Oxidative Stress and Endoplasmic Reticulum Stress Are Involved in the Protective Effect of Alpha Lipoic Acid Against Heat Damage in Chicken Testes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Care and Experimental Treatment
2.2. Histological Sections of Testes
2.3. Analysis of Oxidative Stress Markers
2.4. Western Blotting Analysis
2.5. Analysis of Serum Testosterone Levels
2.6. Statistical Analysis
3. Results
3.1. Histological Observations in the Testicular Tissue of Chickens Exposed to Heat Stress
3.2. The Effect of Alpha Lipoic Acid on Oxidative Stress in the Testes of Chickens Exposed to Heat Stress
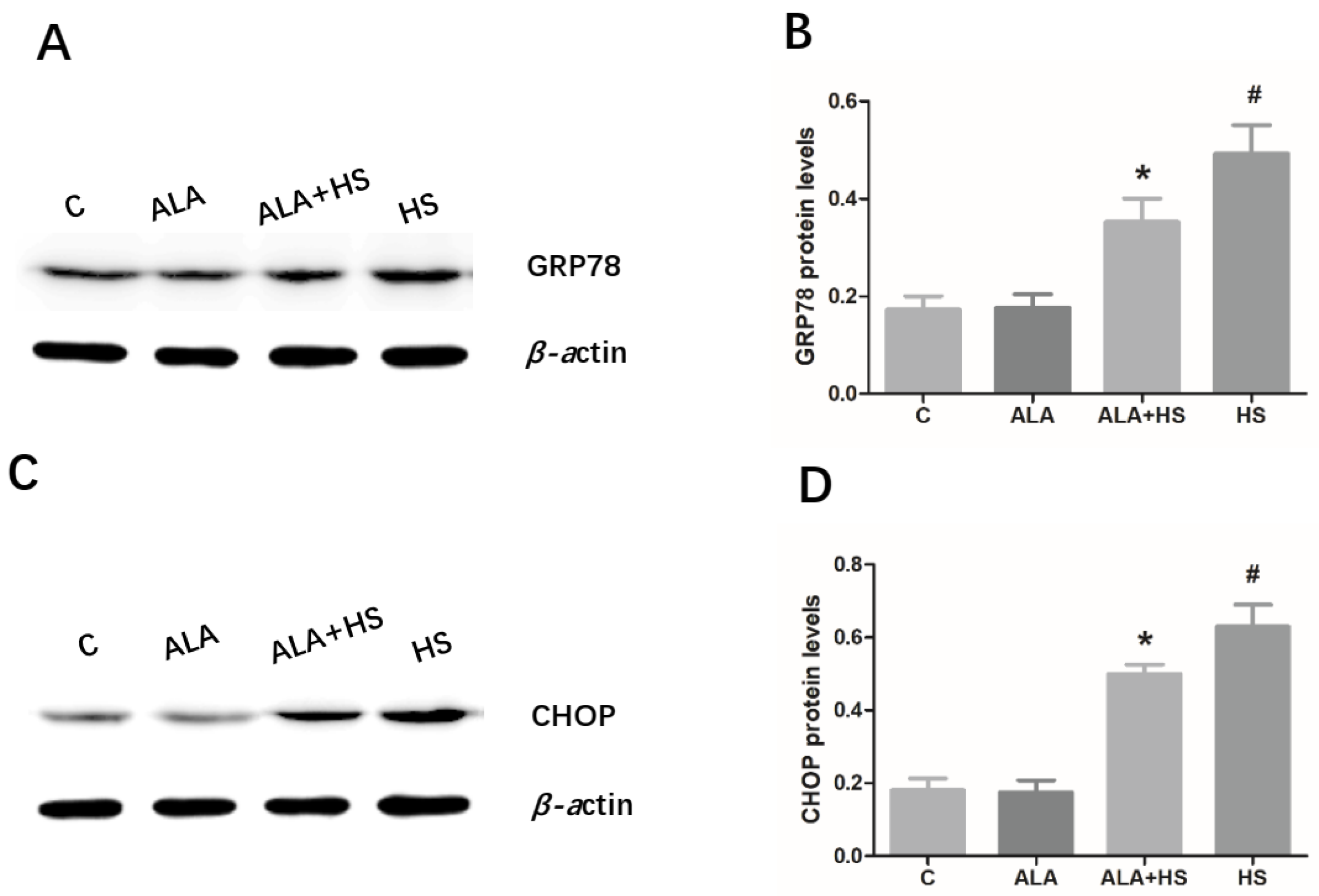
3.3. The Effect of Alpha Lipoic Acid on Er Stress in the Testes of Chicken Exposed to Heat Stress
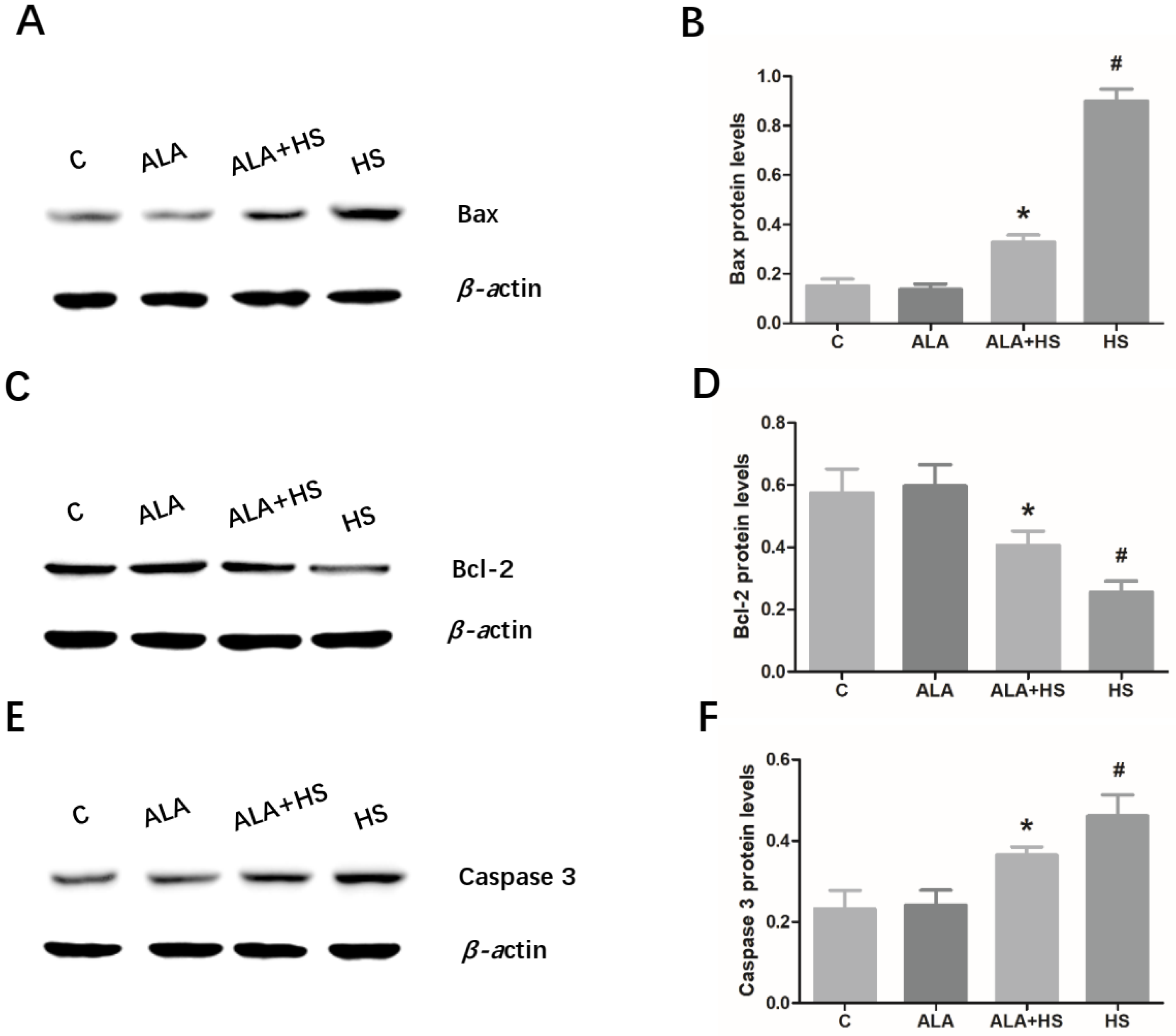
3.4. The Effect of Alpha Lipoic Acid on the Expression of Key Apoptosis-Related Modulators in the Testes of Chickens Exposed to Heat Stress
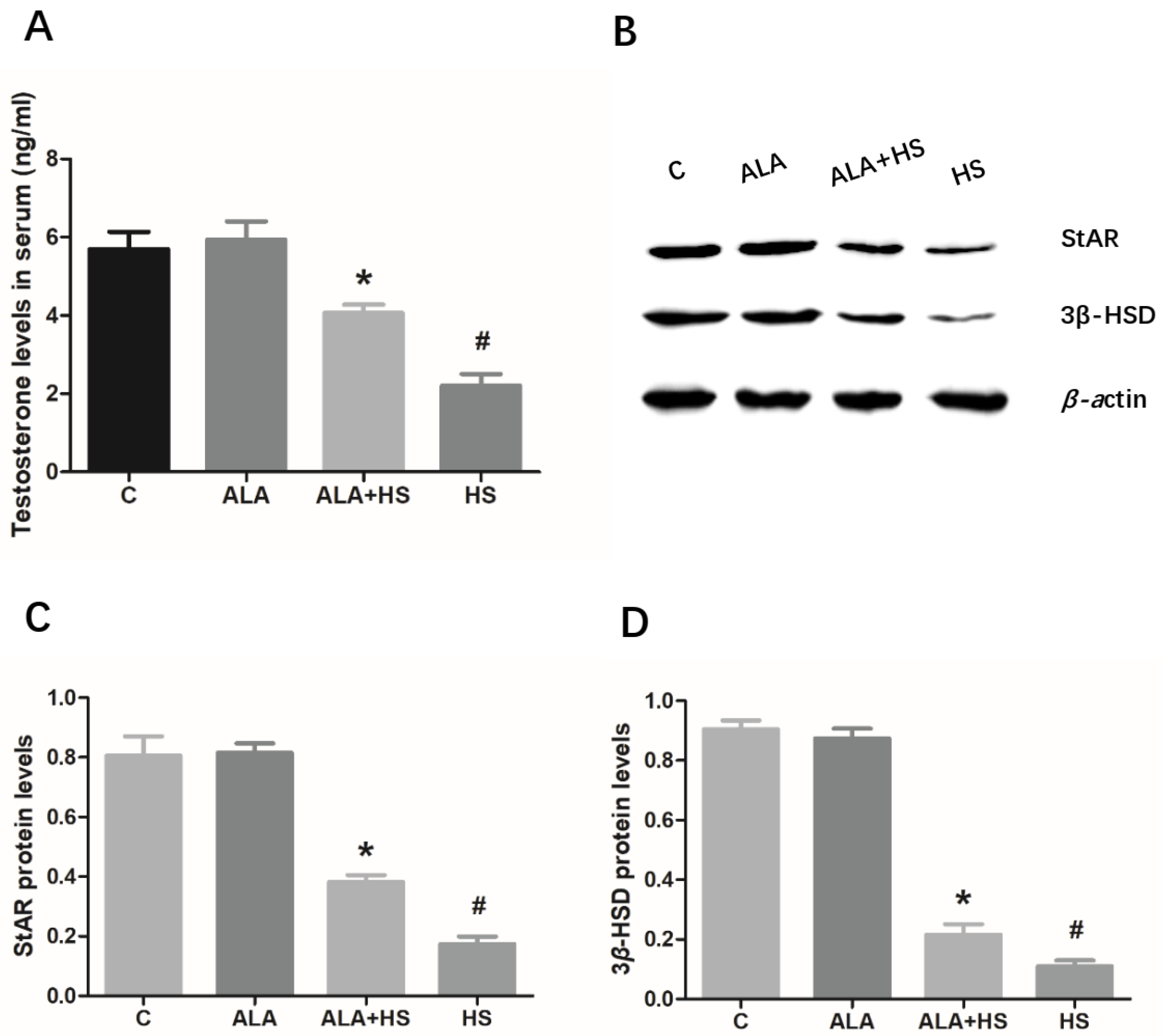
3.5. The Effect of Alpha Lipoic Acid on Serum Testosterone Levels in Chickens Exposed to Heat Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2019, 162, 108025. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Wilde, R.E.; Bielli, A.; Genovese, P.; Rizzoto, G.; Thundathil, J. Hyperthermia is more important than hypoxia as a cause of disrupted spermatogenesis and abnormal sperm. Theriogenology 2019, 131, 177–181. [Google Scholar] [CrossRef]
- Nash, S.; Rahman, M.S. Short-term heat stress impairs testicular functions in the American oyster, Crassostrea virginica: Molecular mechanisms and induction of oxidative stress and apoptosis in spermatogenic cells. Mol. Reprod. Dev. 2019, 86, 1444–1458. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.U.; Sheikh, T.A. Endoplasmic reticulum stress and Oxidative stress in the pathogenesis of Non-alcoholic fatty liver disease. Free Radic. Res. 2015, 49, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.; Chen, F.; Sun, J.; Zhou, J.; Wang, X.; Wang, N.; Li, X.; Zhang, Z.; Wang, A.; Jin, Y. Mycotoxin zearalenone induces apoptosis in mouse Leydig cells via an endoplasmic reticulum stress-dependent signalling pathway. Reprod. Toxicol. 2015, 52, 71–77. [Google Scholar] [CrossRef]
