1. Introduction
Environmental stressors have highly relevant negative impacts on the health condition and productivity of the chickens in broiler farming. Several technological and nutritional problems may increase stress and contribute to immunomodulation, such as overstocking, excess dust, wet litter, improper or contaminated feed, and heat stress. Among the numerous concerns, heat stress is an important issue in intensive poultry production, with increasing significance due to the climate change. Elevated environmental temperature, especially when combined with higher air humidity, can cause suffering and remarkably deteriorates the health, welfare, and growth performance of broilers [
1].
On cellular level, a specific stress response pathway, called heat shock response (HSR) is initiated by heat stress acting for the restoration of cell homeostasis by complex alterations of several signaling and metabolic pathways [
2]. Heat shock proteins (HSPs) are primarily involved in the successful cellular adaptation to stress conditions, while the synthesis of most other proteins gets discontinued during HSR [
3]. Further, HSR is usually linked to enhanced oxidative stress, reflected by elevated levels of pro-oxidants (such as reactive oxygen species, ROS) and inadequate level of antioxidants [
1]. Heat-triggered oxidative stress of broilers resulted in enhanced lipid peroxidation in the liver and the heart muscle [
4], and increased oxidative damage of muscular proteins due to the dysfunction of the respiratory chain [
5,
6]. Among various tissues, liver was found highly susceptible to heat-provoked oxidative stress in broilers [
4].
Heat stress was also reported to cause functional changes in the immune response by altering the gene expression of pro-inflammatory cytokines, such as increasing splenic interleukin (IL-)4 and IL-12 concentrations in chickens [
7]. Further, the cellular immune system may also get diminished by heat stress, reflected by decreased total white blood cell count [
8] and macrophage activity [
9]. Heat stress also strongly affected the immune response by decreasing the splenic IL-6 and IL-12, and further, caecal IL-1β and IL-10 gene expression in
Salmonella Enteritidis infected chickens [
10].
Based on the aforementioned data, heat-associated distress of the liver, due to its central role in the metabolism of nutrients and xenobiotics, may be critical for the whole organism by destructing the maintenance of metabolic health. In addition to hepatocytes, Kupffer cells as the resident liver macrophages, together with further circulation-derived macrophage cells, are predominantly involved in hepatic inflammatory and stress response [
11]. Further, these cells also play a key role in the regulation of metabolic processes, serving as a link between inflammation and metabolism [
12]. Therefore, monitoring the function of hepatic nonparenchymal (NP) cells, primarily macrophages in the complex regulation of inflammation and stress response could highlight new ways of improving animal health and productivity.
To study the effects of heat stress on the function of different liver cells in chickens, novel hepatic cell culture models were aimed to be developed. Our research group has already established and characterized a primary co-culture comprised of hepatocytes and NP cells (mostly Kupffer cells) of pig origin, which can serve as a proper tool for investigations on the cellular inflammatory and stress response [
13]. Since no similar avian liver cell culture models have been prepared yet, the first main goal of the present study was to develop a hepatic parenchymal—NP cell co-culture from chickens. Due to the difference in size of hepatic cells in birds and mammals, cell isolation procedures had to be adapted to chickens, and separated cell fractions needed to be characterized. Further, the molecular effects of a shorter (1 h) and a longer (2 h) heat exposure on the oxidative status, HSP70 and pro-inflammatory cytokine production were aimed to be assessed on the newly established primary liver cell cultures. Applying mono-cultures of hepatocytes and co-cultures of parenchymal and NP cells may highlight the role of different cell types in stress response. The established co-culture as an inflammatory model can presumably contribute to understand the link between hepatic inflammation and distress.
2. Materials and Methods
2.1. Cell Isolation and Culturing Conditions
Liver cells were freshly isolated from three-week-old male broiler chickens of the Ross-308 strain reared and fed according to the Ross technology [
14], and obtained from Gallus Ltd. (Devecser, Hungary). For setting up the cell isolation and separation procedure, some preliminary studies were carried out using one broiler in each trial (eight totally). For the characterization of cell fractions gained by the finally established method (with immunocytochemistry and flow cytometry) and to study the cellular effect of heat stress, liver cells had to be isolated from the same single chicken in order to ensure the homogeneity of the prepared primary cell cultures. All experimental procedures with the animals were carried out in accordance with the national and EU laws, as well as with the institutional guidelines, and were confirmed by the Local Animal Welfare Committee of the University of Veterinary Medicine, Budapest (permission number: PEI/001/1430-4/2015, approval date: 27 April 2015). All chemicals were purchased from Sigma-Aldrich (Darmstadt, Germany) except when otherwise specified.
