Simple Summary
Rice straw is a widely used forage source for ruminants in most Asian countries; thus, it is important to accurately estimate its nutritional value. Rice straw is typically fed to the animals along with other ingredients, and the associative effects of the combined ingredients may alter the nutritional value of rice straw. We found associative effects on the ruminal fermentability (gas production kinetics and rumen parameters), especially when rice straw was co-fermented with timothy hay and corn grain. We conclude that the nutritional value of rice straw increases when used with timothy hay and corn grain, due to the associative effects among feeds, which should be considered in diet formulations.
Abstract
The objective of this study was to evaluate the associative effects of rice straw with timothy hay and corn grain. Using an automated gas production system, in vitro ruminal fermentation was studied for six substrates: 100% rice straw, 100% timothy hay, 100% corn grain, 50% rice straw and 50% timothy hay, 50% rice straw and 50% corn grain, and 50% rice straw, 25% timothy hay, and 25% corn grain. Incubation was performed in three batches with different rumen fluids to assess the in vitro ruminal gas production kinetics and rumen parameters (pH, NH3-N, volatile fatty acid (VFA), and true dry matter digestibility (TDMD)). The associated effects were tested by comparing the observed values of the composited feeds and the weighted means of individual feeds. There was a significant increase in NH3-N when rice straw was fermented with timothy hay, corn grain, or both (p < 0.05). TDMD increased when corn grain was co-fermented, and the total gas and VFA production increased when all three feeds were co-fermented. We conclude that the feed value of rice straw increases when fed to animals along with timothy hay and corn grain.
1. Introduction
Ration formulation is a mixture of individual feed ingredients, and the metabolizable energy (ME) and net energy (NE) of the ration are assumed to be the sum of the individual ingredients. This assumes that the ME and NE values of the individual ingredients do not change when mixed with other feed products. However, some studies have documented associative effects among feed ingredients [1,2,3], defined as interactions between the ration components that alter the nutritional value of the individual ingredients [4]. Associative effects may be positive, negative, or absent [5], and though widely discussed in theory, they are seldom considered in feed formulation.
Ruminant animals are fed diets of highly fibrous roughage, grains, brans, and hulls. Associative effects may be an important factor in ruminant rations, as interactions among these ingredients could modify the microbial fermentation processes in the rumen [6,7]. Several studies have investigated the associative effects of barley straw and alfalfa (Medicago sativa) [8], grasses and legumes [9], red clover (Trifolium pratense) and kikuyu grass silage [10], and corn stalks and alfalfa (Medicago sativa) [11].
Rice is a major crop in Asia and, consequently, rice straw is a common roughage for ruminant animals in most Asian countries. In 2014, approximately 538.88 million tons of rice straw, accounting for more than 90% of total world production, was produced in Asia [12]. Prior studies have investigated the associative effects of rice straw with alfalfa hay and corn silage [13], and those of chemically treated rice straw with grass hay or mulberry leaves [6]. To date, however, no studies have tested the associative effects of rice straw with timothy hay and corn grain, which are commonly co-fed ruminants such as beef cattle. In particular, timothy hay is well known for its good quality and contributes a major proportion of imported forage in Korea [14]. Compared to rice straw, timothy hay contains easily digestible carbohydrates, so that it has higher digestibility of fiber and dry matter [15]. Timothy hay, however, is two to three times more expensive than rice straw, and thus it is mainly used as a forage source for calves and lactating dairy cows and as a supplement for rice straw for growing cattle in Korea [16].
In vitro fermentation methods are widely used to study associative effects, as the digestibility and rumen fermentation of feed can be measured in a relatively simple manner, and numerous samples can be evaluated at one time [17]. Among different in vitro methods, the in vitro gas production technique is commonly used to assess the differences between single and mixed feed substrates for different variables [6,7,13,18,19]. An automated in vitro gas production system (AGPS) was first developed by Pell and Schofield [20]—the AGPS continuously measures the gas produced during in vitro ruminal fermentation with electronic pressure transducers, which are sensitive enough to detect small changes due to associative effects. The AGPS developed by Pell and Schofield is inexpensive, easily adopted, and simple to maintain.
The objective of this study was to evaluate the associative effects of rice straw, timothy hay, and corn grain on the ruminal fermentation characteristics using an automated in vitro gas production system.
2. Materials and Methods
The cannulated cattle used in this study were maintained at the Center for Animal Science Research, Chungnam National University, Korea. All animal usage and experimental procedures were conducted with the approval of the Chungnam National University Animal Research Ethics Committee (CNU-00830).
