Effect of Carotenoids, Oligosaccharides and Anthocyanins on Growth Performance, Immunological Parameters and Intestinal Morphology in Broiler Chickens Challenged with Escherichia coli Lipopolysaccharide
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Birds and Housing
2.3. Evaluated Parameters and Used Methods
2.4. Preparation and Determination of Extracts
2.4.1. Carotenoids
2.4.2. Oligosaccharides
2.4.3. Anthocyanins
2.5. Experimental Design, Dietary Treatments and Growth Performance
2.6. Sample Collection and Lymphoid Organ Weight
2.7. RNA Isolation and Reverse Transcription
2.8. qPCR Analysis of Cytokine and Toll-Like Receptor Genes
2.9. Intestine Morphometric Measurements
2.10. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Lymphoid Organ Weight
3.3. Cytokine and Toll-Like Receptor Gene Expression Analysis
3.4. Intestine Morphometric Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klasing, K.C. Nutrition and the immune system. Br. Poult. Sci. 2007, 48, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y.; Wang, Z. The modulating effect of β-1,3/1,6-glucan supplementation in the diet on performance and immunological responses of broiler chickens. Asian-Australas. J. Anim. Sci. 2008, 21, 237–244. [Google Scholar] [CrossRef]
- Hoelzer, K.; Wong, N.; Thomas, J. Antimicrobial drug use in food-producing animals and associated human health risks: What, and how strong, is the evidence? BMC Vet. Res. 2017, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholomew, M.J.; Hollinger, K.; Vose, D. Characterizing the risk of antimicrobial use in food animals: Fluoroquinolone-resistant Campylobacter from consumption of chicken. In Microbial Food Safety in Animal Agriculture: Current Topics; Torrence, M.E., Isaacson, R.E., Eds.; Iowa State Press: Ames, IA, USA, 2003; pp. 293–301. [Google Scholar]
- Wang, Y.; Wu, C.; Zhang, Q.; Qi, J.; Liu, H.; Wang, Y.; He, T. Identification of New Delhi metallo-b-lactamase 1 in Acinetobacter lwoffii of food animal origin. PLoS ONE 2012, 7, e37152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhao, L.; Cao, F.; Ahmad, H.; Wang, G.; Wang, T. Effects of feeding fermented Gingko biloba leaves on small intestinal morphology, absorption, and immunomodulation of early lipopolysaccharide-challenged chicks. Poult. Sci. 2013, 92, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Wang, Y.Q.; QI, Y.X. Influence of procyanidin supplementation on the immune responses of broilers challenged with lipopolysaccharide. Anim. Sci. J. 2017, 88, 983–990. [Google Scholar] [CrossRef]
- Markazi, A.D.; Perez, V.; Sifri, M.; Shanmugasundaram, R.; Selvaraj, R.K. Effect of whole yeast cell product supplementation (CitriStim®) on immune responses and cecal microflora species in pullet and layer chickens during an experimental coccidial challenge. Poult. Sci. 2017, 96, 2049–2056. [Google Scholar] [CrossRef]
- Novak, M.; Vetvicka, V. β-Glucans, history, and the present: Immunomodulatory aspects and mechanisms of actions. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef]