- Fu, X.L.; Gao, D.S. Endoplasmic reticulum proteins quality control and the unfolded protein response: The regulative mechanism of organisms against stress injuries. Biofactors 2014, 40, 569–585. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Ibtisham, F.; Niu, Y.F.; Wang, Z.; Li, G.H.; Zhao, Y.; Nawab, A.; Xiao, M.; An, L. Curcumin inhibits heat-induced oxidative stress by activating the MAPK-Nrf2/ARE signaling pathway in chicken fibroblasts cells. J. Therm. Biol. 2019, 79, 112–119. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Liu, J.; Shao, Y.; Li, J.; Luo, L.; Xing, M. Copper and arsenic-induced oxidative stress and immune imbalance are associated with activation of heat shock proteins in chicken intestines. Int. Immunopharmacol. 2018, 60, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Shao, Y.; Liu, J.; Li, J.; Xing, M. Copper or/and arsenic induce oxidative stress-cascaded, nuclear factor kappa B-dependent inflammation and immune imbalance, trigging heat shock response in the kidney of chicken. Oncotarget 2017, 8, 98103–98116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemu, T.W.; Pandey, H.O.; Salilew, W.D.; Gebremedhn, S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef]
- Sui, J.; Feng, Y.; Li, H.; Cao, R.; Tian, W.; Jiang, Z. Baicalin protects mouse testis from injury induced by heat stress. J. Therm. Biol. 2019, 82, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.J.; Kim, T.S.; Kim, J.M.; Lee, D.S. Testosterone production by a Leydig tumor cell line is suppressed by hyperthermia-induced endoplasmic reticulum stress in mice. Life Sci. 2016, 146, 184–191. [Google Scholar] [CrossRef]
- Di Tucci, C.; Di Feliciantonio, M.; Vena, F.; Capone, C.; Schiavi, M.C.; Pietrangeli, D.; Muzii, L.; Benedetti, P.P. Alpha lipoic acid in obstetrics and gynecology. Gynecol. Endocrinol. 2018, 34, 729–733. [Google Scholar] [CrossRef]