2.1. Preparation of Experimental Diets
To measure the in vitro rumen fermentation characteristics, the feedstuffs were incubated separately or in combination as follows: 100% rice straw (R), 100% timothy hay (T), 100% corn grain (C), 50% rice straw and 50% timothy hay (RT), 50% rice straw and 50% corn grain (RC), and 50% rice straw, 25% timothy hay, and 25% corn grain (RTC). The chemical compositions of rice straw, timothy hay, and corn grain are presented in Table 1. The feed samples were ground in a cyclone mill (Foss Tecator Cyclotec 1093, Foss, Hillerød, Denmark) with 1 mm sieves prior to the chemical analyses and measurements of in vitro gas production.

Table 1.
Chemical composition of rice straw, timothy hay, and corn grain (g/kg dry matter or as stated).
2.2. Automated Gas Production System
For this study, we developed and used a computerized in vitro gas production system, similar to Pell and Schofield [20]. The system consisted of an incubator (DS-110S, Daewon science, Bucheon-si, Korea), two 10 multi-plate stirrers (MS-52M, Jeio-Tech, Seoul, Korea), pressure sensors attached to the incubation bottles (four arrays of five 125 mL serum bottles, for a total 20 bottles), and a 20-channel data logger. The dimensions of the incubator were 600 × 580 × 650 (W × D × H, unit: mm), the internal temperature was maintained at 39 °C, and a pressure sensor (WIKA A-10; Wika, Lawrenceville, GA, USA) measured pressure increases up to 15 psi (pound-force per square inch; i.e., 1 standard atmosphere, atm). The data logger converted the analog signals from the pressure sensors into digital signals and stored them, and the data were transferred to a computer (via a USB drive) for processing using Microsoft Excel Macros.
In the in vitro fermentation study, the gas produced during anaerobic fermentation accumulated in the bottles. A leakage test and sensor calibration were performed prior to each run of the system, and the contents of the fermentation bottles were stirred continuously with a magnetic bar (90 rpm). The internal pressure of the bottles was measured by pressure sensors, and the data logger automatically recorded the values every 20 min.
2.3. In Vitro Ruminal Fermentation
Two cannulated, non-lactating Holstein cows were used as donor animals. Both were fed the same diet ad libitum twice daily (08:00 and 18:00): 600 g/kg corn silage (232 g/kg DM on as-fed basis; 121 g/kg CP, 527g/kg neutral detergent fiber (NDF) analyzed with a heat-stable amylase and expressed inclusive of residual ash (aNDF), 338 g/kg ADF, 27 g/kg EE, and 78 g/kg ash on DM basis) and 400 g/kg commercial concentrate (878 g/kg DM on as-fed basis; 171 g/kg CP, 339 g/kg aNDF, 192 g/kg ADF, 35 g/kg EE, and 59 g/kg ash on DM basis). The rumen fluid from the two donors was mixed and transferred to a Duran bottle on ice, measured for pH with a general pH meter (EcoMet P25, Istek, Inc., Seoul, Korea), and transported to the laboratory. The rumen contents were strained through eight layers of cheesecloth and glass wool and then combined with four times the volume of the in vitro solution described by Goering and Van Soest [21] under strictly anaerobic conditions. Twenty milliliters of the rumen fluid and buffer mixture was transferred to 125 mL serum bottles (Wheaton, Millville, NJ, USA) containing 0.2 g of the feed samples under continuous O2-free CO2 flow to maintain anaerobic conditions. The 125 mL serum bottles were sealed with septum stoppers and aluminum caps and connected to a three-way stopcock and 20 G needle set. The serum bottles were then connected to the sensor and incubated for 48 h at 39 °C. The contents of the fermentation bottles were stirred continuously with a magnetic bar at 90 rpm. In each fermentation batch, the incubation samples were performed in triplicate, and two blanks contained the buffered rumen fluid without the feed substrate.
2.4. Measurements and Chemical Analysis
After 48 h of incubation, the pH was measured and then volatile fatty acid (VFA) analysis was performed following the protocol proposed by Erwin et al. [22], using a gas chromatograph (HP 6890, Hewlett-Packard CO., Palo Alto, CA, USA) equipped with a flame ionization detector and capillary column (Nukol fused silica capillary column 30 m × 0.25 mm × 0.25 μm, Supelco, Inc., Bellefonte, PA, USA). The temperatures of the oven, injector, and detector were 90–180, 185, and 210 °C, respectively. Nitrogen was used as the carrier gas at a flow rate of 40 mL/min. NH3-N was analyzed as in Chaney and Marbach [23], where the NH3-N concentration was determined by measuring the absorbance at 630 nm with a spectrophotometer (UV-1800, Shimadzu Inc., Kyoto, Japan). The remaining undigested samples and fluid were used to measure aNDF to determine the NDF digestibility and true dry matter digestibility (TDMD) using a modified version of the methods proposed by Goering and Van Soest [21].