The animals were slaughtered in carbon dioxide narcosis by decapitation, and the liver was perfused via the gastropancreaticoduodenal vein of the hepatic portal system. All perfusion buffers were previously warmed up to 40 °C and were freshly oxygenated with Carbogen (95% O2, 5% CO2); the velocity of the perfusion was set to 30 mL/min. In the first stage of the multi-step perfusion, 150 mL ethylene glycol bis (2-aminoethyl ether) tetraacetic acid (EGTA, 0.5 mM) containing Hanks’ balanced salt solution (HBSS) buffer (previously supplemented with 0.035% NaHCO3) was applied, followed by 150 mL EGTA-free HBSS. In the final step, 100 mL HBSS buffer, freshly supplemented with 100 mg collagenase type IV (Nordmark, Uetersen, Germany), 7 mM CaCl2, and 7 mM MgCl2 was perfused into the liver to disintegrate hepatic parenchymal cells.
After excision of the liver, the capsule was disrupted, and the gained liver cell suspension was filtered through three layers of sterile gauze and was incubated in bovine serum albumin (BSA, 2.5%)—containing HBSS buffer on ice for 45 min to avoid cell aggregate formation. Thereafter, the cell suspension was centrifuged three times at 100× g for 3 min, and the hepatocyte-enriched sediment was resuspended in Williams’ Medium E, previously supplemented with 0.22% NaHCO3, 50 mg/L gentamycin, 2 mM glutamine, 4 µg/L dexamethasone, 20 IU/L insulin, and 5% foetal bovine serum (FBS).
The NP cell fraction (containing mostly macrophages, primarily Kupffer cells) was separated from the supernatants gained in the low-speed (100× g) centrifugation steps. The supernatants were centrifuged at 350× g for 10 min to sediment the remaining hepatocytes, cell detritus and red blood cells, and the newly gained supernatant was centrifuged again at 800× g for 10 min. The final sediment, containing NP cells, was also resuspended in Williams’ Medium E. The viability of hepatocytes and NP cells was confirmed by the trypan blue exclusion test, and cell yield was examined by cell counting in Bürker’s chamber to adjust the appropriate cell concentrations (hepatocyte mono-cultures: 106 cells/mL; co-cultures: 8.5 × 105 cells/mL hepatocytes; 1.5 × 105 cells/mL NP cells).
All cell cultures were prepared on 6-well, 96-well (Greiner Bio-One, Frickenhausen, Germany), and lumox x-well (Sarstedt, Nümbrecht, Germany) cell culture dishes, previously coated with collagen type I according to the manufacturer’s instructions. The NP cells were seeded at first, and after their rapid attachment to the plate surface in 20 min, to prepare hepatocyte—NP cell co-cultures, the culture medium was removed and hepatocytes were seeded in the cell ratio of 6:1 (hepatocyte to NP cells). Hepatocyte mono-cultures were also prepared by seeding hepatocyte-enriched fraction onto cell culture dishes. The seeding volume was 1.5 mL/well on 6-well plates, 100 µL/well on 96-well plates, and 300 µL/well on lumox x-well dishes. All cell cultures were incubated at 38.5 °C in humid atmosphere with 5% CO2. Culture media were changed 4 h after seeding, and confluent monolayers were gained following 24 h culturing.
2.2. Characterizing Cell Cultures with Giemsa Staining and Immunocytochemistry
To confirm cell morphology, 48-h-cultured confluent monolayers on 6-well plates were stained with Giemsa, and to assess the presence and the ratio of various liver cell types in different cell culture models, immunocytochemical analyses were applied with chicken specific antibodies. Albumin was detected with a chicken specific, fluorescein isothiocyanate (FITC) coupled anti-albumin antibody (Cedarlane, Burlington, Canada). The macrophages (mainly Kupffer cells as the resident liver macrophages) in NP cell fractions were labelled by using a chicken macrophage specific phycoerythrin (PE) coupled antibody (Southern Biotech, Uden, The Netherlands).