- Imik, H.; Ozlu, H.; Gumus, R.; Atasever, M.A.; Urcar, S.; Atasever, M. Effects of ascorbic acid and alpha-lipoic acid on performance and meat quality of broilers subjected to heat stress. Br. Poult. Sci. 2012, 53, 800–808. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Li, J.L.; Zhang, L.; Jiang, Y.; Gao, F.; Zhou, G.H. Effects of alpha-lipoic acid supplementation in different stages on growth performance, antioxidant capacity and meat quality in broiler chickens. Br. Poult. Sci. 2014, 55, 635–643. [Google Scholar] [CrossRef]
- He, S.J.; Ding, J.X.; Xiong, Y.J.; Liu, D.Y.; Dai, S.F.; Hu, H. Effects of dietary N-acetylcysteine on rectal temperature, respiratory rate, growth performance and blood redox parameters in 22- to 42-day old broilers exposed to chronic heat stress. Europ. Poult. Sci. 2019, 83. [Google Scholar] [CrossRef]
- El-Senousey, H.K.; Chen, B.; Wang, J.Y.; Atta, A.M.; Mohamed, F.R.; Nie, Q.H. Effects of dietary vitamin C, vitamin E, and alpha-lipoic acid supplementation on the antioxidant defense system and immune-related gene expression in broilers exposed to oxidative stress by dexamethasone. Poult. Sci. 2018, 97, 30–38. [Google Scholar] [CrossRef]
- Xu, Y.; Lai, X.; Li, Z.; Zhang, X.; Luo, Q. Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poult. Sci. 2018, 97, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Li, Y.; Luo, X.; He, J. Excessive Selenium Supplementation Induced Oxidative Stress and Endoplasmic Reticulum Stress in Chicken Spleen. Biol. Trace Elem. Res. 2016, 172, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Prathima, P.; Venkaiah, K.; Pavani, R.; Daveedu, T.; Munikumar, M.; Gobinath, M.; Valli, M.; Sainath, S.B. Alpha-lipoic acid inhibits oxidative stress in testis and attenuates testicular toxicity in rats exposed to carbimazole during embryonic period. Toxicol. Rep. 2017, 4, 373–381. [Google Scholar] [CrossRef]
- Pinar, N.; Cakirca, G.; Ozgur, T.; Kaplan, M. The protective effects of alpha lipoic acid on methotrexate induced testis injury in rats. Biomed. Pharm. 2018, 97, 1486–1492. [Google Scholar] [CrossRef]
