Answers to the Frequently Asked Questions Regarding Horse Feeding and Management Practices to Reduce the Risk of Atypical Myopathy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Epidemiological Data
3. Results
3.1. Literature Review
3.2. Epidemiological Data
4. Discussion
4.1. FAQ1: “Which Maples Are Toxic? Is this Tree a Maple and If So, Is It Toxic?”
4.2. FAQ2: “How Can AM Be Prevented (at Pasture Level)?”
4.2.1. Avoid Contact with Toxic Plant Materials
4.2.2. Use or Create Low-Risk Pastures
4.3. FAQ3: “How Can AM Be Prevented (at Horse Level)?”
4.3.1. Management of Grazing Time
4.3.2. Feed and Water Supply
4.3.3. Drinking Water
4.4. FAQ4: “ Our Pasture Is Surrounded by Sycamore Maple Trees, but No Case of AM ever Occured in Our Grazing Horses. Does this Mean the Pasture Is Safe for Our Animals?”
4.5. FAQ5: “When Does the Risk of AM Start and Stop in Autumn and Spring?”
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Votion, D.M.; Van Galen, G.; Sweetman, L.; Boemer, F.; De Tullio, P.; Dopagne, C.; Lefère, L.; Mouithys-Mickalad, A.; Patarin, F.; Rouxhet, S.; et al. Identification of methylenecyclopropyl acetic acid in serum of European horses with atypical myopathy. Equine Vet. J. 2014, 46, 146–149. [Google Scholar] [CrossRef]
- Valberg, S.J.; Sponseller, B.T.; Hegeman, A.D.; Earing, J.; Bender, J.B.; Martinson, K.L.; Patterson, S.E.; Sweetman, L. Seasonal pasture myopathy/atypical myopathy in North America associated with ingestion of hypoglycin A within seeds of the box elder tree. Equine Vet. J. 2013, 45, 419–426. [Google Scholar] [CrossRef]
- Fowden, L.; Pratt, H.M. Cyclopropylamino acids of the genus Acer: Distribution and biosynthesis. Phytochemistry 1973, 12, 1677–1681. [Google Scholar] [CrossRef]
- Bochnia, M.; Sander, J.; Ziegler, J.; Terhardt, M.; Sander, S.; Janzen, N.; Cavalleri, J.M.; Zuraw, A.; Wensch-Dorendorf, M.; Zeyner, A. Detection of MCPG metabolites in horses with atypical myopathy. PLoS ONE 2019, 14, e0211698. [Google Scholar] [CrossRef] [Green Version]
- Von Holt, C.; Chang, J.; von Holt, M.; Böhm, H. Metabolism and metabolic effects of hypoglycin. Biochim. Biophys. Acta 1964, 0, 611–613. [Google Scholar] [CrossRef]
- Melde, K.; Jackson, S.; Bartlett, K.; Stanley, H.; Sherratt, H.; Ghisla, S. Metabolic consequences of methylenecyclopropylglycine poisoning in rats. Biochem. J. 1991, 274, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Westermann, C.M.; de Sain-van der Velden, M.G.M.; van der Kolk, J.H.; Berger, R.; Wijnberg, I.D.; Koeman, J.P.; Wanders, R.J.A.; Lenstra, J.A.; Testerink, N.; Vaandrager, A.B.; et al. Equine biochemical multiple acyl-CoA dehydrogenase deficiency (MADD) as a cause of rhabdomyolysis. Mol. Genet. Metab. 2007, 91, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Palencia, P.; Rivero, J.L.L. Short Communications Atypical myopathy in two grazing horses in northern Spain. Vet. Rec. 2007, 161, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Finno, C.J.; Valberg, S.J.; Wünschmann, A.; Murphy, M.J. Seasonal pasture myopathy in horses in the midwestern United States: 14 cases (1998–2005). J. Am. Vet. Med. Assoc. 2006, 229, 1134–1141. [Google Scholar] [CrossRef]