Isolated cells and cell cultures (following 48 h culturing) on lumox x-well plates were fixed in phosphate buffered saline (PBS) containing 4% formaldehyde for 30 min at room temperature (21 °C). After rinsing the fixed cells in PBS (3 times 5 min), they were permeabilized with Triton-X (0.25%) containing PBS for 20 min, and were subsequently blocked in PBS supplemented with 5% goat serum, 3% BSA, and 0.1% Triton-X for 60 min at room temperature. Antibodies were dissolved (in the ratio of 1:100 for anti-albumin and 1:50 for macrophage specific antibody) in PBS containing 1% BSA and were applied overnight at 4 °C. After counterstaining with 4’,6-diamidino-2-phenylindole (DAPI), cell cultures were analyzed with an Olympus CKX-41-type fluorescent microscope and a Canon EOS 1100D camera.
2.3. Characterizing Cell Fractions with Flow Cytometry
Hepatocyte enriched and NP cell containing fractions were examined with flow cytometry. Cell suspensions with approximately 1 × 106/mL cell concentration were filtered prior to data acquisition with Sysmex CellTrics filters (30 µm, Ref No. 04-0042-2316), then subsequently analyzed with a Beckman Coulter FC 500 flow cytometer equipped with an air-cooled 20 mW, 488 nm Argon ion laser. Forward and side scatter values were recorded in the corresponding photodetectors. The flow rate was set to “low” (10 µL/min). A total of 10,000 events were collected per sample.
Data analysis was done with Flowing free software (version 2.5.1,
www.flowingsoftware.com) and FCS Express 7 Plus (version 7.00.0037,
www.denovosoftware.com) by drawing two-dimensional plots, showing forward (FS) versus side scatter (SS). Both parameters were displayed on log axes.
2.4. Treatments and Measurements of Cellular Metabolic Activity, LDH Activity, H2O2, HSP70, IL-6, and IL-8 Concentrations
After 24 h culturing, culture media were changed to fresh FBS-free Williams’ Medium E, and confluent mono- and co-cultures on 6-well and 96-well plates were incubated at 43 °C for 1 or 2 h to mimic heat stress, while control cells were incubated further at 38.5 °C. The incubation conditions were set based on literature data and our pilot studies, considering that birds have higher physiological body temperature than mammals; however, cells isolated from avian species are often cultured at temperatures similar to mammalian cells. Normal incubation temperatures of avian cell cultures are ranging from 37 to 41.5 °C, and temperatures mimicking heat stress are varied between 40 and 45 °C [
15,
16,
17,
18], hence, as a compromise, 38.5 °C was chosen for maintaining control cells, and 43 °C for studying acute heat stress.
Following heat exposure, the metabolic activity of cells on 96-well plates was monitored by the CCK-8 assay according to the manufacturer’s instructions, detecting the amount of NADH+H+ gained in the catabolic pathways. Briefly, 10 µL CCK-8 reagent and 100 µL fresh Williams’ Medium E were given to the cultured cells, and the absorbance was measured at 450 nm with a Multiskan GO 3.2 reader after 2 h incubation at 38.5 °C. In order to monitor cytotoxicity, extracellular lactate dehydrogenase (LDH) activity was measured by a specific photometric assay (Diagnosticum Ltd., Budapest, Hungary). First, 200 µL working reagent (containing 56 mM phosphate buffer, pH = 7.5; 1.6 mM pyruvate, and 240 µM NADH+H+) was mixed with a 10 µL cell culture medium. The enzyme activity was assessed by a kinetic method, measuring the absorbance of samples at 340 nm with a Multiskan GO 3.2 reader.
Culture media of 6-well plates were collected directly after the applied treatments for measuring extracellular ROS (H2O2), HSP70, IL-6, and IL-8 concentrations. Following the removal of media, cultured cells from both 6-well and 96-well plates were gently washed in PBS and were subsequently lysed in a M-PER buffer supplemented with 1% Halt Protease Inhibitor Cocktail and 1% ethylene diamine tetraacetic acid (EDTA) (Thermo Fisher Scientific, Waltham, MA, USA) for assaying total protein concentrations. All culture media and cell lysate samples were stored at −80 °C until further processing.