2.5. Calculation of Associative Effects
Associative effects were calculated using the following the method, which was similar to the one described by Liu [6]. The associative effects were tested by comparing the observed and predicted values of the composited feeds used in this study. The observed values were from the fermentation of the composited feeds (i.e., RT, RC, and RTC), and the predicted values were the weighted mean of the values for the fermentation of the individual feeds (i.e., R, T, and C). For example, the predicted value of RT = (the observed values for R multiplied by the proportion of R in RT; i.e., 0.5) + (the observed values for T multiplied by the proportion of T in RT; i.e., 0.5).
2.6. Statistical Analysis
The dual-pool Logistic equation [24] was fit to the gas production data using the NLIN (nonlinear regression) procedure in SAS (SAS Institute Inc., Cary, NC, USA) as follows:
where Vt is the total gas production at time t, exp is the exponential function, V1max is the maximum gas production of the fast pool (mL), k1 is the maximum rate of gas production of the fast pool (h−1), L is the discrete lag time (h), V2max is the maximum gas production of the slow pool (mL), and k2 is the maximum rate of gas production of the slow pool (h−1).
All data were analyzed using the MIXED procedure in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Differences among the treatments were compared using Tukey’s range test, with a statistical significance threshold at p < 0.05 and a trending threshold at 0.05 ≤ p < 0.10. A paired t-test was used to compare the associative effects.
3. Results
3.1. Rumen Fermentation Characteristics and the Kinetics of Gas Production
The in vitro fermentation characteristics after 48 h incubation are presented in Table 2. There was a significant difference in pH, NH3-N, TDMD, propionate, and butyrate among all of the treatments (p < 0.05). The pH was lowest in the corn grain (p < 0.01) and higher in the rice straw than in the three mixed feed treatments (RT, RC, and RTC; Table 2). Compared to the rice straw and corn grain treatments, the concentration of NH3-N was significantly higher in all of the timothy hay-containing treatments (T, RT, and RTC). The TDMD was highest for corn grain and higher for timothy hay than for rice straw (p < 0.05). Moreover, the TDMD was significantly higher in the mixed feed treatments than in the rice straw treatment (p < 0.05). The butyrate concentration was also significantly higher in the corn mixed treatments than in the rice straw treatment (p < 0.05). However, there were no differences in the acetate and propionate concentrations of rice straw and the mixed feed treatments.

Table 2.
In vitro fermentation characteristics and kinetic parameters of gas production after 48 h incubation.
