Ginger and Its Derivatives as Promising Alternatives to Antibiotics in Poultry Feed
Abstract
Simple Summary
Abstract
1. Introduction
2. The Effect of Ginger Oil
3. Chemical Composition of Ginger Extract
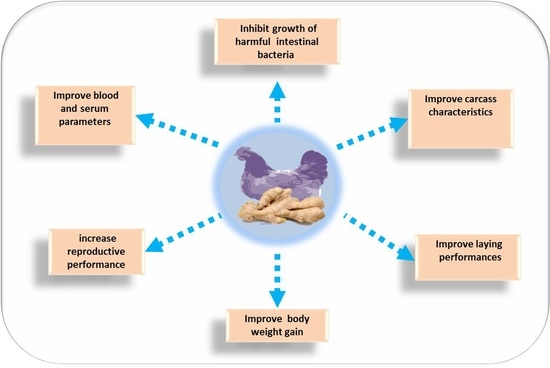
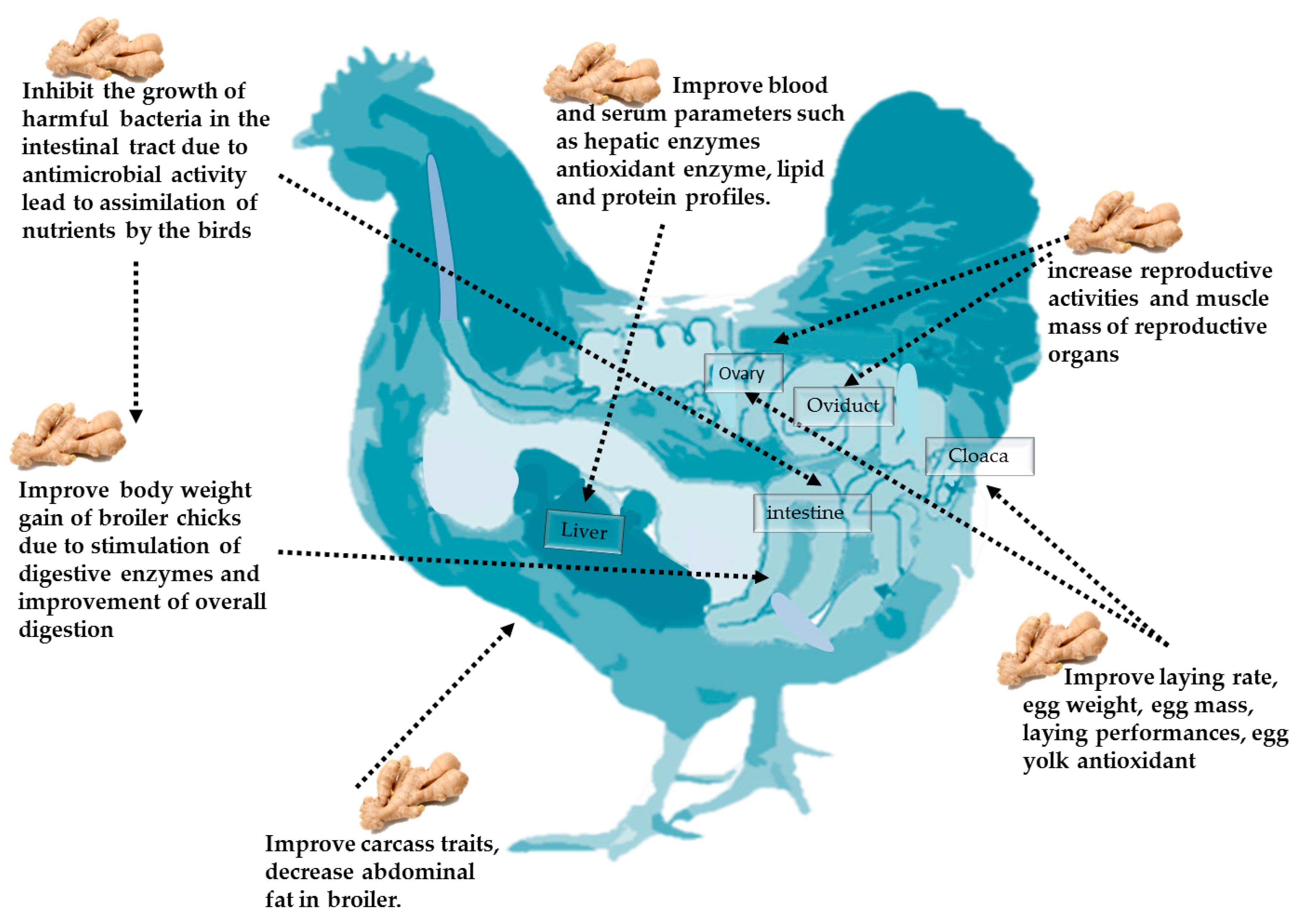
4. Beneficial Application of Ginger and Its Derivatives in Poultry Nutrition
4.1. Effects of Ginger on Body Weight
4.2. Effect of Ginger and Its Derivatives on Carcass Traits
4.3. Effect of Ginger and Its Derivatives on Egg Production and Quality
4.4. Effect of Ginger on Reproductive Performance
4.5. Effect of Ginger on Blood Parameters
4.6. Effect of Ginger and Its Derivatives on Microbiological Aspects
4.7. Effect of Ginger on Meat and Egg Quality
4.8. Effect of Ginger on Economic Efficiency
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abd El-Hack, M.E.; Mahgoub, S.A.; Alagawany, M.; Dhama, K. Influences of dietary supplementation of antimicrobial cold pressed oils mixture on growth performance and intestinal microflora of growing Japanese quails. Int. J. Pharmacol. 2015, 11, 689–696. [Google Scholar]
- Abd El-Hack, M.E.; Mahgoub, S.A.; Hussein, M.M.; Saadeldin, I.M. Improving growth performance and health status of meat-type quail by supplementing the diet with black cumin cold-pressed oil as a natural alternative for antibiotics. Environ. Sci. Pollut. Res. Int. 2018, 25, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Abd El-Hack, M.E.; Mahgoub, S.A.; Saadeldin, I.M.; Swelum, A.A. Effects of clove (Syzygium aromaticum) oil on quail growth, carcass traits, blood components, meat quality, and intestinal microbiota. Poult. Sci. 2018, 98, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kishawy, A.T.; Amer, S.A.; Abd El-Hack, M.E.; Saadeldin, I.M.; Swelum, A.A. The impact of dietary linseed oil and pomegranate peel extract on broiler growth, carcass traits, serum lipid profile, and meat fatty acid, phenol, and flavonoid contents. Asian-Australas J. Anim. Sci. 2019, 32, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, S.A.M.; Abd El-Hack, M.E.; Saadeldin, I.M.; Hussein, M.A.; Swelum, A.A.; Alagawany, M. Impact of Rosmarinus officinalis cold-pressed oil on health, growth performance, intestinal bacterial populations, and immunocompetence of Japanese quail. Poult. Sci. 2019, 98, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, H. Banning antibiotic growth promoters: Learning from the European experience. Poultry Int. 2006, 10–12. [Google Scholar]