- Alexandra, C.; Weaver, M.; See, T.; Kim, S.W. Protective effect of two yeast-based feed additives on pigs chronically exposed to deoxynivalenol and zearalenone. Toxins 2014, 6, 3336–3353. [Google Scholar]
- Collier, C.T.; Carrol, J.A.; Ballou, M.A.; Starkey, J.D.; Sparks, J.C. Oral administration of Saccharomyces cerevisiae boulardii reduces mortality associated with immune and cortisol responses to Escherichia coli endotoxin in pigs. J. Anim. Sci. 2011, 89, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, Z.; Ren, W.; Yue, Y.; Guo, Y. Effects of live yeast supplementation on lipopolysaccharide-induced inflammatory responses in broilers. Poult. Sci. 2016, 95, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, Z.; Tian, X.; Guo, Y.; Zhang, H. Yeast β-d-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int. J. Biol. Macromol. 2016, 85, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Wils-Plotz, E.L.; Klasing, K.C. Effects of immunomodulatory nutrients on growth performance and immune-related gene expression in layer chicks challenged with lipopolyssaccharide. Poult. Sci. 2017, 96, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Feltl, L.; Pacakova, V.; Stulik, K.; Volka, K. Reliability of carotenoid analysis: A review. Curr. Anal. Chem. 2005, 1, 93–102. [Google Scholar] [CrossRef]
- Goodwin, T.W. Metabolism, nutrition, and function of carotenoids. Ann. Rev. Nutr. 1986, 6, 273–297. [Google Scholar] [CrossRef]
- Lee, S.-J.; Bai, S.-K.; Lee, K.-S.; Namkoong, S.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Yim, S.-V.; Chang, K.; Kwon, Y.-G.; et al. Astaxanthin Inhibits Nitric Oxide Production and Inflammatory Gene Expression by Suppressing IκB Kinase-dependent NF-κB Activation. Mol. Cells 2003, 16, 97–105. [Google Scholar]
- Gao, Y.-Y.; Xie, Q.-M.; Jin, L.; Sun, B.-L.; Ji, J.; Chen, F.; Ma, J.-Y.; Bi, Y.-Z. Supplementation of xanthophylls decreased proinflammatory and increased anti-inflammatory cytokines in hens and chicks. Br. J. Nutr. 2012, 108, 1746–1755. [Google Scholar] [CrossRef]
- Gomes, A.M.P.; Malcata, F.X. Bifidobacterium spp. and Lactobacillus acidophilus: Biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci. Technol. 1999, 10, 139–157. [Google Scholar] [CrossRef]
- Cross, D.E.; McDevitt, R.M.; Hillman, K.; Acamovic, T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [CrossRef]
- Yang, Y.; Iji, P.A.; Kocher, A.; Mikkelsen, L.L.; Choct, M. Effects of mannanoligosaccharide and fructooligosaccharide on the response of broilers to pathogenic Escherichia coli challenge. Br. Poult. Sci. 2008, 49, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Shashidhara, R.G.; Devegowda, G. Effect of dietary mannanoligosaccharide on broiler breeder production traits and immunity. Poult. Sci. 2003, 82, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.C.; Kwakkel, R.P.; Williams, B.A.; Parmentier, H.K.; Li, W.K.; Yang, Z.Q.; Verstegen, M.W.A. Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of Eimeria tenella-infected chickens. Poult. Sci. 2004, 83, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Iji, P.A.; Saki, A.A.; Tivey, D.R. Intestinal structure and function of broiler chickens on diets supplemented with a mannan oligosaccharide. J. Sci. Food Agric. 