Investigation of Pre- and Post-Weaning Mortalities in Rabbits Bred in Egypt, with Reference to Parasitic and Bacterial Causes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Ethics
2.2. Period of the Study
2.3. Rabbit Husbandry and Management
2.4. Recording of Production Data
2.5. Reporting Causes of Pre-and Postweaning Mortalities
2.5.1. Preweaning Stage
2.5.2. Postweaning Stage
2.6. Investigation of Pathogenic Causes of Pre-and Postweaning Mortalities in Rabbits
2.6.1. Parasitological Examination of Fecal Samples
2.6.2. Mange Examination
2.6.3. Bacteriological Examination
2.7. Statistical Analysis
3. Results
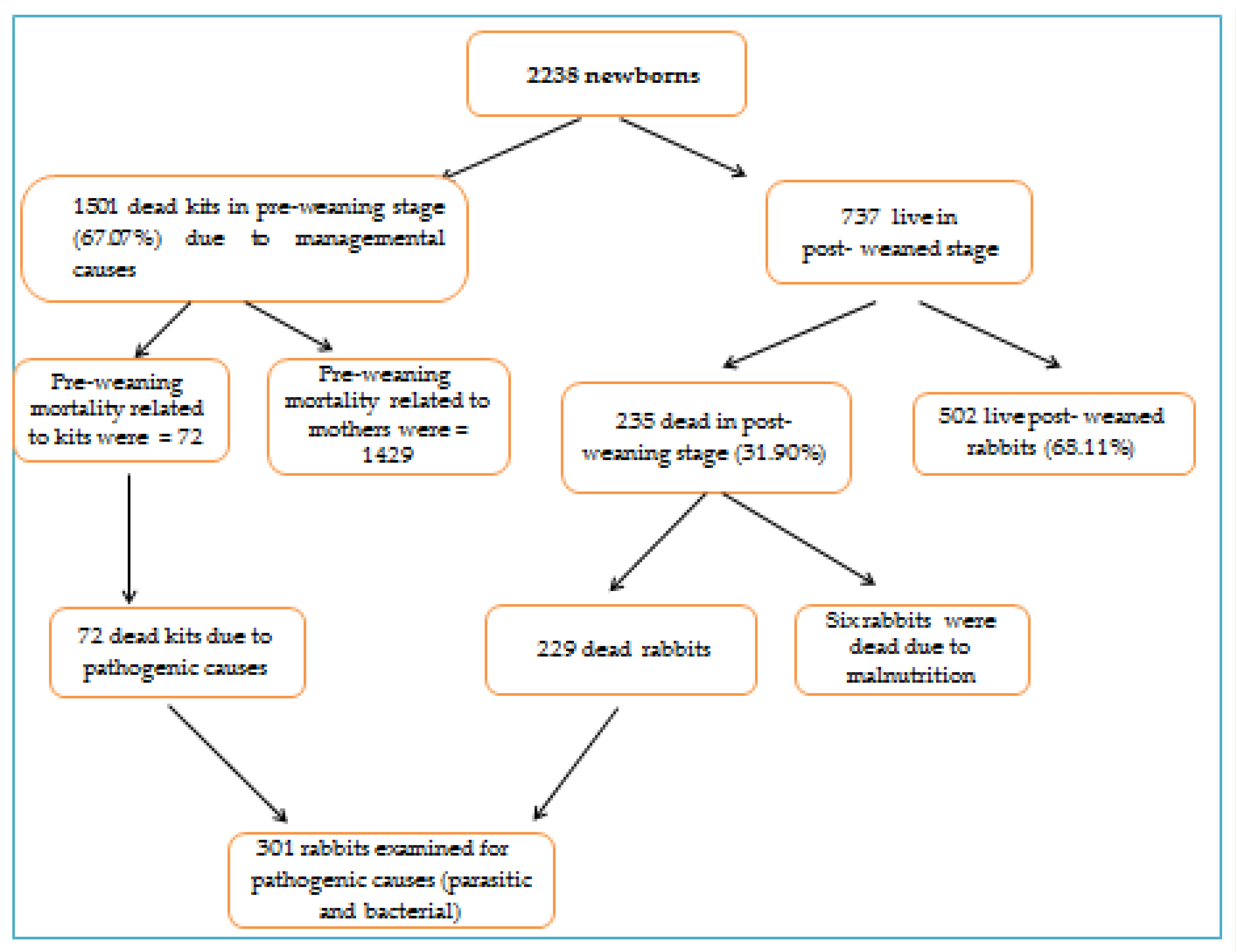
3.1. Mortality Rates
3.2. Managemental Causes of Preweaning Mortality
3.3. Pathogenic Causes of Preweaning Mortality in Rabbits
Bacteriological Causes of Preweaning Mortality
3.4. Seasonal Prevalence of Preweaning Mortality
3.5. Managemental Causes of Postweaning Mortality
3.6. Pathogenic Causes of Postweaning Mortality in Rabbits
3.6.1. Parasitological Findings of Postweaning Mortality
3.6.2. Bacteriological Causes of Postweaning Mortality
3.7. PostMortem Lesions of Postweaning Mortality
3.8. Seasonal Prevalence of Postweaning Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rashwan, A.A.; Ahmed, S.A. Growing rabbit management, housing system, reduction of eating time and feeder space. In Proceedings of the 6th World Rabbit Congress, Toulouse, France, 9–12 July 1996. [Google Scholar]
- Millar, J.S. Nest mortality in small mammals. Ecoscience 2007, 14, 286–291. [Google Scholar] [CrossRef]
- Heppell, S.S.; Caswell, H.; Crowder, L.B. Life histories and elasticity patterns: Perturbation analysis for species with minimal demographic data. Ecology 2000, 81, 654–665. [Google Scholar] [CrossRef]
- Oli, M.K.; Dobson, F.S. The relative importance of life-history variables to population growth rate in mammals: Cole’s prediction revisited. Am. Nat. 2003, 161, 422–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licois, D. Domestic rabbit enteropathogens. In Proceedings of the 8th World Rabbit Congress, Puebla, Mexico, 7–10 September 2004. [Google Scholar]
