4.1. Food Composition and Intake
The present study tested the hypothesis that the interference of some food constituents on the bioavailability of Zn supplied at practical levels could be overcome either by the usage of organic supplemental zinc (chelate Zn proteinate, Bioplex
®) or by the addition of exogenous enzymes from a solid-state fermentation product of
A. niger (Synergen
®). The experimental diets simultaneously covered the requirements in macro and microelements for young adults and the legal limits [
1]. The dietary content of Zn averaged 147 mg/kg DM, ranging from 139 to 159 mg/kg DM, which is within the European maximum legal limit of 227 mg/kg DM [
31]. The amount of supplemental Zn was defined from the Zn content provided by the ingredients and such that the total Zn content was within the range normally found in commercial foods. Indeed, in a total of 162 samples of complete dry dog foods from 22 European countries, the median Zn content was 157.5 mg/kg [
32]. Similarly, Kelly et al. reported a median Zn content of 140 mg/kg DM in 18 dry dog foods [
33], while Pereira et al. obtained a much higher median, of 310 mg/kg DM, for 20 samples of dry dog food belonging to different market segments [
24]. In the present study, the amount of dietary Zn supplied (5.95 to 7.15 mg/kg BW
0.75;
Table 4) was higher than the minimum requirement (2 mg/kg BW
0.75) established by the NRC for adult dogs in maintenance [
1]. Actually, the minimum requirement of dogs would have been ensured by the Zn background level determined in the experimental diets (3 mg/kg BW
0.75), which is defined as the Zn concentration in the complete food delivered by the food ingredients and agreed with those reported by EFSA obtained from CVB feed tables and by data submitted by the industry [
32]. However, the bioavailability of the native Zn in the experimental diets might have been compromised, as the majority of Zn and PA are sourced by wheat and soy concentrate, and as is well known, in cereal and oilseed based diets, the antagonism exerted by phytates applies to native Zn, already bound to it [
34]. Additionally, the considerable interactions between Zn, Ca, Cu and Fe and the impact of fibers on Zn availability through the formation of insoluble complexes must be considered for the definition of the optimal dietary amount of Zn. Due to the difficulties in estimating the Zn bioavailability, in practical situations, the background Zn of the basal diet is assumed to be not available [
32], being the supplementation with a bioavailable Zn source essential to ensure the requirements. All animals remained healthy throughout the study. The intake of DM tended to be higher in dogs fed IZ. The daily food provided was adjusted to ensure an ideal BCS of the dogs in all groups. The observed effects on nutrients intake reflect the slight differences in DM intake and on the chemical composition of diets.
4.2. Effect of Zn Source
Zinc, as other cations, is prone to interact with food components in the gastrointestinal tract and thus form insoluble complexes that lower its bioavailability. The pH, especially in the proximal part of the digestive tract, influences Zn solubility and availability. Inorganic Zn, such as Zn sulfate, is easily dissociated at the acidic pH of the stomach, therefore Zn
2+ remains soluble. When it passes to the intestinal compartment, Zn
2+ can either be bonded to amino acids from the chyme or to carrier proteins of the luminal membranes of the mucosa cells to be transported by passive diffusion or active transport into the bloodstream [
35]. However, it can also form insoluble complexes with food constituents such as PA. The solubility of PA is influenced by pH and it is mostly found negatively charged at pH 3–7. Thus, in the intestine, the chelating effect of phosphate groups causes PA to form insoluble complexes with Zn
2+ and other metal cations [
36]. Conversely, as the pH of the stomach does not affect the coordinate covalent bond between Zn and the organic molecule, Zn chelates are not ionized before absorption, and they are transported across the intestinal barrier using the same mechanism as low molecular weight peptides, being the metal ion separated from the organic molecule only at the site of use [
35].
The results of the present study showed that the digestibility of P was enhanced with Zn proteinate supplementation. Two studies reported that over-supplementation with Zn-oxide decreased P digestibility in pigs [
37,
38], suggesting that P uptake was decreased by inorganic Zn, a phenomenon that was already described in plants [
38], or that precipitation in the intestinal lumen of P and Zn might have occurred. However, in the present study, this is not supported by the zinc biomarkers evaluated and is unlikely to have occurred with the lower level of Zn supplied compared to the earlier studies. Nevertheless, it seems that Zn from Zn proteinate did not interact with P, which may have positive implications for Zn bioavailability. Additionally, it has been suggested that metal proteinates have lower inhibitory effects on phytases than inorganic trace elements or other organic sources [
39] contributing to a higher P digestibility with organic Zn source. In the present study, despite P digestibility have been higher with organic Zn source, no differences were observed between sources when exogenous enzymes were added (interaction between Zn source and enzyme not significant).