2001, 81, 1186–1192. [Google Scholar] [CrossRef]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligsaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Regassa, A.; Kim, J.H.; Kim, W.K. The effect of dietary fructooligosaccharide supplementation on growth performance, intestinal morphology, and immune responses in broiler chickens challenged with Salmonella Enteritidis lipopolysaccharides. Poult.Sci. 2015, 94, 2887–2897. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [Green Version]
- Changxing, L.; Chenling, M.; Alagawany, M.; Jianhua, L.; Dongfang, D.; Gaichao, W.; Wenyin, Z.; Syed, S.F.; Arain, M.A.; Saeed, M.; et al. Health benefits and potential applications of anthocyanins in poultry feed industry. World’s Poult. Sci. J. 2018, 74, 251–264. [Google Scholar] [CrossRef]
- Kaiser, P.; Stӓheli, P. Avian cytokines and chemokines. In Avian Immunol.; Davison, F., Kaspers, B., Schat, K.A., Eds.; Elsevier: London, UK, 2008; p. 203. [Google Scholar]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Lotz, M.; Jirik, F.; Kabouridis, P.; Tsoukas, P.; Hirano, T.; Kishimoto, T.; Carson, D.A. B cell stimulating factor 2/Interleukin 6 is a co-stimulant for human thymocytes and T lymphocytes. J. Exp. Med. 1988, 167, 1253–1268. [Google Scholar] [CrossRef]
- Klasing, K.C. Nutritional aspects of leukocytic cytokine cytokines. J. Nutr. 1988, 118, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Anguita, J.; Hoffmeyer, A.; Zapton, T.; Ihle, J.N.; Fikrig, E.; Rincon, M. Inhibition of Th1 differentation by IL-6 is mediated by SOCSI. Immunity 2000, 13, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Corwin, E.J. Understanding cytokines part I: Physiology and mechanisms of action. Biol. Res. Nurs. 2000, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, T.; Jacob, C.O.; Zhou, D.; Mazurek, N.; Fong, M.; Strassmann, G. Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNF-alpha and IL-6 release by endotoxin-stimulated macrophages. J. Immun. 1995, 155, 4909–4916. [Google Scholar] [PubMed]
- Sekellick, M.J.; Ferrandino, A.F.; Hopkins, D.A.; Marcus, P.I. Chicken interferon gene: Cloning, expression, and analysis. J. Interferon Cytokine Res. 1994, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sick, C.; Schultz, U.; Staeheli, P. A family of genes coding for two serologically distinct chicken interferons. J. Biol. Chem. 1996, 271, 7635–7639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Yang, H.; Kapczynski, D.R. Chicken interferon alpha pretreatment reduces virus replication of pandemic H1N1 and H5N9 avian influenza viruses in lung cell cultures from different avian species. Virol. J. 2011, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Göbel, T.W.; Schneider, K.; Schaerer, B.; Mejri, I.; Puehler, F.; Weigend, S.; Staeheli, P.; Kaspers, B. IL-18 stimulates the proliferation and IFN-gamma release of CD4 + T cells in the chicken: Conservation of a Th1-like system in a non mammalian species. J. Immunol. 2003, 171, 1809–1815. [Google Scholar] [CrossRef] [Green Version]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Keestra, A.M.; de Zoete, M.R.; van Aubel, R.A.M.H.; van Putten, J.P.M. