Effect of Dietary Folic Acid Supplementation during Pregnancy on Blood Characteristics and Milk Composition of Ewes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Experiment Design
2.2. Sampling Procedure and Measurement
2.2.1. Dietary Chemical Analyses
2.2.2. Serum Parameters
2.2.3. Milk Composition
2.3. Statistical Analysis
3. Results
3.1. Serum Variables from Gestation to Parturition
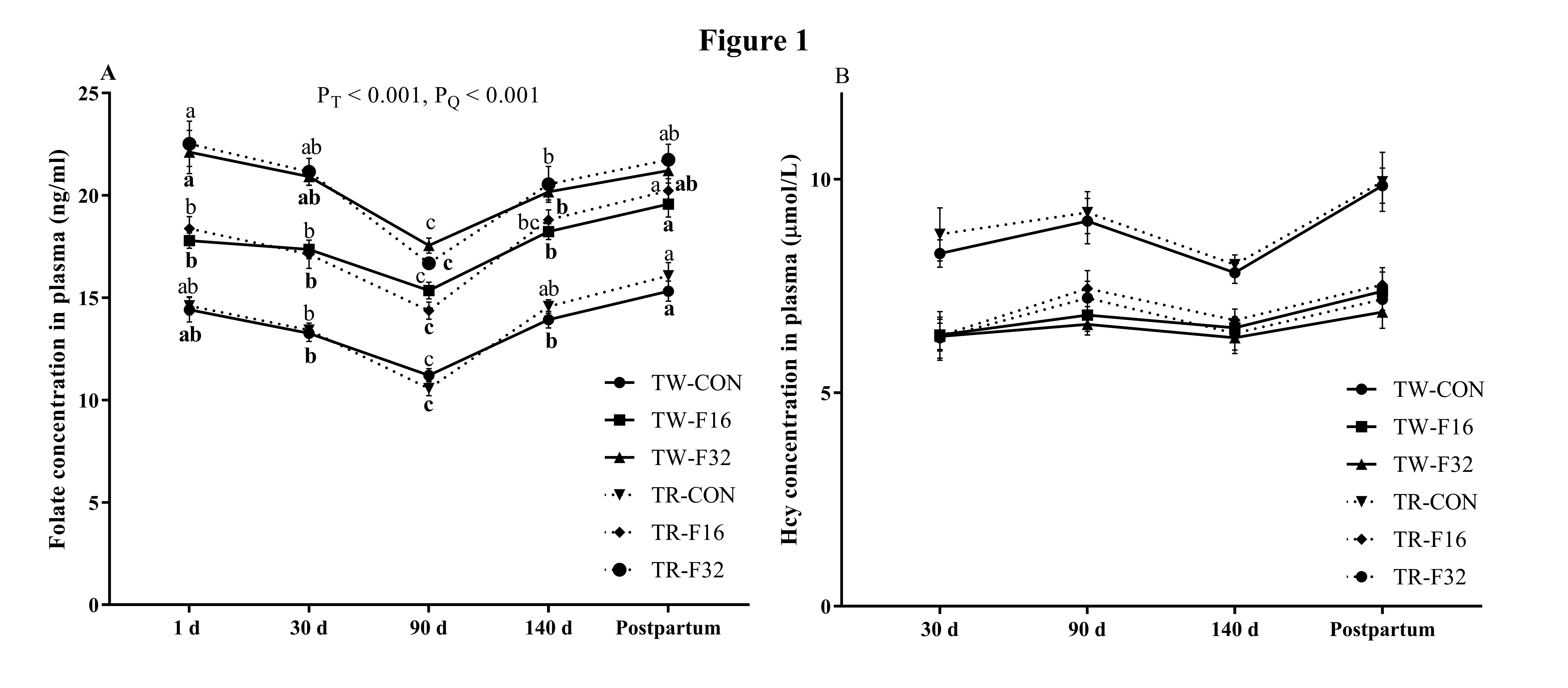
3.1.1. Folate and Hcy
3.1.2. IGF-I and GH
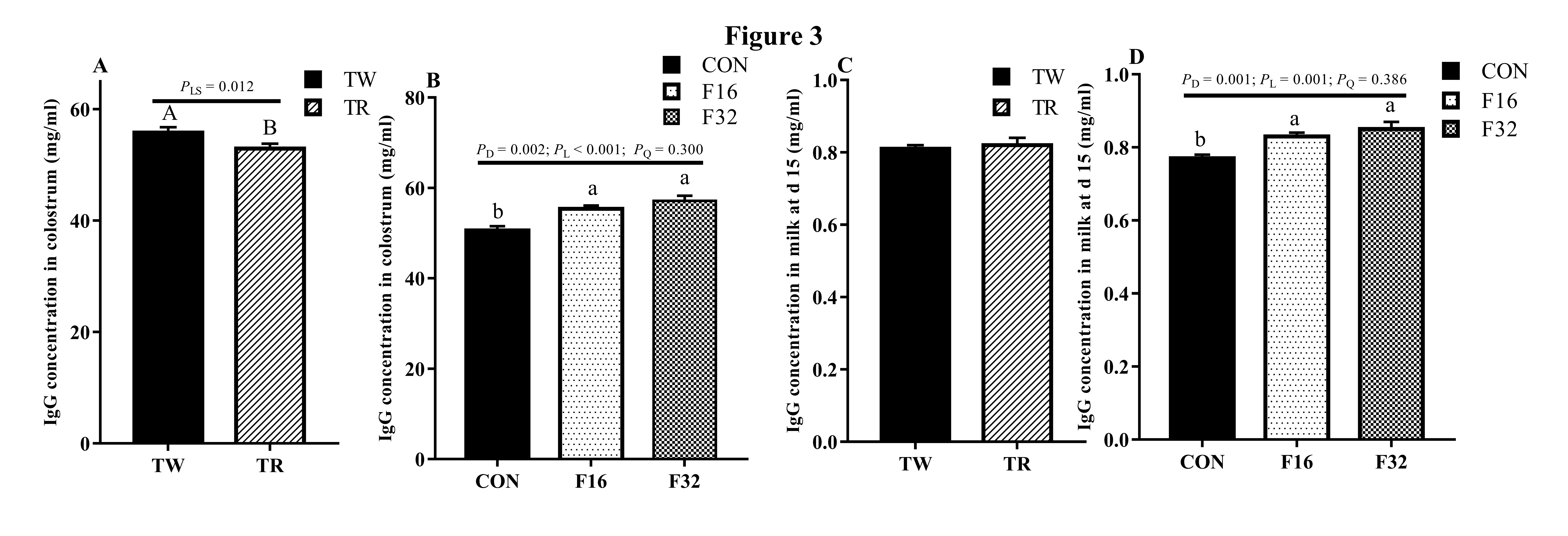
3.1.3. Immunoglobulin
3.2. Milk Components
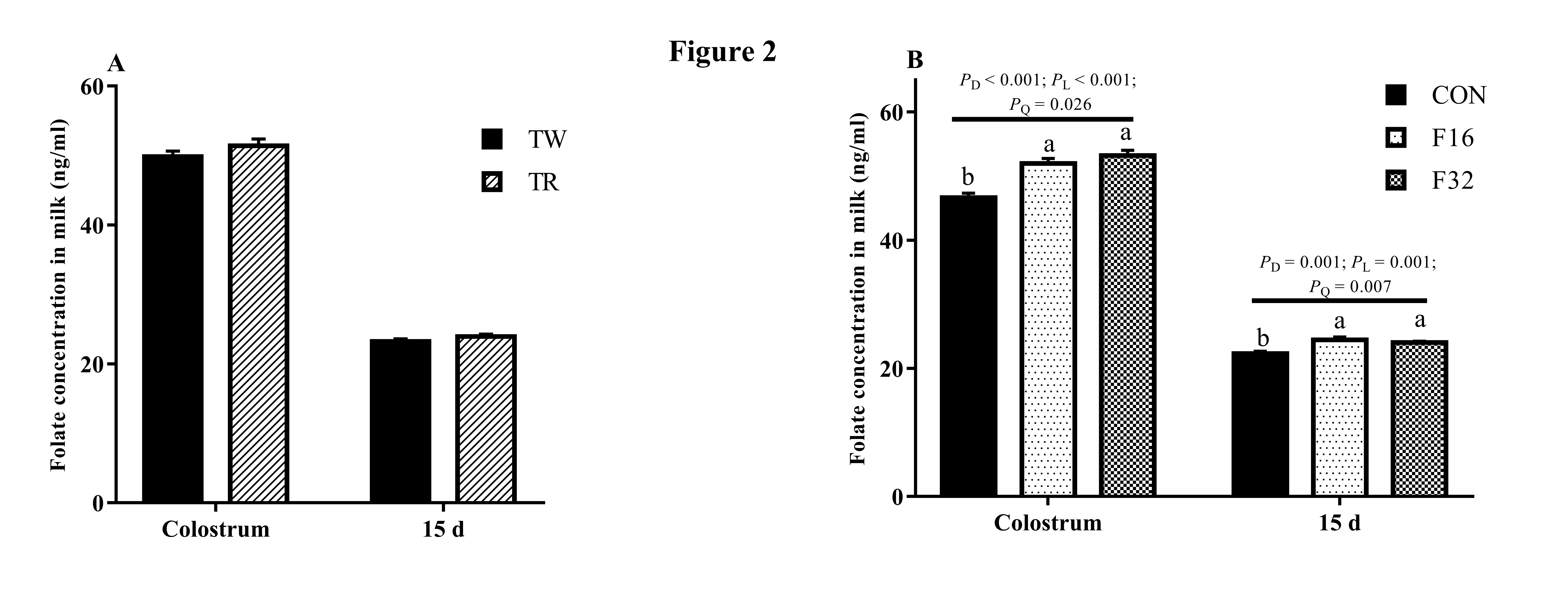
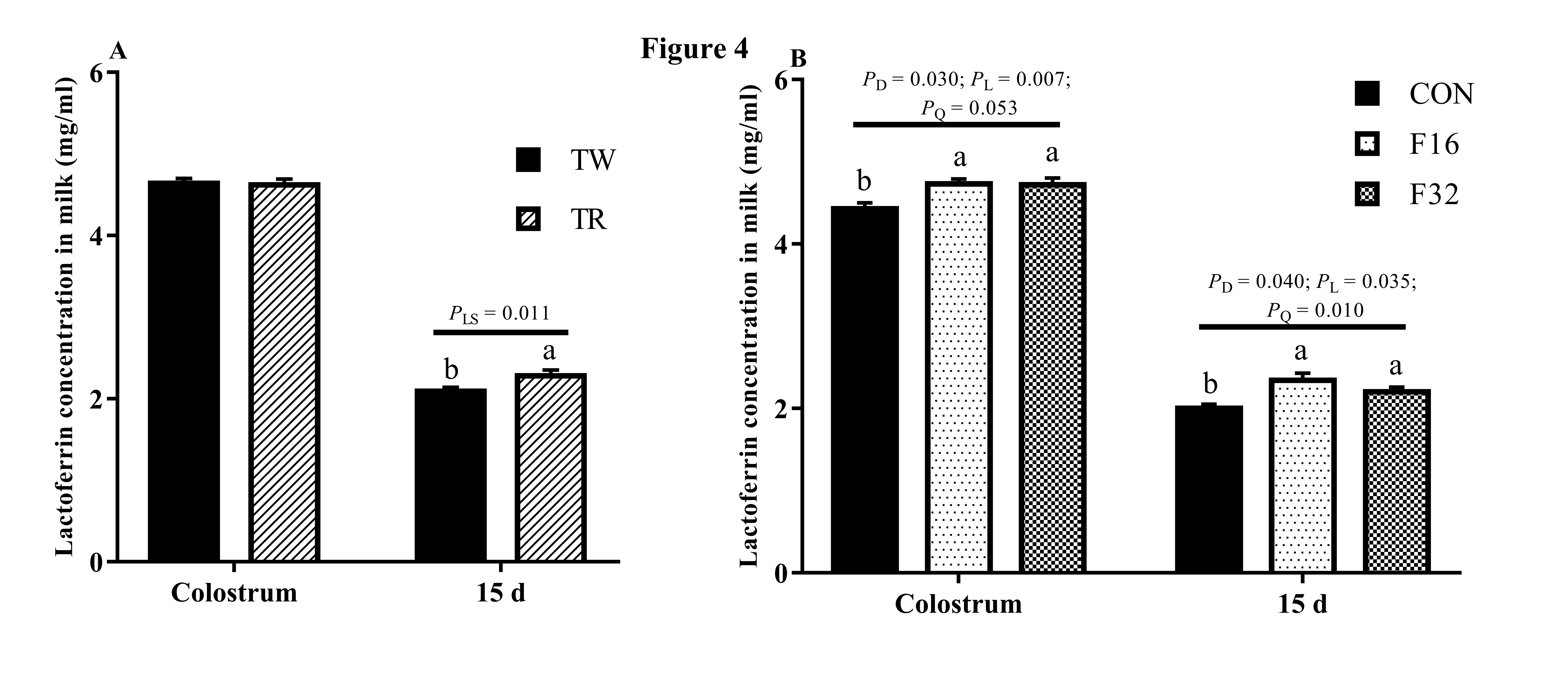
3.2.1. Folate, IgG and Lactoferrin Concentrations
3.2.2. Colostrum and Normal Milk Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fekete, K.; Berti, C.; Trovato, M.; Lohner, S.; Dullemeijer, C.; Souverein, O.W.; Cetin, I.; Decsi, T. Effect of folate intake on health outcomes in pregnancy: A systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr. J. 2012, 11, 75. [Google Scholar] [CrossRef] [Green Version]
- Bailey, L.B.; Gregory, J.F. Folate metabolism and requirements. J. Nutr. 1999, 129, 779–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, C. Biochemical role of folate in cellular metabolism (Reprinted from Folate and Health Disease, pp 23–42, 1995). Clin. Res. Regul. Aff. 2001, 18, 161–180. [Google Scholar] [CrossRef]
- Chou, Y.; Yu, C.; Huang, R.S. Changes in mitochondrial DNA deletion, content, and biogenesis in folate-deficient tissues of young rats depend on mitochondrial folate and oxidative DNA injuries. J. Nutr. 2007, 137, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hsu, Y.C.; Lin, H.L.; Yang, F.L. Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. J. Nutr. 2001, 131, 33–38. [Google Scholar] [CrossRef]
- Asaikkutti, A.; Bhavan, P.S.; Vimala, K. Effects of different levels of dietary folic acid on the growth performance, muscle composition, immune response and antioxidant capacity of freshwater prawn, Macrobrachium rosenbergii. Aquaculture 2016, 464, 136–144. [Google Scholar] [CrossRef]
- Girard, C.L. B-complex vitamins for dairy cows: A new approach. Can. J. Anim. Sci. 1998, 78S, 71–90. [Google Scholar]
- Girard, C.L.; Matte, J.J.; Tremblay, G.F. Gestation and lactation of dairy-cows–A role for folic-acid. J. Dairy Sci. 1995, 78, 404–411. [Google Scholar] [CrossRef]