Aside from the interference of anti-nutritional components of the diet, Zn absorption can be impaired by the interaction with other minerals. Nevertheless, mineral interactions tend to be more deleterious if any is supplied in excess [
40]. Results described here corroborate this, as no differences in the plasma concentration of Zn and other trace elements were observed following an adequate mineral intake. Urinary Zn was similar among dogs fed Zn sulfate and Zn proteinate, which is not surprising since variations on urinary Zn excretion are associated with its excessive intake/deficiency or with the presence of concomitant diseases [
41]. Additionally, urinary excretion is not the preferred route for the control of Zn status, but it is in the gastrointestinal system that the major homeostasis of Zn takes place through the control of absorption of dietary Zn, secretion, and reabsorption of endogenous Zn from pancreatic and biliary and gastroduodenal secretions [
42].
Zinc content in hair and overall coat quality and hair growth were similar in dogs fed Zn sulfate and Zn proteinate. Similarly, Kuhlman and Rompala found no significant differences in Zn hair content among dogs fed inorganic and organic sources of Zn, Mn and Cu, but smoother and less fragmented hair follicles on dogs fed the organic sources [
43]. However, it is not clear whether these findings are only attributable to Zn source or to the replacement of inorganic sources of all three trace elements by organic ones. In another study from Lowe and Wiseman, dogs were subjected to a 30-day adaptation period with no dietary Zn supplementation (only 56 mg Zn/kg from raw materials) and then fed for another 60 days three levels (50, 75 and 100 mg/kg) of three supplementary sources (Zn proteinate, Zn oxide and Zn polysaccharide), with results showing increased growth rate and Zn deposited in hair for Zn proteinate [
43]. This result suggests that Zn proteinate was more efficient in restoring the Zn levels, yet results are not comparable to the ones reported here since the study departs from a Zn depletion status, which may alter the hair retention response to Zn forms. The response of Zn biomarkers to dietary supplementation is not always consistent. When the Zn status is up to a suboptimal level, biomarkers tend to respond positively, enabling to distinguish bioavailability of supplemented forms, as the amount of Zn absorbed and retained increases linearly with supplied Zn. Conversely, when dietary supply exceeds requirements, the amount of Zn absorbed and retained (expressed as a proportion of the ingested Zn amount) decreases with the increase in ingested Zn [
44].
Along with Zn concentration in tissues, Zn-dependent enzymes activity are widely used as biomarkers of Zn status. The results of the present study showed no diet-related differences in the activity of alkaline phosphatase (AP), already considered an inadequate indicator of Zn supply in humans [
45], and in dogs [
46]. Similarly, plasma SOD activity, a Cu and Zn dependent enzyme, was not different in dogs fed Zn sulfate and Zn proteinate. This enzyme might be a more adequate biomarker of Zn supplementation in senior, than in young healthy dogs, since aging involves increased oxidative stress and accelerated cellular senescence, stimulating antioxidant enzymes such as catalase, CAT, glutathione peroxidase and SOD [
47]. Conversely, the plasmatic concentration of alanine aminotransferase (ALT) was significantly higher in dogs fed Zn proteinate compared to Zn sulfate. The clinical importance of increased alanine and aspartate aminotransferase activities, as well as the role of Zn in the regulation of ALT in the presence of liver disease, are well known [
48], whereas the significance of decreased activities is poorly understood. Nevertheless, the values herein obtained fit the interval of normality of healthy dogs [
49].
Zinc can have an anti-inflammatory role by lowering the secretion of CRP, which is an acute-phase protein secreted in response to an increase in pro-inflammatory cytokines, namely of interleukin 6 [
50]. Even though Zn supplementation is more efficient decreasing the CRP of ill patients, basal levels of CRP can also benefit from Zn supplementation [
51]. A study of Jarosz et al. reported that chelate of Zn-glycine reduced the basal level of CRP in comparison to Zn sulfate in chickens from 1 to 42 days of age [
52]. However, in the results reported here, the basal levels of CRP in dogs were not affected by Zn supplementation.