- Rodrigo, R.; Libuy, M.; Feliu, F.; Hasson, D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis. Markers 2013, 35, 773–790. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Yi, G.; Li, L.; Luo, M.; He, X.; Zou, Z.; Gu, Z.; Su, L. Heat stress induces intestinal injury through lysosome- and mitochondria-dependent pathway in vivo and in vitro. Oncotarget 2017, 8, 40741–40755. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Zhang, C.; Gao, C.Y.; Ma, T.; Zhang, H.; Zhou, M.M.; Yang, Y.W.; Yang, L.; Kong, L.Y. Anti-proliferation of triple-negative breast cancer cells with physagulide P: ROS/JNK signaling pathway induces apoptosis and autophagic cell death. Oncotarget 2017, 8, 64032–64049. [Google Scholar] [CrossRef] [Green Version]
- Shadmehr, S.; Fatemi, T.S.; Hosseinifar, S.; Tabandeh, M.R.; Amiri, A. Attenuation of heat stress-induced spermatogenesis complications by betaine in mice. Theriogenology 2018, 106, 117–126. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Mau, T.; O’Brien, M.; Yung, R. Novel role of autophagy-associated Pik3c3 gene in gonadal white adipose tissue browning in aged C57/Bl6 male mice. Aging 2018, 10, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Atalay, E.; Ozdemir, M.T.; Tur, B.K.; Erdogdu, H.I.; Sisman, P. The effect of alpha lipoic acid on oxidative parameters and liver injury in rats with obstructive jaundice. Bratisl. Lek. Listy 2019, 120, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, W.; Wang, L.; Cheng, S.; Zhou, Y.; Ma, L. Protective Effects of alpha-Lipoic Acid on Vascular Oxidative Stress in Rats with Hyperuricemia. Curr. Med. Sci. 2019, 39, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar] [PubMed]
- Doyle, K.M.; Kennedy, D.; Gorman, A.M.; Gupta, S.; Healy, S.J.; Samali, A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J. Cell. Mol. Med. 2011, 15, 2025–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wen, D.; Wang, F.; Wang, C.; Yang, L. Curcumin protects against palmitic acid-induced apoptosis via the inhibition of endoplasmic reticulum stress in testicular Leydig cells. Reprod. Biol. Endocrinol. 2019, 17, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef]