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol. Immunol. 2007, 45, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R. The innate immune response to bacterial flagellin is mediated by Toll-like receptor-5. Nature 2001, 410, 1099. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Iqbal, M.; He, H.; Philbin, V.; Kaiser, P.; Smith, A. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 2005, 29, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, H.A.; Thaxton, J.P.; Dozier, W.A., III; Purswell, J.; Roush, W.B.; Branton, S.L. A rewiev of lightning programs for broiler production. Int. J. Poult. Sci. 2006, 4, 301–308. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Echeverry, H.; Yitbarek, A.; Camelo-Jaimes, G.; Sharif, S.; Guenter, W.; House, J.D.; Rodriguez-Lecompte, J.C. Local and systemic innate immunity in broiler chickens supplemented with yeast-derived carbohydrates. Poult. Sci. 2012, 91, 2164–2172. [Google Scholar] [CrossRef]
- Nagy, Z.; Daood, H.; Koncsek, A.; Molnár, H.; Helyes, L. The simultaneous determination of capsaicinoids, tocopherols, and carotenoids in pungent pepper powder. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 199–209. [Google Scholar] [CrossRef]
- Nemes, A.; Szőllősi, E.; Stündl, L.; Biró, A.; Homoki, J.R.; Szarvas, M.M.; Balogh, P.; Cziáky, Z.; Remenyik, J. Determination of flavonoid and proanthocyanidin profile of Hungarian sour cherry. Molecules 2018, 23, 3278. [Google Scholar] [CrossRef] [Green Version]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef]
- Salim, H.M.; Kang, H.K.; Akter, N.; Kim, D.W.; Kim, J.H.; Kim, M.J.; Na, J.C.; Jong, H.B.; Choi, H.C.; Suh, O.S.; et al. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult. Sci. 2013, 92, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, A.; Jávor, A.; Bai, P.; Oláh, J.; Czeglédi, L. Reference genes selection for reverse transcription quantitative polimerase chain reaction in chicken hypothalamus under different feeding status. J. Anim. Phys. Anim. Nutr. 2018, 102, 286–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, S.; Jahromi, M.F.; Liang, J.B.; Zulkifli, I.; Farjam, A.S.; Laudadio, V.; Tufarelli, V. Effect of oligosaccharides extract from palm kernel expeller on growth performance, gut microbiota and immune response in broiler chickens. Poult. Sci. 2015, 94, 2414–2420. [Google Scholar] [CrossRef]
- Rathgeber, B.M.; Budgell, K.L.; Maclsaac, J.L.; Mirza, M.A.; Doncaster, K.L. Growth performance and spleen and bursa weight of broilers fed yeast beta-glucan. Can. J. Anim. Sci. 2008, 88, 469–473. [Google Scholar] [CrossRef]
- Kamboh, A.A.; Hang, S.-Q.; Khan, M.A.; Zhu, W.-Y. In vivo immunmodulatory effects of plant flavonoids in lipopolysaccharid-challenged broilers. Animal 2016, 10, 1619–1625. [Google Scholar] [CrossRef] [Green Version]
- Vetvicka, V.; Oliveira, C. β(1-3)(1-6)-d-glucan with strong effects on immune status in chicken: Potential importance for efficiancy of commercial farming. J. Nutr. Health Sci. 2014, 1, 309. [Google Scholar]