T-cells express CD4
+ or CD8
+ co-receptors on their surface, defining their function. Upon activation, CD8
+ T-cells can differentiate into cytotoxic T-cells, which recognize and eliminate virally infected and tumor cells, while CD4
+. T-cells have helper function, meaning that they promote the function of other immune cells [
53]. There is growing evidence that Zn mediates the regulation of the immune system by facilitating the transduction of signalling cascades, the physiological mechanisms being discussed elsewhere [
54]. In the present study, dogs fed Zn proteinate had a significantly higher percentage of circulating CD4
+ T-cells suggesting an improved T-cell differentiation. The positive effect of Zn on thymic function has been previously demonstrated and might provide a possible explanation for this observation [
55]. In particular, the improved generation of CD4
+ T cells has been observed in human patients undergoing oral Zn supplementation [
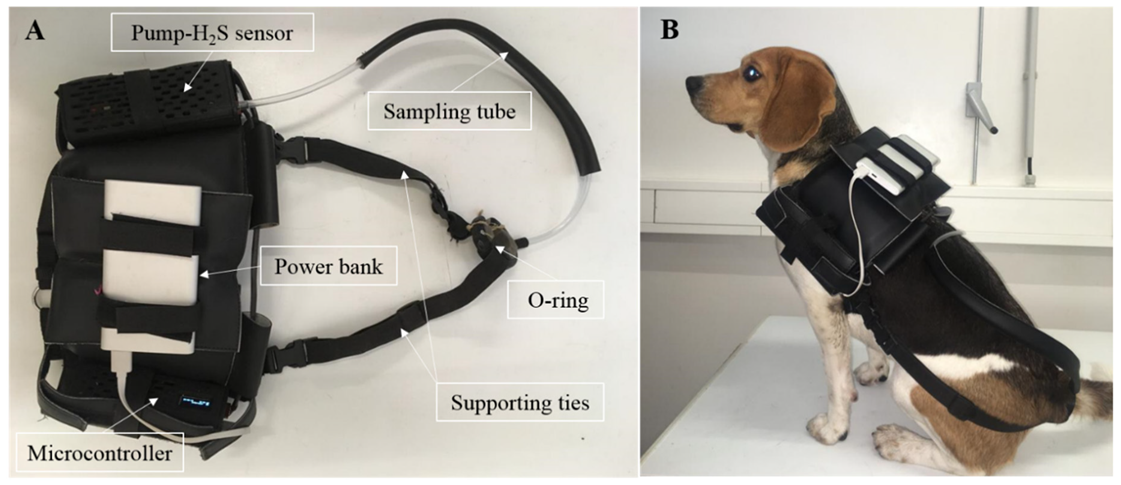
56], in accordance with this study. The Zn source has been suggested to differently impact the number and intensity of flatulence episodes due to different production and release of H
2S in the large intestine. Giffard et al. reported a reduction of 58% in H
2S production with oral administration of Zn acetate, suggesting that the free Zn cations not absorbed in the small intestine bind sulfhydryl compounds such as H
2S and methanethiol to form insoluble salts [
57]. From these results, it was anticipated that inorganic Zn would reduce the number and intensity of flatulence episodes, but the values among diets were similar. This result might be, at least partially, explained by the greater bioavailability of zinc sulfate, used as the inorganic source, compared to zinc acetate used in the earlier study [
58]. Nevertheless, the role of Zn in the large intestine fermentation process of dogs needs to be further investigated.