The H2O2 concentration of cell supernatants was assessed with the Amplex Red Hydrogen Peroxide Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The applied substrate (Amplex Red) reacts with H2O2 in a horseradish peroxidase (HRP) catalyzed reaction, producing highly fluorescent resorufin. After 30 min incubation of 50 µL culture media with 50 µL freshly prepared, Amplex Red (100 µM) and HRP (0.2 U/mL) containing a working solution at room temperature, fluorescence was detected with a Victor X2 2030 fluorometer (λex = 560 nm; λem = 590 nm). The concentrations of HSP70, IL-6, and IL-8 were measured in the culture media of 6-well dishes by chicken specific ELISA kits (Cat. No. MBS734158, MBS268769, and MBS013823, respectively; MyBioSource, San Diego, CA, USA) following the manufacturer’s instructions. The absorbance values were quantified at 450 nm with a Multiskan GO 3.2 reader. Total protein concentration of cell lysates was assessed with the PierceTM Bicinchoninic Acid (BCA) Protein Assay (Thermo Fisher Scientific, Waltham, MA, USA) as indicated by the manufacturer, applying BSA as a standard, adding 25 µL sample to 200 µL reagent mixture and measuring the absorbance after 30 min incubation at 37 °C at 562 nm with a Multiskan GO 3.2 reader.
2.5. Statistics
All treatments were applied in triplicates on 6-well plates, and six wells were included in each group on 96-well plates. Data of metabolic activity, LDH enzyme activity, H2O2, HSP70, IL-6, and IL-8 concentrations of cell culture supernatants were all standardized to the total protein concentrations of cell lysates. Data were analyzed with one-way ANOVA and with Tukey’s post-hoc test, applying the R 2.14.0 software. All results are expressed as mean ± SEM, the level of significance was set at p < 0.05.
4. Discussion
In the present study novel primary hepatic cell culture models have been successfully established from chickens. Based on the investigation of the separated cell fractions with flow cytometry and on the immunofluorescent characterization of cultured cells, hepatocyte mono-cultures and hepatocyte—NP cell co-cultures have been prepared from chicken liver. As justified by immunocytochemistry, the NP cell fraction comprised of mainly macrophages, first of all Kupffer cells as the resident liver macrophages and presumably also circulation-derived macrophages; however, the presence of other NP cell types (such as stellate cells or biliary endothelial cells) can be also suggested.
A monolayer hepatocyte—Kupffer cell and a double-layered enterohepatic co-culture have been also established recently by our research group from swine [
13,
19], but to the best of our knowledge, no similar avian cell cultures were available until now. From chickens, mainly tumorigenic cell lines, such as the Chicken Hepatocellular Carcinoma Cell Line (indicated as LMH cells) [
20,
21] or embryonic liver cell cultures [
22] were used. The newly prepared chicken cell cultures enable studies concerning the specific role of parenchymal and NP cells as the main liver cell fraction, and the hepatocyte—NP cell co-culture model can mimic various inflammatory states by setting different cell type ratios. The applied ratio of 6:1 (hepatocytes to NP cells) refers to a milder hepatic inflammation with moderate intrahepatic macrophage migration [
13]. On this co-culture, the interaction of the inflammatory and stress response can be studied, including molecular alterations of cell function, such as the pro- and anti-inflammatory cytokine production and the redox homeostasis of the cultured liver cells. The main advantage of these models is that they are nontumorigenic primary cell cultures, hence the results can be better extrapolated to the in vivo conditions of the healthy or inflamed chicken liver.