- Graulet, B.; Matte, J.J.; Desrochers, A.; Doepel, L.; Palin, M.F.; Girard, C.L. Effects of dietary supplements of folic acid and vitamin B-12 on metabolism of dairy cows in early lactation. J. Dairy Sci. 2007, 90, 3442–3455. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Q.; Liu, Q.; Wang, C.; Yang, Z.M.; Guo, G.; Huo, W.J.; Pei, C.X.; Zhang, Y.L.; Zhang, S.L.; Wang, H.; et al. Effects of dietary supplements of rumen-protected folic acid on lactation performance, energy balance, blood parameters and reproductive performance in dairy cows. Anim. Feed Sci. Tech. 2016, 213, 55–63. [Google Scholar] [CrossRef]
- Girard, C.L.; Castonguay, F.; Fahmy, M.H.; Matte, J.J. Serum and milk folates during the first two gestations and lactations in Romanov, Finnsheep, and Suffolk ewes. J. Anim. Sci. 1996, 74, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Casella, S.; Lutri, L.; Vazzana, I.; Ferrantelli, V.; Caola, G. Reference values for some haematological, haematochemical, and electrophoretic parameters in the Girgentana goat. Turk J. Vet. Anim. Sci. 2010, 34, 197–204. [Google Scholar]
- Sripad, K.; Shrikant, K.; Raju, M. Hematological profile of Khillar breed of cattle in Karnataka. Veterinary World. 2014, 7, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Weaver, D.M.; Tyler, J.W.; VanMetre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Small Ruminants; The National Academies Press: Washington, DC, USA, 2007.
- AOAC Official. Official Methods of Analysis of the Association of Official Analytical Chemist, 16th ed.; AOAC, International: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Clifford, A.J.; Heid, M.K.; Muller, H.G.; Bills, N.D. Tissue distribution and prediction of total-body folate of rats. J. Nutr. 1990, 120, 1633–1639. [Google Scholar] [CrossRef]
- Duplessis, M.; Lapierre, H.; Pellerin, D.; Laforest, J.P.; Girard, C.L. Effects of intramuscular injections of folic acid, vitamin B12, or both, on lactational performance and energy status of multiparous dairy cows. J. Dairy Sci. 2017, 100, 4051–4064. [Google Scholar] [CrossRef] [Green Version]
- Girard, C.L.; Matte, J.J. Dietary supplements of folic acid during lactation: Effects on the performance of dairy cows. J. Dairy Sci. 1998, 81, 1412–1419. [Google Scholar] [CrossRef]