The kinetics of gas production are shown in Table 2. V1max was greater for corn grain than for the other treatments (p < 0.05), and it was significantly increased in the corn mixed treatments compared to rice straw (p < 0.01). There were no significant differences in the discrete lag time of the rice straw and the mixed treatments. Vmax (the asymptotic total gas production, V1max + V2max) was highest in the corn grain treatment but did not differ between the mixed and rice straw treatments.
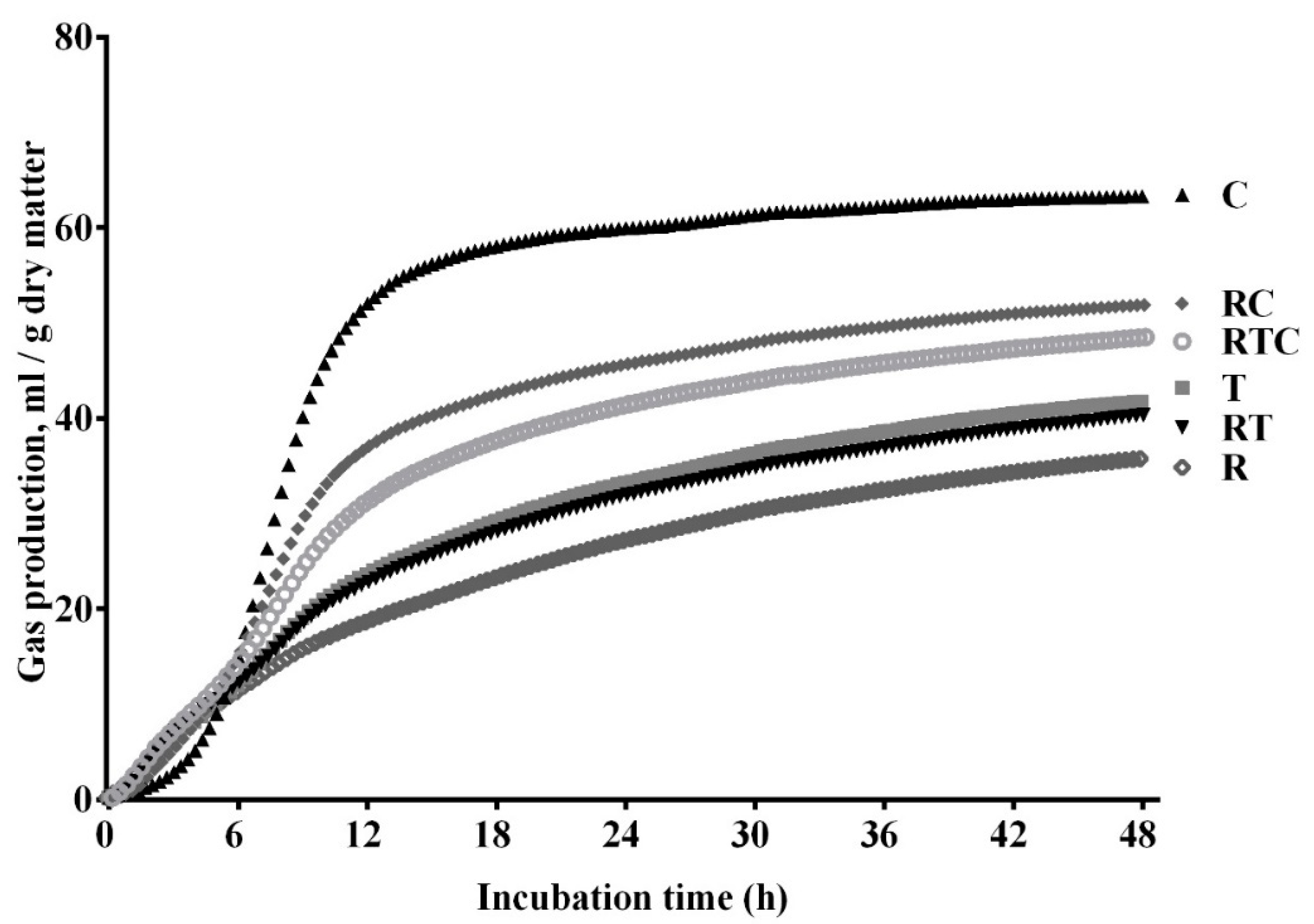
The automated gas production profiles of the individual feedstuffs (rice straw, timothy hay, and corn grain) and the mixed feed treatments (RT, RC, and RTC) are shown in Figure 1. The cumulative volume of gas increased with more incubation time, and after 48 h of fermentation, the gas production was highest in the corn grain, followed by RC, RTC, timothy hay, RT, and rice straw.
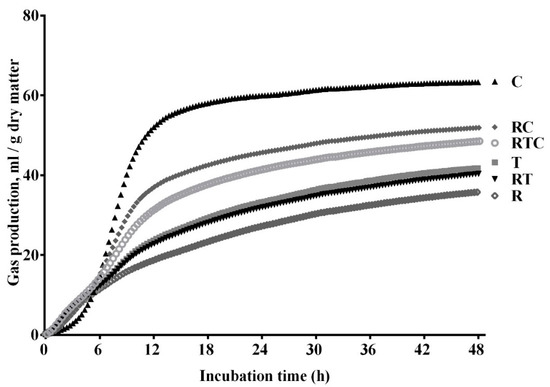
Figure 1.
Gas production profiles of six treatments (R, 100% rice straw; T, 100% timothy hay; C, 100% corn grain; RT, 50% rice straw and 50% timothy hay; RC, 50% rice straw and 50% corn grain; RTC, 50% rice straw, 25% timothy hay, and 25% corn grain).
3.2. Associative Effects on the Rumen Fermentation Characteristics
The probabilities of the significance of associative effects are shown in Table 3. There was a significant difference in the observed and predicted values of NH3-N and TDMD (except for RT) for all three mixed treatments (p < 0.05 for all), indicating a positive associative effect. The observed value of total VFA was significantly higher than the predicted value in the RTC treatment. There were no significant differences in the observed and predicted values of pH, individual VFA, or the acetate to propionate (A/P) ratio for the three mixed treatments. In the RTC treatment, the observed value of the discrete lag time was significantly decreased (p < 0.05), and the observed value of V1max was significantly increased (p < 0.05) compared to the predicted values.

Table 3.
Probability of significance of associative effects after 48 h fermentation.
4. Discussion
Rice straw is a commonly used forage in East Asia, therefore, we were interested in the associative effects of other feeds with rice straw. To date, no studies have investigated the associative effects of rice straw, timothy hay, and corn grain. Corn is an important grain source for cattle in Korea, so there is great interest in the associative effects of corn grain and rice straw. Furthermore, the associative effects of timothy hay, a high-quality forage source, may compensate for the lack of nutrients in rice straw. The objective of this study was to determine the associative effects of rice straw with timothy hay and corn grain using an automated gas production system in an in vitro fermentation experiment.