- Votion, D.M.; Linden, A.; Saegerman, C.; Engels, P.; Erpicum, M.; Thiry, E.; Delguste, C.; Rouxhet, S.; Demoulin, V.; Navet, R.; et al. History and clinical features of atypical myopathy in horses in Belgium (2000–2005). J. Vet. Intern. Med. 2007, 21, 1380–1391. [Google Scholar] [PubMed]
- Van Galen, G.; Marcillaud Pitel, C.; Saegerman, C.; Patarin, F.; Amory, H.; Baily, J.D.; Cassart, D.; Gerber, V.; Hahn, C.; Harris, P.; et al. European outbreaks of atypical myopathy in grazing equids (2006–2009): Spatiotemporal distribution, history and clinical features. Equine Vet. J. 2012, 44, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Medina, S.; Ireland, J.L.; Piercy, R.J.; Newton, J.R.; Votion, D. Equine atypical myopathy in the UK: Epidemiological characteristics of cases reported from 2011 to 2015 and factors associated with survival. Equine Vet. J. 2017, 49, 746–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, K.; Brandtt, K.; Hinrrchs, U.; Schulze, C.; Landes, E. Atypische Myoglobinurie der Weidepferde. Pferdeheilkunde 1997, 13, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Dunkel, B.; Ryan, A.; Haggett, E.; Knowles, E.J. Atypical myopathy in the South-East of England: Clinicopathological data and outcome in hospitalised horses. Equine Vet. Educ. 2018, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Van Galen, G.; Saegerman, C.; Marcillaud Pitel, C.; Patarin, F.; Amory, H.; Baily, J.D.; Cassart, D.; Gerber, V.; Hahn, C.; Harris, P.; et al. European outbreaks of atypical myopathy in grazing horses (2006–2009): Determination of indicators for risk and prognostic factors. Equine Vet. J. Vol. 2012, 44, 621–625. [Google Scholar] [CrossRef]
- Bunert, C.; Langer, S.; Votion, D.M.; Boemer, F.; Muller, A.; Ternes, K.; Liesegang, A. Atypical myopathy in Pere David’s deer (Elaphurus davidianus) associated with ingestion of hypoglycin A. J. Anim. Sci. 2018, 96, 3537–3547. [Google Scholar] [CrossRef]
- Votion, D.M.; Linden, A.; Delguste, C.; Amory, H.; Thiry, E.; Engels, P.; Van Galen, G.; Navet, R.; Sluse, F.; Serteyn, D.; et al. Atypical myopathy in grazing horses: A first exploratory data analysis. Vet. J. 2009, 180, 77–87. [Google Scholar] [CrossRef]
- Westermann, C.M.; van Leeuwen, R.; van Raamsdonk, L.W.D.; Mol, H.G.J. “Hypoglycin A Concentrations in Maple Tree Species in the Netherlands and the Occurrence of Atypical Myopathy in Horses. J. Vet. Intern. Med. 2016, 30, 880–884. [Google Scholar] [CrossRef]
- Renaud, B.; François, A.-C.; Dopagne, C.; Rouxhet, S.; Gustin, P.; Votion, D. Identification of the Maple Tree Responsible for Atypical Myopathy. Available online: http://hdl.handle.net/2268/242221 (accessed on 12 December 2019).
- Van Galen, G.; Dopagne, C.; Rouxhet, S.; Pitel, C.; Votion, D. Etiologie de la myopathie atypique: Conditions de toxicité de l’agent causal—Étude préliminaire. In 40ème Journée de la Recherche Equine; Institut français du cheval et de l’équitation (IFCE): Paris, France, 2014; pp. 101–109. [Google Scholar]
- Search Results—The Plant List. Available online: http://www.theplantlist.org/tpl/search?q=Acer&_csv=on (accessed on 12 December 2019).