The other major goal of the present study was to investigate the cellular effects of acute heat stress on the newly established cell culture models. The applied heat exposure and incubation conditions were set based on previously available literature data and our pilot studies. In spite of the higher physiological body temperature of birds, avian cells are often cultured at relatively lower temperatures, similarly to mammalian cultured cells. For instance, the aforementioned hepatic LMH cells, chicken embryonic fibroblast (CEF) cells, or chicken primary myocardial cells were cultured at 37 °C [
15,
16,
17], while a chicken macrophage-like cell line was maintained at 41.5 °C [
18]. Similarly, different protocols do exist for the in vitro modelling of heat stress. An elevation of the ambient temperature from 37 to 43 °C could trigger maximal heat stress response in LMH cells [
15,
23], while CEF cells were heat stressed by increasing temperature from 37 to 40–44 °C [
16]. Further, heat stress response was evoked in myocardial cells by elevating temperature from 37 to 42 °C [
17], and heat stress was modelled in chicken macrophage-like cells by incubating cells at 45 °C in contrast to control cells cultured at 41.5 °C [
18]. The time course of heat exposure was also different in various studies; the incubation time mostly ranged between 1 and 5 h to provoke short or medium-term heat stress [
15,
17,
18]. Based on the parameters of these avian in vitro experiments, considering also the characteristics and the morphology of primary liver cells (continuously monitored by phase contrast microscopy), in our study control cells were incubated at 38.5 °C and heat exposed cell cultures at 43 °C for 1 and 2 h to mimic the cellular effects of intense, shorter, and longer heat stress.
According to our results, heat stress strongly influenced the metabolic activity, redox state, HSP70, and pro-inflammatory cytokine production of cultured chicken liver cells. The short-term, 1 h heat exposure remarkably increased the catabolic activity of both hepatocyte mono-cultures and hepatocyte—NP cell co-cultures, with a higher extent in the latter case. Heat-associated enhanced metabolic rate may contribute to a better accommodation of cells to the increased temperature, which may be reflected in the alleviation of metabolic activity after 2 h of heat stress. On hepatocyte mono-cultures, cellular metabolic activity was still increased after the longer heat exposure compared to controls, but with a lower extent; however, on co-cultures it was already slightly lower after 2 h of heat treatment than in control cells. These results suggest a time-dependent metabolic adaptation to heat stress, hence cultured liver cells tended to recover after the longer heat exposure, also reflected in the other parameters measured, such as HSP70, IL-6, and IL-8 concentrations of cell supernatants. The heat-triggered changes in the metabolic