In this study, the pH was significantly lower for the three mixed feed treatments (RT, RC, and RTC) than for the rice straw (p < 0.05), corresponding to higher total VFA concentrations in the mixed treatments (which contained more fermentable carbohydrates than the rice straw). The non-fiber carbohydrate (NFC) content of rice straw, timothy hay, and corn grain were 86, 204, and 792 g/kg DM, respectively (Table 1). NFCs provide ruminants with fermentable carbohydrates such as starch, sugar, and pectin, and promote the growth of rumen microorganisms. It has been shown that high dietary NFC increases the total VFA production, decreases ruminal pH, and modifies the molar proportions of VFAs by decreasing the ratio of acetic acid/propionate acid and increasing the proportion of butyrate [25]. Our results are consistent with Haddad [8], who supplemented barley straw diets with 300 and 450 g alfalfa hay (i.e., more NFC) and observed higher total VFA and lower pH values than in non-supplemented barley straw.
The NH3-N concentration, TDMD, and V1max (except for RT) were significantly higher in the mixed feed treatments (RT, RC, and RTC) than in the rice straw treatment (p < 0.05). This can be explained by the higher NFC and lower NDF contents in the mixed feed treatments, which provided more fermentable carbohydrates to the rumen microorganisms [26]. Previous studies have also reported a positive correlation between gas and VFA production, and total gas production at 48 h was primarily determined by the NFC content [27,28]. The ash content of rice straw in this study was 12.2%, higher than both timothy hay (7.3%) and corn (1.2%). Normally, 80% of the ash contained in rice straw is silica [29], and silica limits the digestion of fiber because it prevents bacterial colonization [30]. Thus, the replacement of rice straw with timothy hay or corn gain significantly increased the growth and fermentation of rumen microbes.
When considering the associative effects, the observed NH3-N concentrations were significantly higher than the predicted concentrations in the RT, RC, and RTC treatments. An increase in the NH3-N concentration reflects greater catabolism of proteins and non-proteins [31,32], and may also indicate improvements in the rumen environment [33,34]. The growth of rumen microbes is stimulated by the digestibility of substrates that increases the NH3-N concentration [35]. Niderkorn et al. [36] tested the associative effects of legumes and grass and found that the observed NH3-N content was significantly higher than the predicted value in the legume-grass mixture. The mixture provided the rumen microorganisms with more nitrogen and other chemicals, and the interactions of these chemicals enhanced microbial growth and fermentation [36].
The observed TDMD values were significantly higher than the predicted values (p < 0.05; except RT), demonstrating a positive associative effect on dry matter digestibility; this result is consistent with other reports. Cho et al. [37] reported positive associative effects on the dry matter digestibility, organic matter digestibility, and organic matter effective degradability at an appropriate feeding ratio of corn grain and pasture forage. The authors attributed these results to improved ruminal digestion and the asynchrony of nutrient release by the rumen microorganisms, e.g., the release of soluble carbohydrate from grain seemed to improve the digestibility of the cell wall [37]. A corn grain-supplemented fescue hay diet also resulted in improved nutrient digestibility and rumen microorganism growth [38].
In the RTC treatment, the observed value of total VFA was significantly higher than the predicted value. This might be due to a higher NFC content from the corn grain and easily fermentable cellulose and hemicellulose from the timothy hay. Other work has shown that easily fermentable cellulose and hemicellulose increased the number of cellulolytic bacteria, stimulating the digestibility of other less degradable fiber sources in the diet [39]. Sun et al. [13] reported positive associative effects for the in vitro gas, total VFA, and microbial protein production when alfalfa hay was fermented with corn silage. Together, these results suggest that the combination of rice straw, timothy hay, and corn increases the NFCs from corn grain and the easily fermentable cellulose and hemicellulose from timothy hay, which influences the rumen microorganisms and fermentation environment and produces more fermentable substances to increase the total VFA.
There was a trend for the observed values to be higher than predicted values for Vmax, k1, and k2 in the RTC treatment. Within the same treatment, the observed V1max was also significantly higher than the predicted value, and the discrete lag time observed was significantly lower than the predicted value. A higher V1max and lower lag time indicate that the soluble fraction constitutes a substrate of rapid fermentation that facilitated the adhesion and colonization of microorganisms, thereby increasing fermentation and reducing the lag period [40]. Other studies have observed similar results, documenting a reduced lag time in the initiation of the in vitro digestion of fibers due to the positive associative effects of tropical grasses and legumes [41,42]. Measuring the in vitro gas production is a popular technique for determining the forage digestion characteristics and kinetics of fermentation. Rumen microorganisms ferment substrates into CO2, CH4, H2, and VFA [6], and the increase in gas production may be related to increased VFA, caused by the interaction of fiber and non-fiber decomposing microorganisms or ruminal bacteria and protozoa [6,7]. When the rice straw was fermented with timothy hay and corn grain, interactions among the rumen microorganisms increased substrate fermentation and produced more gas and VFA, which resulted in the higher V1max and a shorter lag time.