- Elgeddawy, S.A.; Shaheen, H.M.; El-Sayed, Y.S.; Abd Elaziz, M.; Darwish, A.; Samak, D.; Batiha, G.E.; Mady, R.A.; Bin-Jumah, M.; Allam, A.A.; et al. Effects of the dietary inclusion of a probiotic or prebiotic on florfenicol pharmacokinetic profile in broiler chicken. J. Anim. Physiol. Anim. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Mohamed, L.A.; El-Hindawy, M.M.; Alagawany, M.; Salah, A.S.; El-Sayed, S.A. Effect of low- or high-CP diet with cold-pressed oil supplementation on growth, immunity and antioxidant indices of growing quail. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1380–1387. [Google Scholar] [CrossRef]
- Reda, F.M.; Alagawany, M.; Mahmoud, H.K.; Mahgoub, S.A.; Elnesr, S.S. Use of red pepper oil in quail diets and its effect on performance, carcass measurements, intestinal microbiota, antioxidant indices, immunity and blood constituents. Animal 2019, 1–9. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R. The role of exogenous enzymes in promoting growth and improving nutrient digestibility in poultry. Iranian J. Vet. Res. 2018, 19, 157–164. [Google Scholar]
- Abd El-Hack, M.; Alagawany, M.; Farag, M.; Tiwari, R.; Karthik, K.; Dhama, K.; Zorriehzahra, J.; Milad, A. Beneficial impacts of thymol essential oil on health and production of animals, fish and poultry: A review. J. Essent. Oil Res. 2016, 28, 365–382. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Ayasan, T.; Abdel-Daim, M.M. Herbs as thermoregulatory agents in poultry: An overview. Sci. Total Environ. 2019, 134399. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Sachan, S.; Karthik, K.; Khama, K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018, 25, 10611–10618. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Marappan, G.; et al. Licorice (Glycyrrhiza glabra) herb as an eco-friendly additive to promote poultry health—Current Knowledge and Prospects. Animals 2019, 9, 536. [Google Scholar] [CrossRef]
- Gado, A.R.; Ellakany, H.F.; Elbestawy, A.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Mahgoub, S.A. Herbal medicine additives as powerful agents to control and prevent avian influenza virus in poultry–a review. Ann. Anim. Sci. 2019. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Ropy, A.; Abdel-Razik, A.H. Effect of dietary sodium butyrate supplementation on growth, blood biochemistry, haematology and histomorphometry of intestine and immune organs of Japanese quail. Animal 2019, 13, 1234–1244. [Google Scholar] [CrossRef]
- Kothari, D.; Lee, W.D.; Niu, K.M.; Kim, S.K. The Genus Allium as Poultry Feed Additive: A Review. Animals 2019, 9, 1032. [Google Scholar] [CrossRef]
- Zhang, G.F.; Yang, Z.B.; Wang, Y.; Yang, W.R.; Jiang, S.Z.; Gai, G.S. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 2009, 88, 2159–2166. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- Onu, P. Evaluation of two herbal spices as feed additives for finisher broilers. Biotechnol. Anim. Husb. 2010, 26, 383–392. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, Z.M. Studies of commonly used traditional medicine-ginger. Zhongguo Zhong Yao Za Zhi 2005, 30, 1569–1573. [Google Scholar] [PubMed]