- Hulme, P.E.; Bacher, S.; Kenis, M.; Klotz, S.; Kühn, I.; Minchin, D.; Nentwig, W.; Olenin, S.; Panov, V.; Pergl, J.; et al. Grasping at the routes of biological invasions: A framework for integrating pathways into policy. J. Appl. Ecol. 2008, 45, 403–441. [Google Scholar] [CrossRef]
- Unger, L.; Nicholson, A.; Jewitt, E.M.; Gerber, V.; Hegeman, A.; Sweetman, L.; Valberg, S. Hypoglycin A Concentrations in Seeds of Acer Pseudoplatanus Trees Growing on Atypical Myopathy-Affected and Control Pastures. J. Vet. Intern. Med. 2014, 28, 1289–1293. [Google Scholar] [CrossRef] [Green Version]
- Baise, E.; Habyarimana, J.A.; Amory, H.; Boemer, F.; Douny, C.; Gustin, P.; Marcillaud-Pitel, C.; Patarin, F.; Weber, M.; Votion, D.M. Samaras and seedlings of Acer pseudoplatanus are potential sources of hypoglycin A intoxication in atypical myopathy without necessarily inducing clinical signs. Equine Vet. J. 2016, 48, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Zuraw, A.; Dietert, K.; Kühnel, S.; Sander, J.; Klopfleisch, R. “Equine atypical myopathy caused by hypoglycin A intoxication associated with ingestion of sycamore maple tree seeds. Equine Vet. J. 2016, 48, 418–442. [Google Scholar] [CrossRef] [PubMed]
- Votion, D.M.; Habyarimana, J.A.; Scippo, M.L.; Richard, E.A.; Marcillaud-Pitel, C.; Erpicum, M.; Gustin, P. Potential new sources of hypoglycin A poisoning for equids kept at pasture in spring: A field pilot study. Vet. Rec. 2019, 184, 740. [Google Scholar] [CrossRef] [PubMed]
- Katul, G.G.; Porporato, A.; Nathan, R.; Siqueira, M.; Soons, M.B.; Poggi, D.; Horn, H.S.; Levin, S.A. Mechanistic analytical models for long-distance seed dispersal by wind. Am. Nat. 2005, 166, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Votion, D. Atypical myopathy: An update. Practice 2016, 38, 241–246. [Google Scholar] [CrossRef]
- Gonzalez-Medina, S.; Montesso, F.; Chang, Y.-M.; Hyde, C.; Piercy, R.J. Atypical myopathy-associated hypoglycin A toxin remains in Sycamore seedlings despite mowing, herbicidal spraying or storage in hay and silage. Equine Vet. J. 2019, 51, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Renaud, B.; Francois, A.C.; Marcillaud-Pitel, C.; Gustin, P.; Votion, D. Myopathie atypique: Les différentes sources d’intoxication. Comment gérer le risque? In Journées Sciences et Innovations Équines; Institut français du cheval et de l’équitation (Ifce): Saumur, France, 2019; p. 9. [Google Scholar]
- Cooper, J.J.; Mason, G.J. The identification of abnormal behaviour and behavioural problems in stabled horses and their relationship to horse welfare: A comparative review. Equine Vet. J. Suppl. 1998, 30, 5–9. [Google Scholar] [CrossRef]