- Girard, C.L.; Castonguay, F.; Matte, J.J. Response of serum concentrations of folates to dietary supplements of folic acid given to ewes during gestation. Can. J. Anim. Sci. 1999, 79, 387–389. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Yu, B.; Mao, X.; Huang, Z.; Chen, D. Effect of folic acid supplementation on hepatic antioxidant function and mitochondrial-related gene expression in weanling intrauterine growth retarded piglets. Livest. Sci. 2012, 146, 123–132. [Google Scholar] [CrossRef]
- Blüher, S.; Kratzsch, J.; Kiess, W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best. Pract. Res. Cl. En. 2005, 19, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol. Metab. 2009, 20, 349–356. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, J.P. Nutritional and developmental roles of insulin-like growth factors in poultry. J. Nutr. 1998, 128S, 302S–305S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Harding, J.E.; Evans, P.C.; Gluckman, P.D. Maternal insulin-like growth-factor-I infusion alters fetus placental carbohydrate and protein-metabolism in pregnant sheep. Endocrinology 1994, 135, 895–900. [Google Scholar] [CrossRef]
- Rosario, F.J.; Nathanielsz, P.W.; Powell, T.L.; Jansson, T. Maternal folate deficiency causes inhibition of mTOR signaling, down-regulation of placental amino acid transporters and fetal growth restriction in mice. Sci. Rep-UK. 2017, 7, 3982. [Google Scholar] [CrossRef]
- Lassarre, C.; Hardouin, S.; Daffos, F.; Forestier, F.; Frankenne, F.; Binoux, M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr. Res. 1991, 29, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.J.; Zhang, F.; Tian, W.J.; Kong, Y.Q.; Li, Q.; Yu, N.; Du, Z.Y.; Wu, Q.Q.; Qin, J.G.; Chen, L.Q. Effects of dietary folic acid on growth, antioxidant capacity, non-specific immune response and disease resistance of juvenile Chinese mitten crabEriocheir sinensis (Milne-Edwards, 1853). Aquaculture 2016, 22, 567–574. [Google Scholar] [CrossRef]
- Duthie, S.J.; Horgan, G.; de Roos, B.; Rucklidge, G.; Reid, M.; Duncan, G.; Pirie, L.; Basten, G.P.; Power, H.J. Blood Folate Status and Expression of Proteins Involved in Immune Function, Inflammation, and Coagulation: Biochemical and Proteomic Changes in the Plasma of Humans in Response to Long-Term Synthetic Folic Acid Supplementation. J. Proteome Res. 2010, 9, 1941–1950. [Google Scholar] [CrossRef]