- Khan, R.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Javdani, M.; Qureshi, M.; Laudadio, V. Potential applications of ginger (Zingiber officinale) in poultry diets. World’s Poult. Sci. J. 2012, 68, 245–252. [Google Scholar] [CrossRef]
- Asghar, A.; Farooq, M.; Mian, M.; Khurshid, A. Economics of broiler production in Mardan division. J. Rural Dev. Adm. 2000, 32, 56–65. [Google Scholar]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clinical Phytoscience 2019, 5, 6. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Kuttan, R. Antioxidant, anti-inflammatory and antinociceptive activities of essential oil from ginger. Indian J. Physiol. Pharmacol. 2013, 57, 51–62. [Google Scholar] [PubMed]
- Govindarajan, V. Ginger-Chemistry, Technology and Quality Evaluation: Part I-CRC Critical Reviews in Food Science and Nutrition. Quensland 1982, 17, 98. [Google Scholar]
- Singh, G.; Maurya, S.; Catalan, C.; De Lampasona, M.P. Studies on essential oils, Part 42: Chemical, antifungal, antioxidant and sprout suppressant studies on ginger essential oil and its oleoresin. Flavour Fragr. J. 2005, 20, 1–6. [Google Scholar] [CrossRef]
- Sharma, P.K.; Singh, V.; Ali, M. Chemical composition and antimicrobial activity of fresh rhizome essential oil of Zingiber officinale Roscoe. Pharmacogn. J. 2016, 8, 185–190. [Google Scholar] [CrossRef]
- Sasidharan, I.; Venugopal, V.V.; Menon, A.N. Essential oil composition of two unique ginger (Zingiber officinale Roscoe) cultivars from Sikkim. Nat. Prod. Res. 2012, 26, 1759–1764. [Google Scholar] [CrossRef]
- Bhattarai, K.; Pokharel, B.; Maharjan, S.; Adhikari, S. Chemical Constituents and Biological Activities of Ginger Rhizomes from Three Different Regions of Nepal. J. Nutri. Diet Probiotics. 2018, 1, 180005. [Google Scholar]
- Stoyanova, A.; Konakchiev, A.; Damyanova, S.; Stoilova, I.; Suu, P.T. Composition and antimicrobial activity of ginger essential oil from Vietnam. J. Essent. Oil-Bear. Plants. 2006, 9, 93–98. [Google Scholar] [CrossRef]
- El-Baroty, G.S.; El-Baky, H.A.; Farag, R.S.; Saleh, M.A. Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr. J. Biochem. Res. 2010, 4, 167–174. [Google Scholar]
- Dieumou, F.E.; Teguia, A.; Kuite, J.R.; Tamokou, J.D.; Fonge, B.N.; Dongmo, M.C. Effects of ginger (Z. officinale) and garlic (Allium sativum) essential oils on growth performance and gut microbial population of broiler chickens. Livestock Res. Rural Dev. 2009, 21, 23–32. [Google Scholar]
- Shanoon, A.K.; Jassim, M.S.; Amin, Q.H.; Ezaddin, I.N. Effects of Ginger (Zingiber officinale) Oil on Growth Performance and Microbial Population of Broiler Ross 308. Inter. J. Poultry Sci. 2012, 11, 589–593. [Google Scholar] [CrossRef]