Nutrient deficiencies in the roughage may be detrimental to the growth of rumen microorganisms (e.g., nitrogen or sulfur) or the ruminant itself (e.g., phosphorus), but positive associative effects were commonly observed when other feed materials contained the needed nutrients. Some studies have suggested that the supplementation of forage with rapidly fermentable carbohydrate sources (i.e., corn grain) would improve microbial growth and feed degradability [43], even in suboptimal nitrogen conditions, because of adaptations in the microbial populations [44,45]. Niderkorn et al. [46] studied the associative effects of temperate climate grass and legumes and found an influence on rumen protein degradation between the legume tannins and grass protein. Improvements to the rumen microflora were also demonstrated by the associative effects of cocksfoot (Dactylis glomerata) and sainfoin (Onobrychis viciifolia) [36]. Thus, when rice straw was fermented with timothy hay and corn, non-fiber carbohydrate from the corn grain, and easily fermented cellulose and hemicellulose from the timothy hay may have contributed to the positive associative effects.
5. Conclusions
This study demonstrated associative effects in ruminal fermentability (gas production kinetics and rumen parameters) when rice straw was co-fermented with timothy hay and corn grain. There was a trend of positive associative effect between rice straw and timothy hay in ruminal digestion; the increase in ruminal fermentability of rice straw was more significant when corn was supplemented. Our results suggest that the feed value of rice straw can be increased by the associative effects of different ingredients and that such factors should be considered in diet formulations.
Author Contributions
L.S. conducted the experiment and prepared the original draft, M.L. performed the statistical analyses and system development, S.J. reviewed the draft, S.S. designed the experiment, and reviewed and edited the draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01422003)”, Rural Development Administration, Republic of Korea.
Acknowledgments
The authors wish to express their gratitude to Lin for his help with Endnote and Hyunjin Cho for his help with cattle management.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dixon, R.M.; Stockdale, C.R. Associative effects between forages and grains: Consequences for feed utilisation. Aust. J. Agric. Res. 1999, 50, 757–773. [Google Scholar] [CrossRef]
- Franci, O.; Antongiovanni, M.; Acciaioli, A.; Bruni, R.; Martini, A. Response surface analyses of the associative effects of lucerne hay, wheat straw and maize gluten feed on growing lambs. Anim. Feed. Sci. Technol. 1997, 67, 279–290. [Google Scholar] [CrossRef]
- Niderkorn, V.; Baumont, R. Associative effects between forages on feed intake and digestion in ruminants. Animal 2009, 3, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Mould, F.; Ørskov, E.; Mann, S. Associative effects of mixed feeds. I. effects of type and level of supplementation and the influence of the rumen fluid pH on cellulolysis in vivo and dry matter digestion of various roughages. Anim. Feed. Sci. Technol. 1983, 10, 15–30. [Google Scholar] [CrossRef]
- Mould, F.; Ørskov, E.; Gauld, S.A. Associative effects of mixed feeds. II. The effect of dietary addition of bicarbonate salts on the voluntary intake and digestibility of diets containing various proportions of hay and barley. Anim. Feed. Sci. Technol. 1983, 10, 31–47. [Google Scholar] [CrossRef]
- Liu, J.X.; Susenbeth, A.; Südekum, K.H. In vitro gas production measurements to evaluate interactions between untreated and chemically treated rice straws, grass hay, and mulberry leaves. J. Anim. Sci. 2002, 80, 517–524. [Google Scholar] [CrossRef]
- Robinson, P.; Getachew, G.; Cone, J. Evaluation of the extent of associative effects of two groups of four feeds using an in vitro gas production procedure. Anim. Feed. Sci. Technol. 2009, 150, 9–17. [Google Scholar] [CrossRef]
- Haddad, S.G. Associative effects of supplementing barley straw diets with alfalfa hay on rumen environment and nutrient intake and digestibility for ewes. Anim. Feed. Sci. Technol. 2000, 87, 163–171. [Google Scholar] [CrossRef]
- Pizzol, J.D.; Ribeiro-Filho, H.; Quereuil, A.; Le Morvan, A.; Niderkorn, V.; Ribeiro-Filho, H.M.N. Complementarities between grasses and forage legumes from temperate and subtropical areas on in vitro rumen fermentation characteristics. Anim. Feed. Sci. Technol. 2017, 228, 178–185. [Google Scholar] [CrossRef]
- Guzatti, G.C.; Duchini, P.G.; Kozloski, G.V.; Niderkorn, V.; Ribeiro-Filho, H.M.N. Associative effects between red clover and kikuyu grass silage: Proteolysis reduction and synergy during in vitro organic matter degradation. Anim. Feed. Sci. Technol. 2017, 231, 107–110. [Google Scholar] [CrossRef]
- Wang, D.; Fang, J.; Xing, F.; Yang, L. Alfalfa as a supplement of dried cornstalk diets: Associative effects on intake, digestibility, nitrogen metabolisation, rumen environment and hematological parameters in sheep. Livest. Sci. 2008, 113, 87–97. [Google Scholar] [CrossRef]
- FAO. The Multiple Goods and Services of Asian Rice Production Systems; FAO: Rome, Italy, 2014. [Google Scholar]
- Sun, P.F.; Wu, Y.M.; Liu, J.X. In vitro gas production technique to evaluate associative effects among lucerne hay, rice straw and maize silage. J. Anim. Feed. Sci. 2007, 16, 272–277. [Google Scholar] [CrossRef]
- Forage Market Report; USDA Foreign Agricultural Service: Seoul, Korea, 2019.