- Li, S.; Zhi, L.; Liu, Y.; Shen, J.; Liu, L.; Yao, J.; Yang, X. Effect of in ovo feeding of folic acid on the folate metabolism, immune function and epigenetic modification of immune effector molecules of broiler. Brit. J. Nutr. 2016, 115, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Hassan, G.A.; Elnouty, F.D.; Samak, M.A.; Salem, M.H. Relationship between milk production and some blood constituents in egyptian baladi goats. Beiträge Zur Tropischen Landwirtschaft Und Veterinärmedizin 1986, 24, 213–219. [Google Scholar]
- Bahiram, K.B.; Khan, J.R.; Korde, J.P. Correlation between Milk Production Traits, Certain Hormones and Serum Biochemical Parameters in Lactating Murrah Buffaloes. Indian J. Vet Sci. Biotech. 2018, 13, 61–64. [Google Scholar]
- Girard, C.L.; Lapierre, H.; Matte, J.J.; Lobley, G.E. Effects of dietary supplements of folic acid and rumen-protected methionine on lactational performance and folate metabolism of dairy cows. J. Dairy Sci. 2005, 88, 660–670. [Google Scholar] [CrossRef]
- Uruakpa, F.O.; Ismond, M.; Akobundu, E. Colostrum and its benefits: A review. Nutr. Res. 2002, 22, 755–767. [Google Scholar] [CrossRef]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune components of bovine colostrum and milk. J. Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef]
- Vatankhah, M. Relationship between immunoglobulin concentrations in the ewe’s serum and colostrum, and lamb’s serum in Lori-Bakhtiari sheep. Iranian J. Appl. Anim. Sci. 2013, 3, 539–544. [Google Scholar]
- Rosales Nieto, C.A.; Ferguson, M.B.; Macleay, C.A.; Briegel, J.R.; Wood, D.A.; Martin, G.B.; Bencini, R.; Thompson, A.N. Milk production and composition, and progeny performance in young ewes with high merit for rapid growth and muscle and fat accumulation. Animal 2018, 12, 2292–2299. [Google Scholar] [CrossRef]
- Bencini, R.; Pulina, G. The quality of sheep milk: A review. Aust. J. Exp. Agr. 1997, 37, 485–504. [Google Scholar] [CrossRef]
- Preynat, A.; Lapierre, H.; Thivierge, M.C.; Palin, M.F.; Cardinault, N.; Matte, J.J.; Desrochers, A.; Girard, C.L. Effects of supplementary folic acid and vitamin B (12) on hepatic metabolism of dairy cows according to methionine supply. J. Dairy Sci. 2010, 93, 2130–2142. [Google Scholar] [CrossRef] [Green Version]