- Cooper, J.J.; Albentosa, M.J. Behavioural adaptation in the domestic horse: Potential role of apparently abnormal responses including stereotypic behavior. Livest. Prod. Sci. 2005, 92, 177–182. [Google Scholar] [CrossRef]
- Hosie, B.D.; Gould, P.W.; Hunter, A.R.; Low, J.C.; Munro, R.; Wilson, H.C. Acute myopathy in horses at grass in east and south east Scotland. Vet. Rec. 1986, 119, 444–449. [Google Scholar] [CrossRef]
- Harris, P.; Whitwell, K. Atypical myoglobinuria alert. Vet. Rec. 1990, 15, 603. [Google Scholar]
- Westermann, C.M.; Dorland, L.; Votion, D.M.; De Sain-van der Velden, M.G.M.; Wijnberg, I.D.; Wanders, R.J.A.; Spliet, W.G.M.; Testerink, N.; Berger, R.; Ruiter, J.P.N.; et al. Acquired multiple Acyl-CoA dehydrogenase deficiency in 10 horses with atypical myopathy. Neuromuscul. Disord. 2008, 18, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Rooney, D.K. Applied nutrition. In Equine Internal Medicine; Sellon, D.C., Reed, S.M., Bayly, W.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2004; pp. 235–272. [Google Scholar]
- Gonzalez-Medina, S.; Hyde, C.; Lovera, I.; Piercy, R.J. Detection of equine atypical myopathy-associated hypoglycin A in plant material: Optimisation and validation of a novel LC-MS based method without derivatisation. PLoS ONE 2018, 13, 13–15. [Google Scholar] [CrossRef] [PubMed]
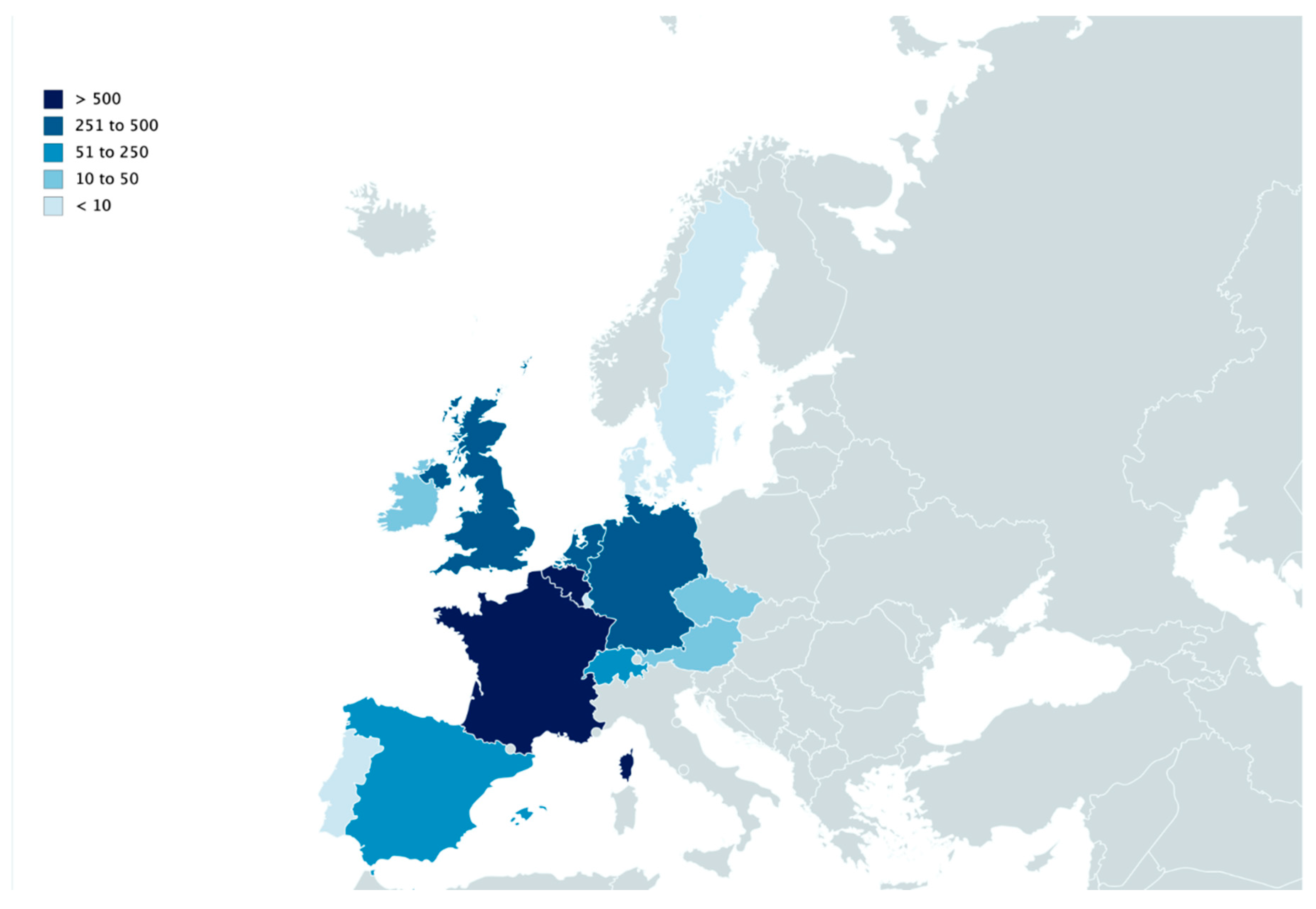



| Year | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 * | 2015 | 2016 | 2017 | 2018 | 2019 | Total/Country | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Countries | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | |
| Austria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 9 | 0 | 0 | 17 |
| Belgium | 46 | 7 | 18 | 0 | 5 | 0 | 69 | 13 | 14 | 3 | 18 | 0 | 1 | 0 | 141 | 8 | 51 | 5 | 6 | 8 | 52 | 20 | 5 | 1 | 191 | 49 | 1 | 732 |
| Czech Republic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 2 | 17 | 0 | 0 | 19 | 2 | 0 | 49 |
| Denmark | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 8 |
| France | 32 | 1 | 11 | 18 | 11 | 0 | 134 | 106 | 32 | 10 | 40 | 16 | 4 | 1 | 64 | 13 | 71 | 9 | 24 | 8 | 194 | 181 | 31 | 18 | 114 | 47 | 26 | 1216 |
| Germany | 7 | 0 | 3 | 5 | 0 | 0 | 93 | 21 | 2 | 2 | 59 | 2 | 0 | 0 | 24 | 1 | 8 | 0 | 0 | 0 | 21 | 20 | 4 | 0 | 33 | 4 | 2 | 311 |
| Ireland | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 38 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 46 |
