0.107 |
| Period 2 | 79.7 | 73.5 | ||||
| Period 3 | 72.9 | 90.3 | ||||
| Period 4 | 94.8 | 83.5 | ||||
| Period 5 | 79.5 | 80.7 | ||||
| Acetic acid, Mol% | ||||||
| Period 1 | 56.9 | 57.9 | 1.8 | 0.117 | 0.001 | 0.053 |
| Period 2 | 51.5 | 56.6 | ||||
| Period 3 | 53.4 | 54.1 | ||||
| Period 4 | 50.6 | 57.3 | ||||
| Period 5 | 53.4 | 56.3 | ||||
| Propionic acid, Mol% | ||||||
| Period 1 | 23.7 | 22.9 | 1.7 | 0.053 | <0.001 | 0.064 |
| Period 2 | 29.5 | 25.3 | ||||
| Period 3 | 29.2 | 27.3 | ||||
| Period 4 | 32.6 | 25.5 | ||||
| Period 5 | 31.3 | 25.9 | ||||
| Butyric acid, Mol% | ||||||
| Period 1 | 14.7 | 14.9 | 0.9 | 0.224 | 0.001 | 0.422 |
| Period 2 | 13.5 | 13.3 | ||||
| Period 3 | 12.1 | 13.8 | ||||
| Period 4 | 11.9 | 13.0 | ||||
| Period 5 | 10.9 | 13.1 | ||||
| Acetate:propionate ratio | ||||||
| Period 1 | 2.49 | 2.63 | 0.22 | 0.103 | <0.001 | 0.131 |
| Period 2 | 1.82 | 2.33 | ||||
| Period 3 | 1.90 | 2.04 | ||||
| Period 4 | 1.57 | 2.34 | ||||
| Period 5 | 1.73 | 2.30 | ||||
| Item + | Grouping Model 1 $ | SD # | p-Value | Grouping Model 2 § | SD # | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| C60 n = 5 | C35 n = 4 | More Efficient n = 5 | Less Efficient n = 4 | |||||
| Daily mean pH | 6.01 | 6.08 | 0.20 | 0.674 | 6.00 | 6.06 | 0.21 | 0.700 |
| Time in min. with pH < 5.8 | 445 | 266 | 250 | 0.400 | 395 | 361 | 288 | 0.873 |
| β0 * | 6.09 | 5.54 | 0.87 | 0.391 | 5.84 | 5.93 | 0.85 | 0.893 |
| β1 † | 6.14 | 5.98 | 0.20 | 0.359 | 5.98 | 6.19 | 0.19 | 0.186 |
| Item + | Grouping Model 1 $ | SD # | p-Value | Grouping Model 2 § | SD # | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| C60 n = 5 | C35 n = 4 | More Efficient n = 5 | Less Efficient n = 4 | |||||
| Microbial crude protein, g/day | 2129 | 2280 | 409 | 0.806 | 2479 | 1922 | 356 | 0.081 |
| Fermented organic matter, kg/day | 14.4 | 12.9 | 3.0 | 0.457 | 12.1 B | 15.8 A | 2.1 | 0.034 |
| Microbial crude protein/fermented organic matter, g/kg | 155 | 186 | 47 | 0.355 | 206 A | 122 B | 17 | <0.001 |
| Duodenal dry matter flow, kg/day | 15.8 | 15.9 | 2.9 | 0.954 | 17.7 A | 13.6 B | 2.2 | 0.032 |
| Duodenal organic matter flow, kg/day | 13.6 | 13.5 | 2.6 | 0.956 | 15.1 A | 11.7 B | 2.0 | 0.037 |
| Rumen ammonia-N *, mg/100 g | 3.0 | 7.5 | 4.0 | 0.176 | 5.12 | 5.44 | 3.95 | 0.932 |
| Item + | Grouping Model 1 $ | SD # | p-Value | Grouping Model 2 § | SD # | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| C60 n = 5 | C35 n = 4 | More Efficient n = 5 | Less Efficient n = 4 | |||||
| Duodenal nutrient digestibility, % | ||||||||
| Neutral detergent fiber | 37 | 40 | 12 | 0.750 | 32 | 46 | 11 | 0.086 |
| Acid detergent fiber | 31 | 35 | 13 | 0.711 | 27 | 40 | 12 | 0.144 |
| Organic matter | 43 | 38 | 10 | 0.490 | 33 B | 51 A | 5 | 0.001 |
| Total digestive tract nutrient digestibility, % | ||||||||
| Neutral detergent fiber | 46 | 42 | 6 | 0.298 | 41 B | 49 A | 4 | 0.036 |
| Acid detergent fiber | 40 | 35 | 6 | 0.338 | 34 B | 43 A | 4 | 0.017 |
| Organic matter | 67 A | 60 B | 4 | 0.003 | 61 B | 68 A | 3 | 0.027 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bünemann, K.; Johannes, M.; Schmitz, R.; Hartwiger, J.; von Soosten, D.; Hüther, L.; Meyer, U.; Westendarp, H.; Hummel, J.; Zeyner, A.; et al. Effects of Different Concentrate Feed Proportions on Ruminal Ph Parameters, Duodenal Nutrient Flows and Efficiency of Microbial Crude Protein Synthesis in Dairy Cows During Early Lactation. Animals 2020, 10, 267. https://doi.org/10.3390/ani10020267
Bünemann K, Johannes M, Schmitz R, Hartwiger J, von Soosten D, Hüther L, Meyer U, Westendarp H, Hummel J, Zeyner A, et al. Effects of Different Concentrate Feed Proportions on Ruminal Ph Parameters, Duodenal Nutrient Flows and Efficiency of Microbial Crude Protein Synthesis in Dairy Cows During Early Lactation. Animals. 2020; 10(2):267. https://doi.org/10.3390/ani10020267
Chicago/Turabian StyleBünemann, Katharina, Maren Johannes, Rolf Schmitz, Julia Hartwiger, Dirk von Soosten, Liane Hüther, Ulrich Meyer, Heiner Westendarp, Jürgen Hummel, Annette Zeyner, and et al. 2020. "Effects of Different Concentrate Feed Proportions on Ruminal Ph Parameters, Duodenal Nutrient Flows and Efficiency of Microbial Crude Protein Synthesis in Dairy Cows During Early Lactation" Animals 10, no. 2: 267. https://doi.org/10.3390/ani10020267