- Herve, T.; Raphaël, K.J.; Ferdinand, N.; Vitrice, L.; Tiwa, F.; Gaye, A.; Moyo, N. 2018 Growth performance, serum biochemical profile, oxidative status, and fertility traits in male Japanese quail fed on ginger (Zingiber officinale, roscoe) essential oil. Vet. Med. Inter. 2018, 2018, 7682060. [Google Scholar] [CrossRef]
- Herve, T.; Raphaël, K.J.; Ferdinand, N.; Victor Herman, N.; Marvel, W.; Moyo, N.; Tiwa, F. Effects of Ginger (Zingiber officinale, Roscoe) Essential Oil on Growth and Laying Performances, Serum Metabolites, and Egg Yolk Antioxidant and Cholesterol Status in Laying Japanese Quail. J. Vet. Med. 2019, 2019, 7857504. [Google Scholar] [CrossRef]
- Habibi, R.; Sadeghi, G.H.; Karimi, A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br.Poultry Sci. 2014, 55, 228–237. [Google Scholar] [CrossRef]
- Mohamed, N.E.S. Response of Broiler Chicks to Diets Containing Mixture Garlic and Ginger Essential Oils as Natural Growth Promoter. Ph.D. Thesis, Sudan University for Science and Technology, Khartoum, Sudan, 2015. [Google Scholar]
- Abd El-khalek, E.; ELnaggar, A.S. Growth and physiological response of gimmizah chicks to dietary supplementation with ginger, black seeds, thyme and oregano oil as natural feed additives. Egypt. Poult. Sci. J. 2016, 36, 1163–1182. [Google Scholar]
- Ghasemi, H.A.; Taherpour, K. Comparison of broiler performance, blood biochemistry, hematology and immune response when feed diets were supplemented with ginger essential oils or mannan-oligosaccharide. Iranian J. Vet. Med. 2015, 9, 195–205. [Google Scholar]
- Tekeli, A.; Celik, L.; Kutlu, H.R.; Gorgulu, M. Effect of dietary supplemental plant extracts on performance, carcass characteristics, digestive system development, intestinal microflora and some blood parameters of broiler chicks. In Proceedings of the 12th European Poultry Conference, Verona, Italy, 10–14 September 2006; pp. 10–14. [Google Scholar]
- Osman, A.; El-Araby, G.M.; Taha, H. Potential use as a bio-preservative from lupin protein hydrolysate generated by alcalase in food system. J. Appl. Biol. Biotechnol. 2016, 4, 076–081. [Google Scholar]
- An, S.; Liu, G.; Guo, X.; An, Y.; Wang, R. Ginger extract enhances antioxidant ability and immunity of layers. Anim. Nutr. 2019, 5, 407–409. [Google Scholar] [CrossRef]
- Tchoffo, H.; Ngoula, F.; Kana, J.R.; Kenfack, A.; Ngoumtsop, V.H.; Vemo, N.B. Effects of ginger (Zingiber officinale) rhizomes essential oil on some reproductive parameters in laying Japanese quail (Coturnix coturnix japonica). Adv. Reprod. Sci. 2017, 5, 64–74. [Google Scholar] [CrossRef]