| Luxembourg | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Portugal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Spain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 52 |
| Sweden | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Switzerland | 0 | 0 | 9 | 0 | 0 | 0 | 31 | 3 | 0 | 0 | 6 | 0 | 0 | 1 | 12 | 0 | 0 | 0 | 0 | 0 | 9 | 3 | 0 | 0 | 7 | 0 | 0 | 81 |
| The Netherlands | 13 | 0 | 3 | 0 | 2 | 0 | 34 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 1 | 4 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 9 | 10 | 1 | 107 |
| United Kingdom | 1 | 0 | 13 | 0 | 0 | 0 | 39 | 20 | 3 | 0 | 33 | 6 | 2 | 2 | 52 | 13 | 154 | 20 | 2 | 1 | 11 | 11 | 3 | 1 | 13 | 3 | 5 | 408 |
| Total/season | 102 | 8 | 57 | 23 | 18 | 0 | 406 | 171 | 51 | 16 | 213 | 24 | 7 | 4 | 322 | 41 | 326 | 34 | 34 | 17 | 292 | 256 | 43 | 26 | 398 | 115 | 35 | 3039 |
| Total/year | 102 | 65 | 41 | 406 | 222 | 229 | 31 | 326 | 367 | 68 | 309 | 299 | 424 | 150 | ||||||||||||||
| Category | Risk Factors | Odds Ratio | 95% Cl for Odds Ratio |
|---|---|---|---|
| Demographic data | Young horses (<3 years) | ||
| Thin (or normal weight *) | 3.08 [17] (b) (3.85 [15]/2.20 [17] (b)) | 1.01–9.39 [17] (b) (1.77–8.37 [15]/1.01–4.79 [17] (b)) | |
| Horse management | At pasture 24 h a day all year round | 5.42 [15] 3.07 [17] (b) in winter 3.78 [17] (b) in spring 23.2 [17] (b) in summer 10.9 [17] (b) in autumn | 2.577–11.42 [15] 1.45–6.50 [17] (b) in winter 1.49–9.59 [17]] (b) in spring 1.41–382 [17] (b) in summer 3.56–33.4 [17]] (b) in autumn |
| Not physically active | 11.8 [17] (b) | 5.02–27.8 [17] (b) | |
| Feeding practice | Hay given in autumn | 4.09 [17]] (a) | 1.18–14.1 [17] (a) |
| Pasture | History of previous death of horse(s) on the pasture | 4.45 [17]] (b) | 1.61–12.29 [17] (b) |
| Lush pasture in winter | 3.95 [17] (b) | 1.49–10.46 [17] (b) | |
| Sloping pasture/steep slope | 3.43 [15] 3.70 [17] (b) | 1.52–7.77 [15] 1.58–8.68 [17] (b) | |
| Access to dead leaves piled up in autumn | 11.11 [15] 10.47 [17] (b) | 4.82–25.59 [15] 2.82–40.88 [17] (b) | |
| Presence of trees at pasture * | 7.82 [15] | 1.99–30.73 [15] | |
| Dead wood at pasture | 3.12 [15] | 1.42–6.84 [15] | |
| Humid pasture | 2.63 [17] (b) | 1.29–5.36 [17] (b) | |
| Pasture surrounded by or containing a stream/river | 2.78 [17] (b) | 1.24–6.19 [17] (b) | |
| Spreading of manure | 5.73 [17] (b) | 2.40–13.69 [17] (b) |
| Category | Protective Factors | Odds Ratio | 95% Cl for Odds Ratio |
|---|---|---|---|
| Demographic data | Overweight | 0.25 [17] (b) | 0.09–0.69 [17] (b) |