- Takujiro Homma, J.F. Heat stress promotes the down-regulation of IRE1alpha in cells: An atypical modulation of the UPR pathway. Exp. Cell. Res. 2016, 349, 128–138. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.; Chen, J.; Zhao, S.; Wang, T.; Zou, H.; Wang, Y.; Gu, J.; Liu, X.; Bian, J.; et al. Alpha-lipoic acid protects against cadmium-induced neuronal injury by inhibiting the endoplasmic reticulum stress eIF2alpha-ATF4 pathway in rat cortical neurons in vitro and in vivo. Toxicology 2019, 414, 1–13. [Google Scholar] [CrossRef]
- Hu, H.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, K.; Sun, H. Alpha-lipoic acid defends homocysteine-induced endoplasmic reticulum and oxidative stress in HAECs. Biomed. Pharm. 2016, 80, 63–72. [Google Scholar] [CrossRef]
- Sok, J.; Wang, X.Z.; Batchvarova, N.; Kuroda, M.; Harding, H.; Ron, D. CHOP-Dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol. Cell Biol. 1999, 19, 495–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwarha, G.; Raza, S.; Prasanthi, J.R.; Ghribi, O. Gadd153 and NF-kappaB crosstalk regulates 27-hydroxycholesterol-induced increase in BACE1 and beta-amyloid production in human neuroblastoma SH-SY5Y cells. PLoS ONE 2013, 8, e70773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scorrano, L.; Oakes, S.A.; Opferman, J.T.; Cheng, E.H.; Sorcinelli, M.D.; Pozzan, T.; Korsmeyer, S.J. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science 2003, 300, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Glamoclija, V.; Vilovic, K.; Saraga-Babic, M.; Baranovic, A.; Sapunar, D. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil. Steril. 2005, 83, 426–431. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, H.I.; Kim, D.H.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Alpha-lipoic acid attenuates p-cresyl sulfate-induced renal tubular injury through suppression of apoptosis and autophagy in human proximal tubular epithelial cells. Biomed. Pharm. 2019, 112, 108679. [Google Scholar] [CrossRef]