- Saleh, N.; Allam, T.; El-Latif, A.A.; Ghazy, E. The effects of dietary supplementation of different levels of thyme (Thymus vulgaris) and ginger (Zingiber officinale) essential oils on performance, hematological, biochemical and immunological parameters of broiler chickens. Global Vet. 2014, 12, 736–744. [Google Scholar]
- Al-Tahtawy, R.H.M.; El-Bastawesy, A.M.; Monem, M.A.; Zekry, Z.K.; Al-Mehdar, H.A.; El-Merzabani, M.M. Antioxidant activity of the volatile oils of Zingiber officinale (ginger). Spatula DD 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Natta, L.; Orapin, K.; Krittika, N.; Pantip, B. Essential oil from five Zingiberaceae for anti food-borne bacteria. Inter. Food Res. J. 2008, 15, 337–346. [Google Scholar]
- Majolo, C.; Nascimento, V.P.; Chagas, E.C.; Chaves, F.C.M. Antimicrobial activity of essential oil from Curcuma longa and Zingiber officinale rhizomes against enteric Salmonella isolated from chicken. Rev. bras. Plantas Med. 2014, 16, 505–512. [Google Scholar] [CrossRef]
- Qorbanpour, M.; Fahim, T.; Javandel, F.; Nosrati, M.; Paz, E.; Seidavi, A.; Ragni, M.; Laudadio, V.; Tufarelli, V. Effect of Dietary Ginger (Zingiber officinale Roscoe) and Multi-Strain Probiotic on Growth and Carcass Traits, Blood Biochemistry, Immune Responses and Intestinal Microflora in Broiler Chickens. Animals 2018, 8, 117. [Google Scholar] [CrossRef]
- Alizadeh-Navaei, R.; Roozbeh, F.; Saravi, M.; Pouramir, M.; Jalali, F.; Moghadamnia, A.A. Investigation of the effect of ginger on the lipid levels. A double blind controlled clinical trial. Saudi Med. J. 2008, 29, 1280–1284. [Google Scholar]
- Hong, J.C.; Steiner, T.; Aufy, A.; Lien, T.F. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest. Sci. 2012, 144, 253–262. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Baliga, M.S.; Venkatesh, P.; Ulloor, J.N. Influence of ginger rhizome (Zingiber officinale Rosc) on survival, glutathione and lipid peroxidation in mice after whole-body exposure to gamma radiation. Radiat. Res. 2003, 160, 584–592. [Google Scholar] [CrossRef]
- Shewita, R.S.; Taha, A.E. Influence of dietary supplementation of ginger powder at different levels on growth performance, haematological profiles, slaughter traits and gut morphometry of broiler chickens. South Afr. J. Anim. Sci. 2018, 48, 997–1008. [Google Scholar] [CrossRef]
- Azhir, D.; Zakeri, A.; Rezapour, A.K. Effect of ginger powder rhizome on humeral immunity of broiler chickens. Eur. J. Exp. Biol. 2012, 2, 2090–2092. [Google Scholar]
- Arkan, B.M.; Mohammed, A.M.; Ali, J. Effect of ginger (Zingiber officinale) on performance and blood serum parameters of broilers. Int. J. Poult. Sci. 2012, 91, 143–146. [Google Scholar]
- Zhao, X.; Yang, Z.B.; Yang, W.R.; Wang, Y.; Jiang, S.Z.; Zhang, G.G. Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poult. Sci. 2011, 90, 1720–1727. [Google Scholar] [CrossRef]