- Morales-Lopez, R.; Brufau, J. Immune-modulatory effects of dietary Saccharomices cerevisiae cell wall in broiler chickens inoculated with Escherichia coli lipopolysaccharide. Br. Poult. Sci. 2013, 54, 247–251. [Google Scholar] [CrossRef]
- Shen, Y.B.; Piao, X.S.; Kim, S.W.; Wang, L.; Liu, P.; Yoon, I.; Zhen, Y.G. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 2009, 87, 2614–2624. [Google Scholar] [CrossRef]
- Kumar, S.; Ciraci, C.; Redmond, S.B.; Chuammitri, P.; Andreasen, C.B.; Palic, D.; Lamont, S.J. Immune response gene expression in spleens of diverse chicken lines fed dietary immunomodulators. Poult. Sci. 2011, 90, 1009–1013. [Google Scholar] [CrossRef]
- Sheoran, N.; Sihag, S.; Maan, N.S. Expression analysis of immunity related genes in White Leghorn layers supplemented with probiotics and prebiotics. Pharm. Innov. 2017, 6, 14–18. [Google Scholar]
- Shanmugasundaram, R.; Sifri, M.; Jeyabalan, R.; Selvaraj, R.K. Effect of yeast cell product (CitriStim) supplementation on turkey performance and intestinal immune cell parameters during an experimental lipopolysaccharide injection. Poult. Sci. 2014, 93, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lillehoj, H.S.; Lillehoj, E.P.; Lee, S.H. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection in chickens. Vet. Immunol. Immunopathol. 2006, 114, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Selvaraj, R.K. Lutein supplementation alters inflammatory cytokine production and antioxidant status in F-line turkeys. Poult. Sci. 2011, 90, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.B.; Gutierres, J.M.; Bohnert, C.; Zago, A.M.; Abdalla, F.H.; Vieira, J.M.; Palma, H.E.; Oliveira, S.M.; Spanello, R.M.; Duarte, M.M.; et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. J. Nutr. Biochem. 2015, 26, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.A.; Dilger, R.N.; Hopkins, A.C.; Price, N.P.; Fahej, G.C., Jr. The effects of a galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex in broiler chicks challenged with Eimeria acervulina. Poult. Sci. 2012, 91, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Pourabedin, M.; Chen, Q.; Yang, M.; Zhao, X. Mannan- and xylooligosaccharides modulatecaecal microbiota and expression of inflammatory-related cytokines and reduce caecal Salmonella enteritidis colonization in young chickens. FEMS Microbiol. Ecol. 2017, 93, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Liu, S.; Guo, Y.; Applegate, T.J.; Eicher, S.D. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 2014, 111, 1394–1404. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Roberts, J.; Wu, S.-B. Regulation of Immunity-Related Genes by Infectious Bronchitis Virus Challenge in Spleen of Laying Chickens. Viral. Immunol. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.P.; Yang, M.X.; Zhang, L.L.; Lu, Z.X.; Zhou, Y.M.; Wang, T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult. Sci. 2015, 94, 1504–1511. [Google Scholar] [CrossRef]
- Cox, C.M.; Sumners, L.H.; Kim, S.; McElroy, A.P.; Bedford, M.R.; Dalloul, R.A. Immune responses to dietary β-glucan in broiler chicks during an Eimeria challenge. Poult. Sci. 2010, 89, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Sifri, M.; Selvaraj, R.K. Effect of yeast cell product (CitriStim) supplementation on broiler performance and intestinal immune cell parameters during an experimental coccidial infection. Poult. Sci. 2013, 92, 358–363. [Google Scholar] [CrossRef] [PubMed]