- Sacadura, F.C.; Robinson, P.H.; Evans, E.; Lordelo, M. Effects of a ruminally protected B-vitamin supplement on milk yield and composition of lactating dairy cows. Anim. Feed Sci. Tech. 2008, 144, 111–124. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.Q.; Li, Z.; Jian, L.Y.; Gao, Y.F.; Qu, Y.H.; Liu, C.; Xu, C.C.; Li, Y.X.; Diao, Z.C.; et al. Maternal folic acid supplementation modulates the growth performance, muscle development and immunity of Hu sheep offspring of different litter size. J. Nutr. Biochem. 2019, 70, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Wang, B.; Li, Z.; Luo, H.L.; Wang, Y.J.; Zhang, C.; Jian, L.Y.; Gao, Y.F.; Lu, W.; Liu, M.; et al. Effects of rumen-protected folic acid addition in maternal and post-weaning diets on growth performance, total tract digestibility, ruminal fermentation and blood metabolites in lambs. Anim. Feed Sci. Tech. 2020, 260, 114364. [Google Scholar] [CrossRef]
| Ingredients | Formula of Concentrate | TMR Nutrient Levels | |||
|---|---|---|---|---|---|
| EG | LG | Nutrients | EG | LG | |
| Corn | 53.00 | 50.00 | Dry matter | 92.35 | 90.96 |
| Soybean meal | 9.70 | 22.50 | Crude protein | 9.60 | 10.00 |
| Rapeseed meal | 12.00 | 7.00 | Ether extract | 3.51 | 4.52 |
| Wheat bran | 15.70 | 11.50 | Ash | 11.19 | 8.85 |
| Limestone | 1.00 | 1.00 | Neutral detergent fiber | 49.19 | 34.15 |
| CaHPO4 | 0.60 | 0.60 | Acid detergent fiber | 29.98 | 24.86 |
| NaHCO3 | 1.30 | 1.30 | Metabolizable energy 2) | 1.93 | 2.15 |
| NaCl | 0.80 | 0.40 | Calcium | 0.54 | 0.56 |
| Vitamin E | 0.40 | 0.10 | Phosphorus | 0.37 | 0.26 |
| Soybean oil | 0.30 | 0.40 | |||
| Premix 1) | 5.00 | 5.00 | |||
| De-mold agent | 0.20 | 0.20 | |||
| Total | 100.00 | 100.00 | |||
| Item | Time | LZ | D | SEM | P-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (d) | TW | TR | CON | F16 | F32 | LZ | D | I × | L | Q | ||
| Folate (ng/ml) | 1 | 18.11 | 18.67 | 14.51 c | 18.09 b | 22.57 a | 0.63 | 0.325 | <0.001 | 0.889 | <0.001 | 0.378 |
| 30 | 17.19 | 17.23 | 13.35 c | 17.24 b | 21.04 a | 0.58 | 0.923 | <0.001 | 0.896 | <0.001 | 0.895 | |
| 90 | 14.7 | 13.87 | 10.88 c | 14.86 b | 17.11 a | 0.46 | 0.01 | <0.001 | 0.91 | <0.001 | 0.019 | |
| 140 | 17.44 | 17.97 | 14.25 c | 18.52 b | 20.35 a | 0.5 | 0.241 | <0.001 | 0.967 | <0.001 | 0.006 | |
| P | 18.7 | 19.35 | 15.70 c | 19.90 b | 21.48 a | 0.48 | 0.23 | <0.001 | 0.984 | <0.001 | 0.017 | |