| Horse management | Frequent deworming | 0.11 [17] (a) 0.05 [17] (b) | 0.01–0.67 [17] (a) 0.01–0.16 [17] (b) |
| Regular vaccination | 0.10 [17] (b) | 0.05–0.21 [17] (b) | |
| Regular physical activity | 0.08 [17] | 0.03–0.19 [17] | |
| Weather-dependent pasturing time in spring and in autumn | 0.24 spring [17] 0.10 autumn [17] | 0.06–0.89 spring [17] 0.02–0.56 autumn [17] | |
| <6 h at pasture per day | 0.04 [15] 0.62 [17] | 0.01–0.19 [15] 0.16–2.36 [17] | |
| No access to pasture | 0.03 [15] | 0.00–0.22 [15] | |
| Feeding practice and water supply | Supplementary feeds all year round | 0.17 [15] | 0.05–0.59 [15] |
| Silage and concentrate feed in autumn + corn in winter | 0.20 [17] (a) for silage 0.19 [17] (a) for concentrate feed 0.22 [17] (a) for corn | 0.04–0.94 [17] (a) for silage 0.04–0.87 [17] (a) for concentrate food 0.05–0.93 [17] (a) for corn | |
| Salt block (all year) | 3.52 [12] 0.20 [17] | 1.08–11.47 [12] 0.09–0.40 [17] | |
| Water provision in tank/bathtub | 0.25 [15] | 0.09–0.69 [15] | |
| Water supplied by the distribution network | 0.39 [17] (b) | 0.17–0.88 [17] (b) | |
| Pasture | Gentle slope | 0.34 [17] (b) | 0.14–0.84 [17] (b) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Votion, D.-M.; François, A.-C.; Kruse, C.; Renaud, B.; Farinelle, A.; Bouquieaux, M.-C.; Marcillaud-Pitel, C.; Gustin, P. Answers to the Frequently Asked Questions Regarding Horse Feeding and Management Practices to Reduce the Risk of Atypical Myopathy. Animals 2020, 10, 365. https://doi.org/10.3390/ani10020365
Votion D-M, François A-C, Kruse C, Renaud B, Farinelle A, Bouquieaux M-C, Marcillaud-Pitel C, Gustin P. Answers to the Frequently Asked Questions Regarding Horse Feeding and Management Practices to Reduce the Risk of Atypical Myopathy. Animals. 2020; 10(2):365. https://doi.org/10.3390/ani10020365
Chicago/Turabian StyleVotion, Dominique-Marie, Anne-Christine François, Caroline Kruse, Benoit Renaud, Arnaud Farinelle, Marie-Catherine Bouquieaux, Christel Marcillaud-Pitel, and Pascal Gustin. 2020. "Answers to the Frequently Asked Questions Regarding Horse Feeding and Management Practices to Reduce the Risk of Atypical Myopathy" Animals 10, no. 2: 365. https://doi.org/10.3390/ani10020365
APA StyleVotion, D.-M., François, A.-C., Kruse, C., Renaud, B., Farinelle, A., Bouquieaux, M.-C., Marcillaud-Pitel, C., & Gustin, P. (2020). Answers to the Frequently Asked Questions Regarding Horse Feeding and Management Practices to Reduce the Risk of Atypical Myopathy. Animals, 10(2), 365. https://doi.org/10.3390/ani10020365