activity of mono-cultured hepatocytes and co-cultured parenchymal and NP cells showed a similar pattern, but co-cultures seemed to accommodate faster to the altered temperature conditions than hepatocyte mono-cultures. Based on our findings, extracellular LDH activity was not affected by heat stress indicating that the applied heat exposures were not cytotoxic and did not induce necrosis of the cultured cells. Compared to the hepatocyte mono-cultures, the observed lower LDH activity of co-cultures may relate to the higher metabolic activity of these cultures.
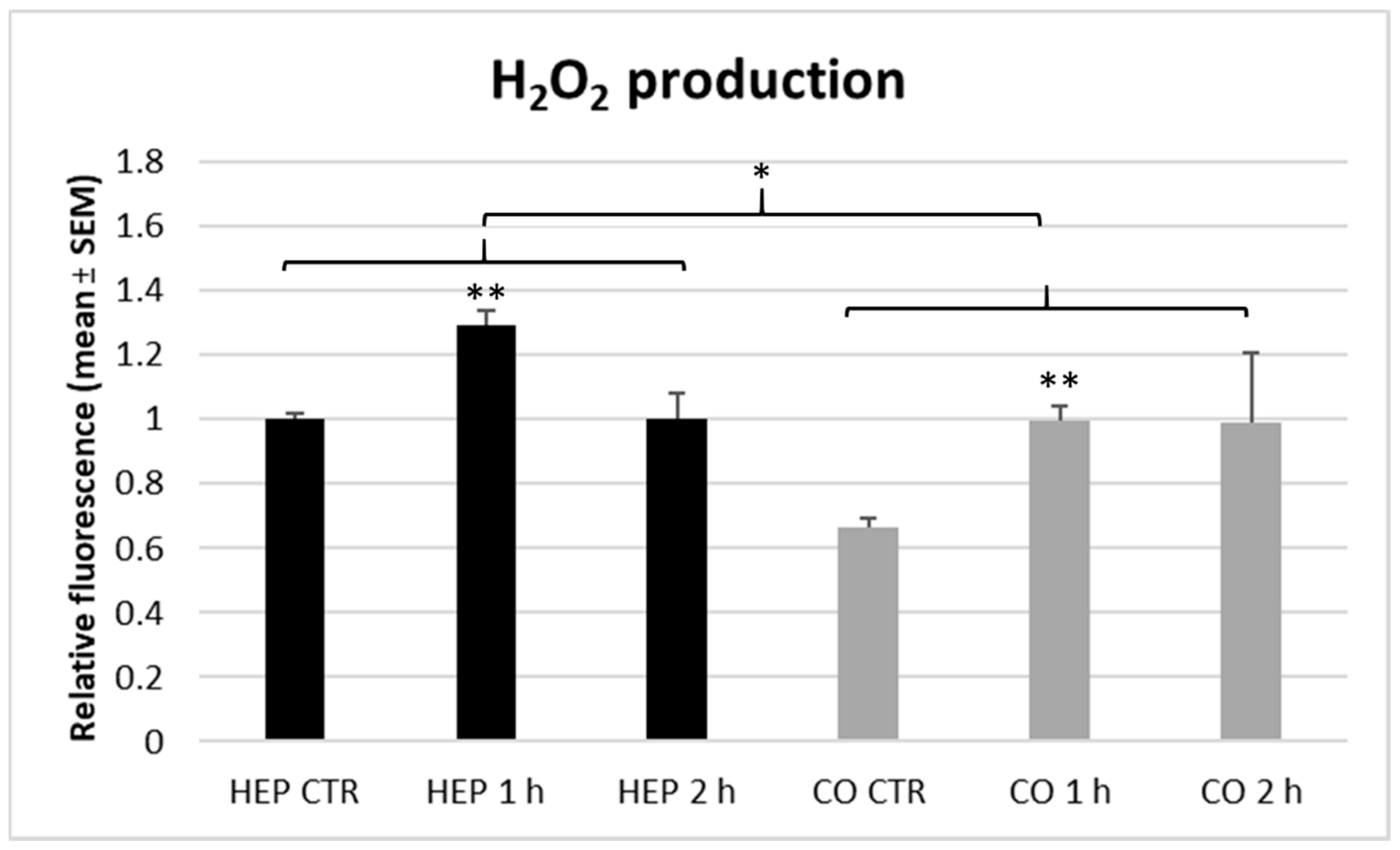
Numerous studies reported that heat stress is commonly associated with increased oxidative stress caused by elevated ROS production or inadequate amount of antioxidants [
1]. In comparison with other organs, liver was found the most sensitive to heat-triggered oxidative stress response in chickens [
4]. In the present study, 1 h lasting heat stress increased the H
2O
2 concentration of cell supernatants on both cell culture models, indicating an elevation in hepatocellular ROS release and presumably contributing to increased oxidative stress in the liver. Similarly to the metabolic activity, extracellular ROS levels tended to be normalized after 2 h of heat exposure. When comparing hepatocyte mono-cultures with hepatocyte—NP co-cultures, both models responded in the same manner as no significant differences could be found in the H
2O
2 concentration of culture media gained from different cell cultures.
The concentrations of HSP70, IL-6, and IL-8 in culture media were altered similarly by heat exposure. After the shorter (1 h) heat treatment, the level of extracellular HSP70 and those of the measured pro-inflammatory cytokines were intensively decreased, with an average extent of approx. 90% on both hepatocyte mono-cultures and hepatocyte—NP cell co-cultures. However, all these concentrations tended to be normalized following the longer, 2 h heat exposure. A similar heat stress associated decline in the HSP70 level was reported in a previous study with rat myocardial cells, where the intracytoplasmic HSP70 concentration was reduced by a short term, 1 h long heat exposure, whereas it was restored after 2 h heat incubation [
24]. It can be suggested that the utilization of heat-shock proteins in an intense short term heat stress exceeded their synthesis, resulting in decreased HSP70 levels, but after 2 h, cells could produce a sufficient amount of HSP70 to fulfill the increased requirements and contribute to the restoration of physiological cell function, reflected by normalized metabolic activity and ROS levels. Intracellular HSP70 protein expression was gradually increased by heat stress of 1 to 5 h in another in vitro study done on chicken myocardial cells [
21]. In addition, a 2 h long heat stress induced the expression of the HSPA2 gene encoding HSP70 on a chicken macrophage-like cell line [
18].