- Naveena, B.M.; Mendiratta, S.K. Tenderisation of spent hen meat using ginger extract. Br. Poult. Sci. 2001, 42, 344–349. [Google Scholar] [CrossRef]
- Attia, Y.A.; Saber, S.H.; Abd-El-Hamid, E.A.; Radwan, M.W. Response of broiler chickens to dietary supplementation of ginger (Zingiber officinale) continuously or intermittently in comparison with prebiotics. Egypt. Poult. Sci. 2017, 37, 523–543. [Google Scholar]
- Herawati; Marjuki. The effect of feeding red ginger (Zingiber officinale Rosc.) as phytobiotic on broiler slaughter weight and meat quality. Inter. J. Poult. Sci. 2011, 10, 983–985. [Google Scholar] [CrossRef]
- Wen, C.; Gu, Y.; Tao, Z.; Cheng, Z.; Wang, T.; Zhou, Y. Effects of Ginger Extract on Laying Performance, Egg Quality, and Antioxidant Status of Laying Hens. Animals 2019, 9, 857. [Google Scholar] [CrossRef]
- Yang, C.W.; Ding, X.; Zhao, X.; Guo, Y.X.; Mu, A.L.; Yang, Z.B. Effects of star anise (Illicium verum Hook. f.), salvia miltiorrhiza (Salvia miltiorrhiza Bge) and ginger root (Zingiber officinale Roscoe) on laying performance, antioxidant status and egg quality of laying hens. Europ. Poult. Sci. 2017, 81. [Google Scholar] [CrossRef]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Yao, L.; Ma, P.; Chen, Z.; Han, T.L.; Yuan, C.; Zhang, J.; Jiang, L.; Liu, L.; et al. 6-gingerol ameliorates age-related hepatic steatosis: Association with regulating lipogenesis, fatty acid oxidation, oxidative stress and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2019, 362, 125–135. [Google Scholar] [CrossRef]
- Gurbuz, Y.; Salih, Y.G. Influence of sumac (Rhus Coriaria L.) and ginger (Zingiber officinale) on egg yolk fatty acid, cholesterol and blood parameters in laying hens. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1316–1323. [Google Scholar] [CrossRef]
- Abdollahi, A.M.; Virtanen, H.E.K.; Voutilainen, S.; Kurl, S.; Tuomainen, T.P.; Salonen, J.T.; Virtanen, J.K. Egg consumption, cholesterol intake, and risk of incident stroke in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 110, 169–176. [Google Scholar] [CrossRef]
- Oleforuh-Okoleh, V.U.; Chukwu, G.C.; Adeolu, A.I. Effect of ground ginger and garlic on the growth performance, carcass quality and economics of production of broiler chickens. Glob. J. Bio-Dcience Biotechnol. 2014, 3, 225–229. [Google Scholar]
- Karangiya, V.K.; Savsani, H.H.; Patil, S.S.; Garg, D.D.; Murthy, K.S.; Ribadiya, N.K.; Vekariya, S.J. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Veterinary World 2016, 9, 245–250. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Yusuf, M. Evaluation of ginger (Zingiber officinale) as a feed additive in broiler diets. Livest. Res. Rural Dev. 2011, 23, 202. Available online: http://www.lrrd.org/lrrd23/9/moha23202.htm (accessed on 4 November 2015).