4.3. Exogenous Enzymes
Enzyme supplementation has long been used in poultry and pig diets to improve the nutritional value and decrease the anti-nutritional effects of NSPs, but information in dogs is much scarcer. Since enzymes degrade dietary NSPs, thus reducing their anti-nutritional effects, their use allows the decrease in dietary cost through increasing the inclusion of NSP-containing ingredients. The commercial solid-state fermentation product used in the present study comprises a multi-enzymatic complex, including phytase, protease, xylanase, ß-glucanase, cellulase, amylase and pectinase [
59]. Thus, it is expected that its residual enzymatic activity could enhance the digestibility of macronutrients and minerals by degrading PA and NSP. However, the results presented here showed that CTTAD of macronutrients and energy and flatulence of dogs remained unaffected by the addition of exogenous enzymes. Conversely, a previous study with turbot juveniles observed an increase in DM digestibility, and posterior intestinal activity of lipase and protease with the addition of 400 mg/kg of the same commercial multi-enzymatic complex (Synergen
®) used in the present study [
8]. Similarly, the addition of 200 mg/kg of Synergen
® was related to a higher feed ratio conversion in broilers [
9]. Although studies in other species have shown positive effects, the results reported here are in accordance with digestibility trials performed in adult/senior Beagle dogs. Sá et al. reported that the addition of an enzyme blend composed by 4.5 U β-glucanase/kg, 16 U xylanase/kg, 1.5 U cellulase/kg, 198 U glucoamylase/kg, 1.9 U phytase/kg, and 9000 U α-amylase/kg did not improve the digestibility of diets without and with wheat bran (25%) [
60]. In line with this, Pacheco et al. reported no effects of exogenous enzymes (12/24 U amylase/kg, 16/32 U cellulase/kg, 40/80 U xylanase/kg, 80/160 U β-glucanase/kg, 120/240 U phytase/kg, 280/560 U protease/kg, and 1600/3200 U pectinase/kg) on CTTAD, fecal scores and urinary pH of dogs fed diets with 20% and 40% of full-fat rice bran [
16]. The disparity in the substrate for degradation (ingredient composition of the diets) and on the level of enzymes added in each experiment preclude a direct comparison of the studies published. In the present study, the multi-enzymatic complex was added according to the recommendations for other animal species and agreeing with another study [
9], in which positive effects were seen, as no recommendations are set for dogs.
In the present study, the addition of exogenous enzymes also retained the Zn bioavailability. Conversely, earlier studies reported improved Zn availability through the use of microbial phytase in broilers [
61] and in pigs [
62]. The results have been more evident with pigs than in broilers [
63] and less consistent in dogs, which might be related to the different physiology of the gastrointestinal compartments that limits the action of the exogenous enzymes. Indeed, the success of exogenous enzymes depends upon the pH of the gastrointestinal compartment, retention times and protease activity of the host, namely pepsin, trypsin, and chymotrypsin [
64,
65]. Sagawa et al. reported that gastric pH of fasted dogs was 2.03, while dogs fed 10 and 200 g of dry food had on average a pH of 1.08 and 1.26, and a gastric emptying time of 562 and 1212 min, respectively [
66]. Mahar et al. corroborate the inexistence of any elicit change in gastric pH 60 min after a meal (300 g of dry dog food) as pH was between 1.4 and 2.5 with occasional peaks ranging from 3.5 to 4.5 [
67], whereas, another study reported that mean intestinal pH of fasted dogs was 7.3 ± 0.09 [
68]. Nielsen et al. stressed the differences of activity of microbial phytases in a simulated gastric environment, highlighting the double optimum pH (3 and 6) and the decrease in activity rate of
A. niger phytase in the presence of pepsin [
69]. An in vitro study showed that the activity of β-glucanase, xylanase, amylase, and protease was optimal at pH 3–5, 5.3–6.5, 4.8 and 2, respectively [
70]. Along with pH, the retention time is important for enzyme activity. According to Cuyper et al., who tested diets for dogs consisting exclusively of chunked day-old chicks, the mean total transit total time was 1692 min, of which 48.8% of the time the digest was on the stomach, 8.7% on the small intestine and 42.5% on the colon [
71]. Similarly, Mahar et al. reported a gastric empty time of 971 min with 300 g of dry dog food [
67]. However, ingredient composition affects retention times as reported by Pedreira et al. [
72] that found increased gastric empty time after the inclusion of 10% fiber (sugarcane). It seems that the acidic pH of dogs’ stomach and the retention time might be adequate for proteases and probably reasonably adequate for phytases, being, however, detrimental for carbohydrases. In contrast, broilers have pH and retention times of 5.5 and 10–15 min in the crop, 2.5–3.5 and 30–90 min in the gizzard, 5–7 and 25–40 min in the duodenum/jejunum [
73], which appears to be beneficial in terms of acidity, yet challenging due to the lower length of contact between enzymes and digesta in the various compartments. Apart from the differences in the digestion of monogastric animals, one has to consider that the success of exogenous enzymes may also vary within dogs due to the differences in the gastrointestinal physiology of breeds [
74,
75]. Another hypothesis that may explain the lack of effects of phytase in Zn bioavailability is the natural dissociation that acidic stomach pH of dogs allows even in the absence of phytases. If that is so, it is likely that animals with a higher stomach pH, such as pigs, could have a more clear effect of phytase activity on phytate hydrolysis [
63].