The effects of heat stress on the immune response have been described in certain studies, but limited data is available concerning heat-triggered changes in hepatic immune function. Heat stress could provide controversial immunomodulatory action on the pro-inflammatory cytokine production as it stimulated splenic IL-4 and IL-12 [
7], but decreased splenic IL-6, IL-12 gene expression, and also that of IL-1β and IL-10 in caecal tonsils of chickens [
10]. However, it should be underlined that these data were gained after chronic in vivo heat stress. The latter immunosuppressive actions were found only in
Salmonella Enteritidis infected chickens, reflecting a possible interaction of infection with inflammatory and stress response [
10]. Heat stress can also suppress cellular immunity by reducing total white blood cell count and macrophage activity [
8,
9]. In the present study, 1 h of heat stress downregulated the hepatic production of both measured pro-inflammatory cytokines, IL-6 and IL-8, but their levels returned to the baseline after 2 h of heat exposure.
The connection of HSPs and inflammatory mediators has been already studied by analyzing the liver transcriptome of chickens [
25]. Most HSPs, such as HSP70, were upregulated by a 3 h long heat stress, while the expression of pro-inflammatory cytokines was mostly low and not affected by heat exposure [
25]. However, it can be suggested that HSPs possess a key regulatory role in mitigating immune and inflammatory response under heat stress [
26]. In our present cell culture study, HSP70 and pro-inflammatory cytokines responded to heat stress in a similar manner as they were downregulated after 1 h, but normalized within 2 h of heat exposure.
Based on our results and in line with previous studies [
25], it can be suggested that the potential impact of a shorter heat stress on liver cell function is much higher than that of a longer heat exposure. Both 1 and 2 h incubation at 43 °C can be considered as acute heat stress, but there were significant differences between the shorter and longer heat exposure. The 1 h lasting intense heat stress could provoke dramatic changes, such as stimulating catabolic metabolism and ROS release and strongly decreasing HSP70 and pro-inflammatory cytokine production, but the majority of these alterations could be mitigated within 2 h of exposure by successful cellular adaptation. Hence, the critical role of short term heat stress in broiler farming has to be emphasized as it can impair liver function and the health of chickens.
When comparing the applied different cell culture models, mostly similar results were received on hepatocyte mono-cultures and hepatocyte—NP cell co-cultures. In case of metabolic activity, the co-culture serving as an inflammatory model showed higher baseline level, which was altered by heat stress to the same extent as on mono-cultured hepatocytes. However, it can be suggested that the metabolic rate of co-cultures was normalized faster as already a moderate decrease was observable after 2 h of heat stress compared to controls. These results indicate an increasing action of inflammation (mimicked by including mostly macrophages as NP cells in co-cultures) on cell metabolic rate and suggest a better capability of inflamed liver cells for accommodation to short term acute heat stress. This is consistent with the observed lower baseline level of extracellular ROS concentration in the hepatocyte—NP cell co-cultures compared to the hepatocyte monoculture.
In conclusion, the established novel primary cell culture models provide useful tools for studying the inflammatory and stress response in the liver of chickens. The successful separation of hepatocytes and NP cells and the preparation of a hepatocyte—NP cell co-culture from chickens enable investigations on the role of different cell types and on the interaction of stress and hepatic immune response. According to the 3R principle, it is also noteworthy, that using the above described and newly established primary cell culture models, considerable amount of data can be achieved using only one chicken, instead of setting up a complete in vivo study applying a large number of animals. Based on our results, a short term (1 h) intense heat stress can largely influence liver cell functions by increasing metabolism and extracellular H2O2 release, and by decreasing HSP70, IL-6, and IL-8 production. However, all these alterations were restored after 2 h of heat exposure, indicating a fast recruitment of liver cells. These data highlight the great impact of short term heat stress on the functions of chicken liver cells, underline the mediatory role of oxidative stress in acute stress response and imply a fast cellular adaptation potential of liver cells.