- Hetta, M.; Martinsson, K.; Cone, J.W.; Gustavsson, A. The effect of additives in silages of pure timothy and timothy mixed with red clover on chemical composition and in vitro rumen fermentation characteristics. Grass Forage Sci. 2003, 58, 249–257. [Google Scholar] [CrossRef]
- Ki, K.S.; Park, S.B.; Lim, D.H.; Seo, S. Evaluation of the nutritional value of locally produced forage in Korea using chemical analysis and in vitro ruminal fermentation. Asian-Australas. J. Anim. Sci. 2017, 30, 355–362. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Tagliapietra, F.; Cattani, M.; Guadagnin, M.; Haddi, M.L.; Sulas, L.; Muresu, R.; Squartini, A.; Schiavon, S.; Bailoni, L. Associative effects of poor-quality forages combined with food industry byproducts determined in vitro with an automated gas-production system. Anim. Prod. Sci. 2015, 55, 1117–1122. [Google Scholar] [CrossRef]
- Westreicher-Kristen, E.; Blank, R.; Schulz, F.; Susenbeth, A. Replacing maize silage with red clover silage in total mixed rations for dairy cows: In vitro ruminal fermentation characteristics and associative effects. Anim. Feed. Sci. Technol. 2017, 227, 131–141. [Google Scholar] [CrossRef]
- Pell, A.; Schofield, P. Computerized Monitoring of Gas Production to Measure Forage Digestion In Vitro. J. Dairy Sci. 1993, 76, 1063–1073. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); Agriculture. Handbook No. 379; USDA-ARS: Washington, DC, USA, 1970. [Google Scholar]
- Erwin, E.; Marco, G.; Emery, E. Volatile Fatty Acid Analyses of Blood and Rumen Fluid by Gas Chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified Reagents for Determination of Urea and Ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef]
- Gao, X.; Oba, M. Effect of increasing dietary nonfiber carbohydrate with starch, sucrose, or lactose on rumen fermentation and productivity of lactating dairy cows. J. Dairy Sci. 2016, 99, 291–300. [Google Scholar] [CrossRef]
- Forbes, J.M.; Provenza, F.D. Integration of learning and metabolic signals into a theory of dietary choice and food intake. In Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction; Cronje, P., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 3–19. [Google Scholar]
- Seo, S.; Lee, S.C.; Lee, S.Y.; Seo, J.G.; Ha, J.K. Degradation Kinetics of Carbohydrate Fractions of Ruminant Feeds Using Automated Gas Production Technique. Asian-Australas. J. Anim. Sci. 2009, 22, 356–364. [Google Scholar] [CrossRef]
- Lee, M.; Jeong, S.; Seo, J.; Seo, S. Changes in the ruminal fermentation and bacterial community structure by a sudden change to a high-concentrate diet in Korean domestic ruminants. Asian-Australas. J. Anim. Sci. 2019, 32, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; El-Samni, T.M. Physical and Chemical Properties of Rice Straw Ash and Its Effect on the Cement Paste Produced from Different Cement Types. J. King Saud Univ.-Eng. Sci. 2006, 19, 21–29. [Google Scholar]
- Bae, H.; McAllister, T.A.; Kokko, E.; Leggett, F.; Yanke, L.; Jakober, K.; Ha, J.K.; Shin, H.; Cheng, K.-J. Effect of silica on the colonization of rice straw by ruminal bacteria. Anim. Feed. Sci. Technol. 1997, 65, 165–181. [Google Scholar] [CrossRef]
- Griffith, C.L.; Ribeiro, G.; Oba, M.; McAllister, T.A.; Beauchemin, K.A. Fermentation of Ammonia Fiber Expansion Treated and Untreated Barley Straw in a Rumen Simulation Technique Using Rumen Inoculum from Cattle with Slow versus Fast Rate of Fiber Disappearance. Front. Microbiol. 2016, 7, 534. [Google Scholar] [CrossRef]
- Witzig, M.; Lengowski, M.B.; Zuber, K.H.; Möhring, J.; Rodehutscord, M. Effects of supplementing corn silage with different nitrogen sources on ruminal fermentation and microbial populations in vitro. Anaerobe 2018, 51, 99–109. [Google Scholar] [CrossRef]