| Hcy (μmol/L) | 30 | 6.98 | 7.04 | 8.48 a | 6.24 b | 6.31 b | 0.24 | 0.857 | <0.001 | 0.746 | <0.001 | 0.008 |
| 90 | 7.48 | 7.96 | 9.12 a | 7.13 b | 6.91 b | 0.23 | 0.171 | <0.001 | 0.855 | <0.001 | 0.018 | |
| 140 | 6.88 | 7.03 | 7.91 a | 6.61 b | 6.34 b | 0.16 | 0.555 | <0.001 | 0.986 | <0.001 | 0.048 | |
| P | 8.04 | 8.22 | 9.89 a | 7.46 b | 7.04 b | 0.27 | 0.62 | <0.001 | 0.972 | <0.001 | 0.007 | |
| Item | Time | LZ | D | SEM | P-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (d) | TW | TR | CON | F16 | F32 | LZ | D | I × | L | Q | ||
| IGF-I | 30 | 242.59 | 248.17 | 241.25 | 252.52 | 242.37 | 4.64 | 0.592 | 0.619 | 0.94 | 0.87 | 0.328 |
| 90 | 263.55 | 273.01 | 252.68 b | 276.12 a | 275.99 a | 3 | 0.053 | <0.001 | 0.611 | 0.001 | 0.023 | |
| 140 | 246.7 | 256.96 | 237.17 | 258.23 | 260.09 | 4.25 | 0.225 | 0.076 | 0.897 | 0.023 | 0.215 | |
| P | 265.57 | 272.84 | 255.93 b | 278.24 a | 273.43 a | 3.55 | 0.284 | 0.035 | 0.54 | 0.046 | 0.058 | |
| GH | 30 | 10.41 | 10.33 | 10.39 | 10.2 | 10.53 | 0.22 | 0.876 | 0.846 | 0.924 | 0.571 | 0.691 |
| 90 | 12.12 | 12.46 | 11.82 | 12.4 | 12.66 | 0.17 | 0.324 | 0.151 | 0.493 | 0.035 | 0.56 | |
| 140 | 12.12 | 12.29 | 11.97 | 12.44 | 12.21 | 0.2 | 0.673 | 0.655 | 0.304 | 0.44 | 0.307 | |
| P | 13.19 | 13.52 | 12.57 | 13.98 | 13.51 | 0.23 | 0.482 | 0.061 | 0.843 | 0.092 | 0.044 | |
| Item | Groups | Time (d) | SEM | P-Values | |||||
|---|---|---|---|---|---|---|---|---|---|
| 30 | 90 | 140 | P | T | L | Q | |||
| IGF-I | TW-CON | 239.09 | 250.93 | 233.4 | 257.83 | 5.45 | 0.414 | 0.447 | 0.571 |
| TW-F16 | 247.34 | 271.3 | 250.51 | 271.66 | 4.48 | 0.082 | 0.168 | 0.865 | |
| TW-F32 | 241.33 | 268.41 | 256.2 | 267.2 | 4.83 | 0.175 | 0.127 | 0.391 | |
| TR-CON | 243.4 | 254.42 | 240.94 | 254.02 | 5.87 | 0.83 | 0.758 | 0.938 | |
| TR-F16 | 257.70 b | 281.04 a | 265.94 ab | 284.83 a | 3.76 | 0.034 | 0.043 | 0.737 | |
| TR-F32 | 243.39 b | 283.57 a | 263.98 ab | 279.66 a | 4.83 | 0.003 | 0.012 | 0.111 | |
| GH | TW-CON | 10.54 | 11.45 | 11.51 | 12.43 | 0.27 | 0.109 | 0.02 | 0.977 |
| TW-F16 | 10.12 c | 12.15 b | 12.28 b | 13.66 a | 0.33 | <0.001 | <0.001 | 0.447 | |
| TW-F32 | 10.57 b | 12.76 a | 12.56 a | 13.49 a | 0.32 | 0.003 | 0.001 | 0.199 | |
| TR-CON | 10.22 b | 12.19 a | 12.42 a | 12.71 a | 0.34 | 0.03 | 0.007 | 0.132 | |