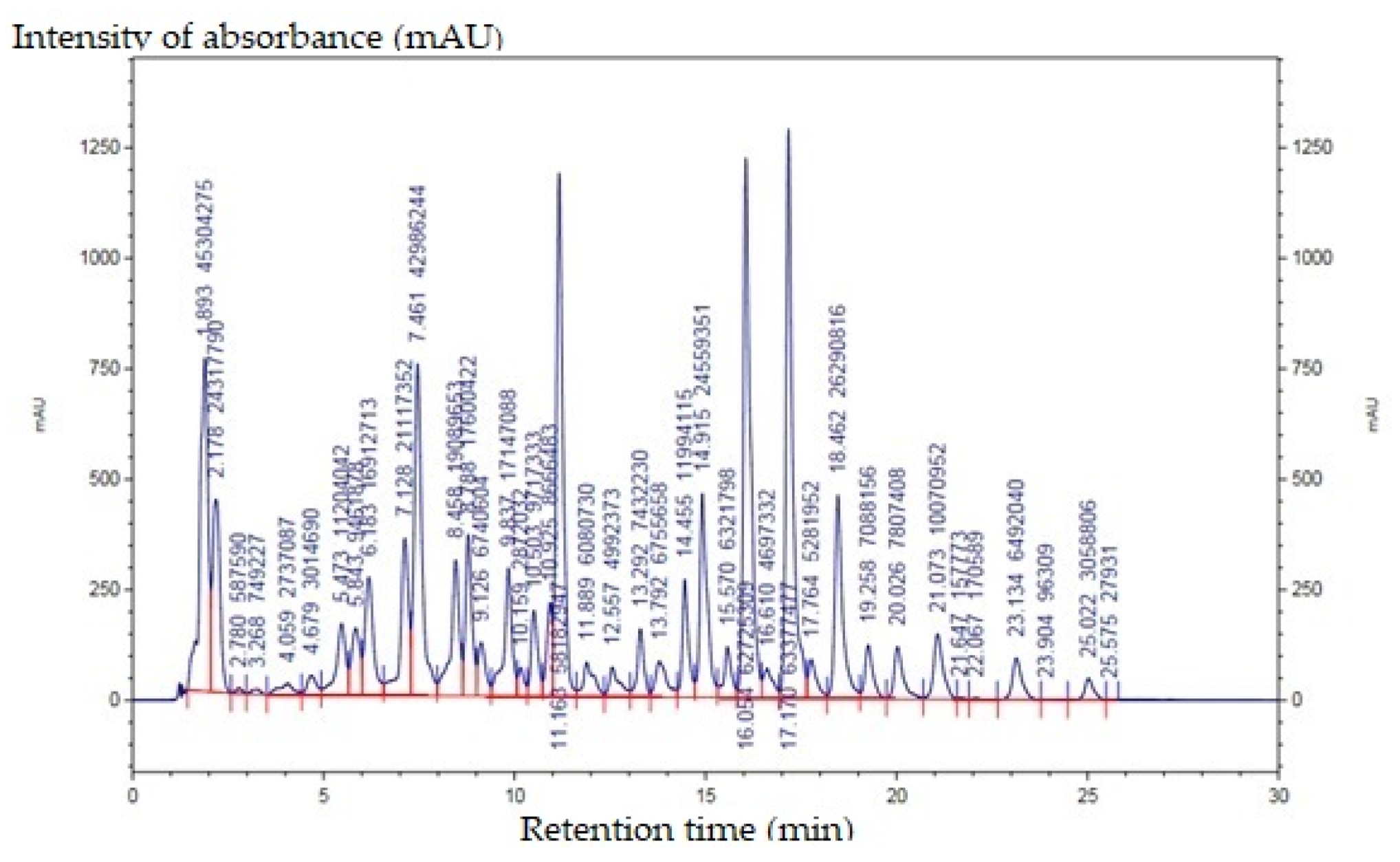





| Retention Time | Name of Compound | Relative Percentage of Areas (%) |
|---|---|---|
| 11.163 | β-carotene | 9.965 |
| 16.054 | cis-capsanthin | 10.743 |
| 17.17 | capsanthin | 10.854 |
| 18.462 | zeaxanthin | 4.503 |
| Name of Monomers | Relative Percentage of Areas (%) |
|---|---|
| Glucose | 71.310 |
| Arabinose | 8.993 |
| Xylose | 8.697 |
| Galactose | 6.815 |
| Mannose | 4.185 |
| Name of Anthocyanin Compounds | Quantity (mg/100 g) |
|---|---|
| cyanidin-3-O-glucosyl-rutinoside | 2.77–10.31 |
| cyanidin-3-O-rutinoside | 4.93–14.56 |
| cyanidin-3-O-monoglucoside | 2.02–7.79 |
| Basal Ingredients | Value | |||
|---|---|---|---|---|
| Pre-Starter (Day 1–9) | Starter (Day 10–21) | Grower (Day 22–31) | Finisher (Day 32–42) | |
| Corn, % | 33 | 34 | 33 | 32 |
| Wheat, % | 27 | 29 | 31 | 32 |
| Soybean meal, solvent extracted (46.0% CP), % | 29 | 24 | 20 | 16 |
| Soybean meal, extruded (46.0% CP), % | 4 | 6 | 4 | 4 |
| Sunflower meal, extracted, % | 1 | 3 | 4 | |
| Feed yeast, % | 1 | |||
| DDGS, % | 1 | 3 | 5 | |
| Plant fats, % | 2 | 1 | 3 | 4 |
| Premix, % | 4 | 4 | 3 | 3 |
| Total, % | 100 | 100 | 100 | 100 |
| Nutrient Level | ||||
| Dry matter, % | 89.06 | 89.03 | 89.15 | 89.15 |
| AMEn poultry, MJ/kg | 12.23 | 12.47 | 12.81 | 13.01 |
| Crude protein, % | 21.58 | 20.28 | 19.05 | 18.28 |
| Crude fat, % | 4.61 | 4.83 | 6.22 | 6.83 |
| Crude fibre, % | 3.37 | 3.51 | 3.7 | 3.88 |
| Lysine, % | 1.37 | 1.27 | 1.17 | 1.09 |
| Methionine, % | 0.57 | 0.54 | 0.53 | 0.49 |
| Methionine + Cysteine, % | 0.94 | 0.9 | 0.87 | 0.83 |
| Calcium, % | 0.85 | 0.73 | 0.71 | 0.67 |
| Phosphorus, % | 0.63 | 0.55 | 0.52 | 0.49 |
| Accesion No. or Reference | Primer Sequences (5′→3′) | Gene | Amplicon Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| XM_015297469.1 | F: TGCTTCGTGCTGGAGTCACCC | IL-1β | 98 | 59.93 |
| R: GGCCGGTACAGCGCAATGTT | 59.02 | |||
| XM_015281283.2 | F: AGCGAAAAGCAGAACGTCGAGTC | IL-6 | 107 | 58.73 |
| R: GCCGAGTCTGGGATGACCACTTC | 59.94 | |||
| AM049251.1 | F: ACTTCAGCTGCCTCCACACCTT | IFN-α | 92 | 59.14 |
| R: CAGGAACCAGGCACGAGCTT | 57.74 | |||
| NM_205149.1 | F: AACAACCTTCCTGATGGCGTGA | IFN-γ | 89 | 57.46 |
| R: GCTTTGCGCTGGATTCTCAAGT | 57.02 | |||
| NM_001030693.1 | F: ACCCGAACTGCAGTTTCTGGAT | TLR-4 | 120 | 57.20 |
| R: AGGTGCTGGAGTGAATTGGC | 55.61 | |||
| XM_025148815.1 | F: ATGAGCTGAGGCTTTAGTTGGAGA | TLR-5 | 108 | 56.61 |
| R: CCAGCTAGTGCTATTCCAAAGACA | 55.62 | |||
| [55] | F: GCTGGCATTGCACTGAATGAC | GAPDH | 113 | 55.73 |
| R: CACTCCTTGGATGCCATGT | 52.42 | |||
| [55] | F: AGATCACAGCCCTGGCACCTAG | ACTB | 61 | 58.80 |
| R: TTGCGCTCAGGTGGGGCAAT | 60.22 |
| Diet | ||||||
|---|---|---|---|---|---|---|
| Parameters | Control | β-Glucan | Carotenoids | Oligosaccharides | Anthocyanins | RMSE * |
| BW (g/bird) | ||||||
| Day 1 | 38.9 | 37.9 | 38.6 | 38.6 | 38.5 | 0.5 |
| Day 10 | 232 | 226 | 221 | 222 | 227 | 14 |