- Xu, N.N.; Wang, D.M.; Wang, B.; Wang, J.K.; Liu, J. Different endosperm structures in wheat and corn affected in vitro rumen fermentation and nitrogen utilization of rice straw-based diet. Animal 2018, 13, 1607–1613. [Google Scholar] [CrossRef]
- Ndlovuu, L.R.; Buchanan-Smith, J.G. Utilization of poor quality roughages by sheep: Effects of alfalfa supplementation on ruminal parameters, fiber digestion and rate of passage from the rumen. Can. J. Anim. Sci. 1985, 65, 693–703. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Degradation of quillaja saponins by mixed culture of rumen microbes. Lett. Appl. Microbiol. 1997, 25, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Niderkorn, V.; Baumont, R.; Le Morvan, A.; Macheboeuf, D. Occurrence of associative effects between grasses and legumes in binary mixtures on in vitro rumen fermentation characteristics. J. Anim. Sci. 2011, 89, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.S.; Ueda, K.; Kondo, S. Evaluation of associative effects on ruminal digestion kinetics between pasture and grains using in vitro gas production method. Anim. Sci. J. 2012, 83, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Trotta, R.; Klotz, J.L.; Harmon, D.L. Effects of source and level of dietary energy supplementation on in vitro digestibility and methane production from tall fescue-based diets. Anim. Feed. Sci. Technol. 2018, 242, 41–47. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Schmitz-Esser, S.; Klevenhusen, F.; Podstatzky-Lichtenstein, L.; Wagner, M.; Zebeli, Q. Grain-rich diets differently alter ruminal and colonic abundance of microbial populations and lipopolysaccharide in goats. Anaerobe 2013, 20, 65–73. [Google Scholar] [CrossRef]
- Shen, Y.; Ran, T.; Saleem, A.; Wang, H.; Yang, W. Short communication: Ground corn steeped in citric acid modulates in vitro gas production kinetics, fermentation patterns and dry matter digestibility. Anim. Feed. Sci. Technol. 2019, 247, 9–14. [Google Scholar] [CrossRef]
- Brown, W.F.; Pitman, W.D. Concentration and degradation of nitrogen and fibre fractions in selected tropical grasses and legumes. Trop. Grassl. 1991, 25, 305–312. [Google Scholar]
- Brown, W.F.; Pitman, W.D. In vitro fibre digestion: Associative effects in tropical grass–legume mixtures. Trop. Grassl. 1991, 25, 297–304. [Google Scholar]
- Zicarelli, F.; Calabrò, S.; Cutrignelli, M.I.; Infascelli, F.; Tudisco, R.; Bovera, F.; Piccolo, V. In vitro fermentation characteristics of diets with different forage/concentrate ratios: Comparison of rumen and faecal inocula. J. Sci. Food Agric. 2011, 91, 1213–1221. [Google Scholar] [CrossRef]
- Belanche, A.; Doreau, M.; Edwards, J.E.; Moorby, J.; Pinloche, E.; Newbold, C.J. Shifts in the Rumen Microbiota Due to the Type of Carbohydrate and Level of Protein Ingested by Dairy Cattle Are Associated with Changes in Rumen Fermentation. J. Nutr. 2012, 142, 1684–1692. [Google Scholar] [CrossRef]
- Guadagnin, M.; Tagliapietra, F.; Cattani, M.; Schiavon, S.; Worgan, H.J.; Belanche, A.; Newbold, C.J.; Bailoni, L. Rumen fermentation and microbial yield of high- or low-protein diets containing ground soybean seeds or homemade rapeseed expellers evaluated with RUSITEC. Can. J. Anim. Sci. 2013, 93, 363–371. [Google Scholar] [CrossRef]
- Niderkorn, V.; Irene, M.H.; Le Morvan, A.; Jocelyne, A. Synergistic effects of mixing cocksfoot and sainfoin on in vitro rumen fermentation. Role of condensed tannins. Anim. Feed. Sci. Technol. 2012, 178, 48–56. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).