| TR-F16 | 10.28 c | 12.65 b | 12.59 b | 14.31 a | 0.37 | 0.001 | <0.001 | 0.54 | |
| TR-F32 | 10.49 b | 12.55 a | 11.86 ab | 13.53 a | 0.36 | 0.01 | 0.004 | 0.744 | |
| Item | Time | LZ | D | SEM | P-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (d) | TW | TR | CON | F16 | F32 | LZ | D | I × | L | Q | ||
| IgG | 30 | 10.95 | 11 | 11.01 | 11.3 | 10.61 | 0.22 | 0.91 | 0.442 | 0.526 | 0.597 | 0.192 |
| 90 | 11.94 | 11.91 | 12.16 | 11.9 | 11.71 | 0.21 | 0.944 | 0.74 | 0.897 | 0.422 | 0.944 | |
| 140 | 11.74 | 12.25 | 10.42 b | 12.68 a | 12.88 a | 0.28 | 0.237 | 0.002 | 0.219 | <0.001 | 0.033 | |
| P | 13.46 | 14.48 | 12.20 b | 14.98 a | 14.73 a | 0.32 | 0.033 | <0.001 | 0.303 | 0.001 | 0.006 | |
| IgM | 30 | 1.61 | 1.57 | 1.57 | 1.57 | 1.64 | 0.03 | 0.524 | 0.524 | 0.925 | 0.394 | 0.532 |
| 90 | 1.58 | 1.6 | 1.56 | 1.59 | 1.64 | 0.03 | 0.696 | 0.46 | 0.457 | 0.25 | 0.843 | |
| 140 | 1.63 | 1.64 | 1.64 | 1.62 | 1.64 | 0.03 | 0.917 | 0.963 | 0.979 | 0.919 | 0.762 | |
| P | 1.73 | 1.77 | 1.62 b | 1.83 a | 1.80 a | 0.03 | 0.459 | 0.023 | 0.517 | 0.025 | 0.058 | |
| IgA | 30 | 0.45 | 0.44 | 0.43 | 0.45 | 0.45 | 0.02 | 0.849 | 0.689 | 0.845 | 0.543 | 0.594 |
| 90 | 0.43 | 0.43 | 0.41 | 0.45 | 0.43 | 0.01 | 0.988 | 0.345 | 0.964 | 0.323 | 0.229 | |
| 140 | 0.45 | 0.44 | 0.42 | 0.46 | 0.46 | 0.01 | 0.856 | 0.087 | 0.879 | 0.035 | 0.337 | |
| P | 0.43 | 0.43 | 0.41 b | 0.44 a | 0.44 a | 0.01 | 0.9 | 0.049 | 0.95 | 0.015 | 0.225 | |
| Item | Groups | Time (d) | SEM | P-Values | |||||
|---|---|---|---|---|---|---|---|---|---|
| 30 | 90 | 140 | P | T | L | Q | |||
| IgG | TW-CON | 10.67 | 12.13 | 10.67 | 12.22 | 0.3 | 0.089 | 0.217 | 0.934 |
| TW-F16 | 11.62 b | 11.83 b | 12.4 b | 14.26 a | 0.32 | 0.005 | 0.001 | 0.11 | |
| TW-F32 | 10.55 b | 11.88 b | 12.14 b | 13.92 a | 0.37 | 0.004 | 0.001 | 0.691 | |
| TR-CON | 11.35 | 12.2 | 10.18 | 12.17 | 0.35 | 0.156 | 0.876 | 0.388 | |
| TR-F16 | 10.98 c | 11.98 bc | 12.96 b | 15.71 a | 0.45 | <0.001 | <0.001 | 0.105 | |
| TR-F32 | 10.66 c | 11.55 c | 13.62 b | 15.55 a | 0.48 | <0.001 | <0.001 | 0.345 | |
| IgM | TW-CON | 1.6 | 1.59 | 1.65 | 1.65 | 0.03 | 0.893 | 0.522 | 0.995 |
| TW-F16 | 1.6 | 1.54 | 1.61 | 1.77 | 0.04 | 0.143 | 0.073 | 0.131 | |
| TW-F32 | 1.65 | 1.62 | 1.63 | 1.77 | 0.04 | 0.626 | 0.378 | 0.382 | |
| TR-CON | 1.55 | 1.52 | 1.64 | 1.6 | 0.03 | 0.691 | 0.455 | 0.937 | |