| Day 21 | 759 a,b | 795 b | 769 a,b | 715 a,b | 726 a | 38 |
| Day 32 | 1713 | 1767.7 | 1709.9 | 1735.3 | 1705.3 | 66 |
| Day 42 | 2758 | 2727 | 2748 | 2618 | 2590 | 98 |
| ADG (g/day/bird) | ||||||
| Pre-starter (Day 1–9) | 19 | 19 | 18 | 18 | 19 | 1 |
| Starter (Day 10–21) | 47.9 a,b,c | 51.8 c | 49.8 a,c | 44.9 b | 45.3 a,b | 3 |
| Grower (Day 22–31) | 87 | 88 | 86 | 93 | 89 | 5 |
| Finisher (Day 32–42) | 104 | 96 | 104 | 88 | 89 | 10 |
| Day 1–42 | 65 | 64 | 65 | 61 | 61 | 2 |
| ADFI (g/day/bird) | ||||||
| Pre-starter (Day 1–9) | 4 | 3 | 3 | 4 | 5 | 2 |
| Starter (Day 10–21) | 50 | 58 | 58 | 56 | 61 | 15 |
| Grower (Day 22–31) | 130 a | 144 a,b | 148 a,b | 149 a,b | 159 b | 16 |
| Finisher (Day 32–42) | 114 | 132 | 124 | 127 | 132 | 15 |
| Day 1–42 | 73 a | 83 b | 81 a,b | 82 b | 88 b | 6 |
| Ileum Morphology | Diet | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Control (LPS) | Control (Saline) | β-Glucan | Carotenoids | Oligosaccharides | Anthocyanins | |||
| Villus height (µm) | 774.31 a | 712.02 a | 998.93 b | 908.94 b | 977.08 b | 921.84 b | 27.42 | <0.0001 |
| Crypt depth (µm) | 140.38 b,c | 107.31 a | 120.43 a,b | 160.27 c | 179.90 d | 134.47 b | 8.086 | <0.0001 |
| VH:CD ratio | 5.83 a,b | 6.81 b,c | 8.57 d | 6.19 a,b,c | 5.70 a | 7.12 c | 0.3625 | <0.0001 |
| Total mucosa thickness (µm) | 1156.89 a,c | 1137.47 a | 1350.07 b | 1251.06 b,c | 1346.49 b | 1286.38 b | 35.63 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csernus, B.; Biró, S.; Babinszky, L.; Komlósi, I.; Jávor, A.; Stündl, L.; Remenyik, J.; Bai, P.; Oláh, J.; Pesti-Asbóth, G.; et al. Effect of Carotenoids, Oligosaccharides and Anthocyanins on Growth Performance, Immunological Parameters and Intestinal Morphology in Broiler Chickens Challenged with Escherichia coli Lipopolysaccharide. Animals 2020, 10, 347. https://doi.org/10.3390/ani10020347
Csernus B, Biró S, Babinszky L, Komlósi I, Jávor A, Stündl L, Remenyik J, Bai P, Oláh J, Pesti-Asbóth G, et al. Effect of Carotenoids, Oligosaccharides and Anthocyanins on Growth Performance, Immunological Parameters and Intestinal Morphology in Broiler Chickens Challenged with Escherichia coli Lipopolysaccharide. Animals. 2020; 10(2):347. https://doi.org/10.3390/ani10020347
Chicago/Turabian StyleCsernus, Brigitta, Sándor Biró, László Babinszky, István Komlósi, András Jávor, László Stündl, Judit Remenyik, Péter Bai, János Oláh, Georgina Pesti-Asbóth, and et al. 2020. "Effect of Carotenoids, Oligosaccharides and Anthocyanins on Growth Performance, Immunological Parameters and Intestinal Morphology in Broiler Chickens Challenged with Escherichia coli Lipopolysaccharide" Animals 10, no. 2: 347. https://doi.org/10.3390/ani10020347
APA StyleCsernus, B., Biró, S., Babinszky, L., Komlósi, I., Jávor, A., Stündl, L., Remenyik, J., Bai, P., Oláh, J., Pesti-Asbóth, G., & Czeglédi, L. (2020). Effect of Carotenoids, Oligosaccharides and Anthocyanins on Growth Performance, Immunological Parameters and Intestinal Morphology in Broiler Chickens Challenged with Escherichia coli Lipopolysaccharide. Animals, 10(2), 347. https://doi.org/10.3390/ani10020347