| Forms and Doses | Results | Author/s |
|---|---|---|
| Ginger oil (100 µl/kg body weight (BW)) | Weight of eggs clearly improved in Japanese quails orally administered with ginger root extract | [36] |
| Ginger roots extracted basic oil (100 or 150 µl/kg BW) | The serum transaminases (alanine transaminase and aspartate transaminases), total cholesterol and LDL-cholesterol were markedly decreased | [36] |
| Ginger oil (40 mg ginger oil/kg/day) | By increasing doses of oil, Escherichia coli or other Enterobacteria (Salmonella and Shigella) populations in the intestinal contents significantly dropped when compared with control data | [35] |
| Mannan-oligosaccharide plus ginger essential oil (200 mg/kg diet) mixture | The birds nourished on this mixture showed an improved body weight gain (BWG) from the first to 42 days of age when compared with control data. | [41] |
| Basal diet + 120 mg, Zingiber officinale essential oil/kg | Z. officinale improved the BWG in comparison with control and other treated groups. | [42] |
| Ginger oil (0, 0.25%, 0.50% and 0.75%) | Egg mass clearly advanced in Japanese quails exposed to ginger roots extracted basic oil without concerning the level of the dose with special attention to the control data. | [43] |
| Oral application of 100–150 µl/kg BW of ginger roots oil. | Use of ginger root oil in laying Japanese quails recorded the highest outcomes on egg mass and lowered egg and serum cholesterol levels without any harmful factors on feed uptake and BW profits. | [36] |
| Ginger oil (100 and 150 µl/kg BW) | This oil caused a clear advancement in fertility ratio in comparison to the control outcomes. | [36] |
| Ginger oil (100 and 150 µl/kg BW) | Fertility, hatchability and chick’s weight were improved in birds treated by ginger oil. Embryonic deaths dropped clearly with any level of the ginger rhizomes essential oil dose. The values of serum total proteins, estradiol, follicle stimulating hormone and luteinizing hormone were greatly improved in a dose-associated pattern. | [45] |
| 0.1% ginger extract | Ginger extract clearly advanced laying rates with daily egg weight | [44] |
| Ginger oil (100 and 150 l/kg BW) | Serum contents of malondialdehyde (MDA), triglycerides and total cholesterol serum features were decreased. Total protein, globulin and antioxidant enzymes were elevated. | [36] |
| Ginger oil at doses of 100, 200 and 300 mg/kg BW. | Ginger oil at dose of 100 mg/kg BW improved serum lipid profile. | [46] |
| 150 mg/kg BW ginger extracted basic oil | Superoxide dismutase (SOD) in hepatic tissue was elevated by ginger compared to control data. MDA values in hepatic tissue were diminished in the groups feeding ginger powder oil. | [38] |
| Ginger extraction | Ginger extract clearly lessened prostaglandin E2 (PGE2) value. | [44] |
| Ginger essential oils (100 mg/kg) | Serum cholesterol values were diminished in the ginger essential oil and mannan-oligosaccharide supplemented diet. | [41] |
| Z. officinale | Blood glucose values were advanced by Z. officinale, at the same time blood triglyceride values was elevated by both Z. officinale and S. aromaticum dietary treatments. | [42] |
| Ginger essential oils. | Escherichia coli populations in the ileo-cæcal contents quantitatively diminished in comparison with the control data in response to increased levels of ginger essential oils. | [34] |
| Ginger essential oil | Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa and fungal (Aspergillus niger and Candida albicans) populations were decreased with ginger oil supplementation. | [29] |
| 8% ginger extraction from fresh ginger and the residue ginger after essential oil extraction. | Staphylococcus aureus, Bacillus subtilis, Klebsiella spp and Escherichia coli were decreased with ginger oil supplementation. | [31,49] |