- Zou, X.L.; Yu, Y.Z.; Yu, H.H.; Wang, G.F.; Pi, R.B.; Xu, Z.; Zhang, C.; Zhou, W.J.; Li, D.D.; Chen, X.G.; et al. Protective effects of lipoic acid-niacin dimers against blue light-induced oxidative damage to retinal pigment epithelium cells. Int. J. Ophthalmol. 2019, 12, 1262–1271. [Google Scholar] [CrossRef]
- Mohajeri, D.; Kaffashi, E.R. Effects of Nigella sativa on heat-induced testis damage in mouse. Bratisl. Lek. Listy 2015, 116, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Ma, Q.G.; Ji, C.; Zhang, J.Y.; Zhao, L.H.; Zhang, Y.; Jie, Y.Z. Dietary Lipoic Acid Influences Antioxidant Capability and Oxidative Status of Broilers. Int. J. Mol. Sci. 2011, 12, 8476–8488. [Google Scholar] [CrossRef] [Green Version]


| Item | 22–42 d |
|---|---|
| Ingredients | |
| Maize | 64.0 |
| Soybean meal | 27.8 |
| Soybean oil | 3.0 |
| Fish power | 2.5 |
| Calcium hydrogen phosphate | 1.4 |
| Sodium chloride | 0.30 |
| Premix 1 | 1.0 |
| Nutrient level | |
| ME/(MJ/kg) 2 | 13.03 |
| Crude protein | 20.02 |
| Lysine | 1.02 |
| Methionine + Cystine | 0.78 |
| Calcium | 1.00 |
| Total phosphorus | 0.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Yin, Q.; Li, J.; He, S. Oxidative Stress and Endoplasmic Reticulum Stress Are Involved in the Protective Effect of Alpha Lipoic Acid Against Heat Damage in Chicken Testes. Animals 2020, 10, 384. https://doi.org/10.3390/ani10030384
Xiong Y, Yin Q, Li J, He S. Oxidative Stress and Endoplasmic Reticulum Stress Are Involved in the Protective Effect of Alpha Lipoic Acid Against Heat Damage in Chicken Testes. Animals. 2020; 10(3):384. https://doi.org/10.3390/ani10030384
Chicago/Turabian StyleXiong, Yongjie, Qirun Yin, Jing Li, and Shaojun He. 2020. "Oxidative Stress and Endoplasmic Reticulum Stress Are Involved in the Protective Effect of Alpha Lipoic Acid Against Heat Damage in Chicken Testes" Animals 10, no. 3: 384. https://doi.org/10.3390/ani10030384
APA StyleXiong, Y., Yin, Q., Li, J., & He, S. (2020). Oxidative Stress and Endoplasmic Reticulum Stress Are Involved in the Protective Effect of Alpha Lipoic Acid Against Heat Damage in Chicken Testes. Animals, 10(3), 384. https://doi.org/10.3390/ani10030384