| TR-F16 | 1.53 b | 1.64 b | 1.63 b | 1.89 a | 0.04 | 0.01 | 0.003 | 0.268 | |
| TR-F32 | 1.64 | 1.66 | 1.64 | 1.83 | 0.03 | 0.1 | 0.046 | 0.185 | |
| IgA | TW-CON | 0.44 | 0.42 | 0.43 | 0.41 | 0.01 | 0.687 | 0.338 | 0.912 |
| TW-F16 | 0.45 | 0.45 | 0.46 | 0.44 | 0.01 | 0.838 | 0.837 | 0.566 | |
| TW-F32 | 0.45 | 0.43 | 0.46 | 0.44 | 0.01 | 0.688 | 0.826 | 0.986 | |
| TR-CON | 0.42 | 0.41 | 0.41 | 0.41 | 0.02 | 0.691 | 0.455 | 0.937 | |
| TR-F16 | 0.46 | 0.45 | 0.46 | 0.44 | 0.01 | 0.843 | 0.526 | 0.849 | |
| TR-F32 | 0.44 | 0.44 | 0.47 | 0.44 | 0.01 | 0.769 | 0.787 | 0.668 | |
| Items | Time | LZ | D | SEM | P-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TW | TR | CON | F16 | F32 | LZ | D | I × | L | Q | |||
| Fat (%) | C | 12.06 | 11.92 | 11.47 | 12.15 | 12.24 | 0.27 | 0.645 | 0.570 | 0.978 | 0.287 | 0.609 |
| 15 d | 5.27 | 5.48 | 4.89 | 5.52 | 5.70 | 0.19 | 0.671 | 0.218 | 0.968 | 0.076 | 0.569 | |
| Protein (%) | C | 13.46 | 13.23 | 12.68 b | 13.72 a | 13.64 a | 0.16 | 0.497 | 0.046 | 0.865 | 0.031 | 0.096 |
| 15 d | 5.59 | 5.47 | 5.26 | 5.66 | 5.67 | 0.10 | 0.496 | 0.098 | 0.845 | 0.042 | 0.242 | |
| Lactose (%) | C | 3.15 | 3.27 | 3.16 | 3.21 | 3.26 | 0.12 | 0.678 | 0.963 | 0.836 | 0.578 | 0.869 |
| 15 d | 5.67 | 5.52 | 5.49 | 5.69 | 5.61 | 0.04 | 0.053 | 0.110 | 0.792 | 0.240 | 0.096 | |
| BUN (mg/dL) | C | 4.81 | 4.74 | 4.91 | 4.71 | 4.71 | 0.38 | 0.693 | 0.619 | 0.955 | 0.313 | 0.512 |
| 15 d | 5.84 | 5.88 | 6.16 | 5.64 | 5.79 | 0.16 | 0.911 | 0.468 | 0.874 | 0.231 | 0.274 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Li, Z.; Li, H.; Luo, H.; Blair, H.T.; Jian, L.; Diao, Z. Effect of Dietary Folic Acid Supplementation during Pregnancy on Blood Characteristics and Milk Composition of Ewes. Animals 2020, 10, 433. https://doi.org/10.3390/ani10030433
Wang B, Li Z, Li H, Luo H, Blair HT, Jian L, Diao Z. Effect of Dietary Folic Acid Supplementation during Pregnancy on Blood Characteristics and Milk Composition of Ewes. Animals. 2020; 10(3):433. https://doi.org/10.3390/ani10030433
Chicago/Turabian StyleWang, Bo, Zhen Li, Heqiong Li, Hailing Luo, Hugh T. Blair, Luyang Jian, and Zhicheng Diao. 2020. "Effect of Dietary Folic Acid Supplementation during Pregnancy on Blood Characteristics and Milk Composition of Ewes" Animals 10, no. 3: 433. https://doi.org/10.3390/ani10030433