| Ginger extracted oil | Ginger extracted oil could show a significant suppressing action against some picked bacterial strains. | [33] |
| Ginger oil for 30 days | Oral application of ginger oil for at least 30 days, critically improved glutathione reductase, glutathione and SOD values. | [26] |
| Ginger powder (0.15, 0.20 and 0.25%) | Antibody titre was higher in birds fed 0.25% ginger than other rations after seven days post injection. The counts of Lactobacillus in ileal content of birds fed 0.20 and 0.25% ginger were higher in comparison with the other treatments. | [50] |
| Ginger powder (receiving ginger capsules 3 g/day in 3 divided doses) | Supplementation of ginger powder significantly decreased the levels of total cholesterol, triglycerides, low density lipoprotein (LDL) and very-low density lipoprotein (VLDL). | [51] |
| Ginger essential oil (125 ppm) | In broiler chickens receiving ginger essential oil greater high density lipoprotein (HDL) and lower VLDL levels, whereas no significant difference was observed in LDL concentration. Ammonia concentration in ileum was the lowest in broiler fed with essential oil supplementation. | [52] |
| Ginger extract | Ginger extract enhances the serological response and had an antioxidant activity (both in vitro and in vivo) mainly attributed to pungent active principles like shogaols and gingerols. | [22] |
| Ginger rhizome (10 mg/kg) | In vitro, ginger extract showed antibacterial activity against Salmonella typhimurium, Pseudomonas aeruginosa, Candida albicans and Escherichia coli. | [53] |
| Basal diet plus 2 g/kg, 4 g/kg and 6 g/kg ginger powder | Ginger powder increased hemagglutination inhibition (HI) titre against Newcastle Disease virus. Also, ginger powder at 6 g/kg increased the leucocytes count and serum total protein, but decreased cholesterol and high-density lipoprotein (HDL) levels. | [54] |
| Aqueous extracts of ginger | Aqueous extract of ginger improved performance and plays an immune stimulant against Newcastle Disease. | [55] |
| 0.1% and 0.2% ginger | Birds fed 0.1% and 0.2% ginger had better feed conversion ratio | [56] |
| Ginger powder (5, 10, 15, or 20 g/kg of diet) for 10 weeks. | Dietary supplementation of ginger powder at 15 or 20 g/kg enhanced performance and egg yolk and serum antioxidant status and improved dietary oxidation stability in a dose-dependent manner in laying hens. | [57] |
| Ginger powder (particle size of 300 µm) at the rate of 5 g/kg. | Ginger powder increased activities of SOD and glutathione peroxidase and reduced MDA content in the serum of birds. | [18] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Hack, M.E.; Alagawany, M.; Shaheen, H.; Samak, D.; Othman, S.I.; Allam, A.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Osman, A.; et al. Ginger and Its Derivatives as Promising Alternatives to Antibiotics in Poultry Feed. Animals 2020, 10, 452. https://doi.org/10.3390/ani10030452
Abd El-Hack ME, Alagawany M, Shaheen H, Samak D, Othman SI, Allam AA, Taha AE, Khafaga AF, Arif M, Osman A, et al. Ginger and Its Derivatives as Promising Alternatives to Antibiotics in Poultry Feed. Animals. 2020; 10(3):452. https://doi.org/10.3390/ani10030452
Chicago/Turabian StyleAbd El-Hack, Mohamed E., Mahmoud Alagawany, Hazem Shaheen, Dalia Samak, Sarah I. Othman, Ahmed A. Allam, Ayman E. Taha, Asmaa F. Khafaga, Muhammad Arif, Ali Osman, and et al. 2020. "Ginger and Its Derivatives as Promising Alternatives to Antibiotics in Poultry Feed" Animals 10, no. 3: 452. https://doi.org/10.3390/ani10030452
APA StyleAbd El-Hack, M. E., Alagawany, M., Shaheen, H., Samak, D., Othman, S. I., Allam, A. A., Taha, A. E., Khafaga, A. F., Arif, M., Osman, A., El Sheikh, A. I., Elnesr, S. S., & Sitohy, M. (2020). Ginger and Its Derivatives as Promising Alternatives to Antibiotics in Poultry Feed. Animals, 10(3), 452. https://doi.org/10.3390/ani10030452