- El-Ashram, A.S.; Aboelhadid, S.M.; Abdel-Kafy, E.M.; Hashem, S.A.; Mahrous, L.N.; Farghly, E.M.; Moawad, U.K.; Kamel, A.A. Prophylactic and Therapeutic Efficacy of Prebiotic Supplementation against Intestinal Coccidiosis in Rabbits. Animals 2019, 9, 965. [Google Scholar] [CrossRef] [Green Version]
- Pakandl, M. Coccidia of rabbit: A review. Folia Parasitol. 2009, 56, 153–166. [Google Scholar] [CrossRef]
- Darzi, M.M.; Mir, M.S.; Shahardar, R.A.; Pandit, B.A. Clinicopathological, histochemical and therapeutic studies on concurrent sarcoptic and notoedric acariosis in rabbits (Orytolagus cuniculus). Vet. Orhiv. 2007, 77, 167–175. [Google Scholar]
- Aboelhadid, S.M.; Mahrous, L.N.; Hashem, S.A.; Abdel-Kafy, E.M.; Miller, R.J. In vitro and in vivo effect of Citrus limon essential oil against sarcoptic mange in rabbits. Parasitol. Res. 2016, 115, 3013–3020. [Google Scholar] [CrossRef]
- Bornstein, S.M.T.; Samuel, W.H. Parasitic Diseases of Wild Mammals; Iowa state University Press: Ames, IA, USA, 2001; pp. 107–119. [Google Scholar]
- Tameem Eldar, A.A.; Elamin, K.M.; Yousif, I.A.; Hassan, H.E.; Musa, A.M. Growth and Mortality in Pre-and Post-Weaning Rearing Periods for Sudanese Local Rabbits. J. Anim. Prod. Adv. 2012, 2, 214–220. [Google Scholar]
- Soulsby, E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals, 7th ed.; Baillere Tindall: London, UK, 1986; pp. 593–614. [Google Scholar]
- Zajac, A.M.; Conboy, G.A. Veterinary Clinical Parasitology; Blackwell Publishing: Malden, MA, USA, 2006. [Google Scholar]
- Huang, G.; Zhang, S.; Zhou, C.; Tang, X.; Li, C.; Wang, C.; Tang, X.; Suo, J.; Jia, Y.; El-Ashram, S.; et al. Influence of Eimeria falciformis infection on Gut Microbiota and Metabolic Pathways in Mice. Infect. Immun. 2018, 86, e00073-18. [Google Scholar] [CrossRef] [Green Version]
- Fichi, G.; Flamini, G.; Zaralli, L.; Perrucci, S. Efficacy of an essential oil of Cinnamomum Zeylanicum against Psoroptes cunicul. Phytomedicine 2007, 14, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinate Bacteriology, 9th ed.; Williams and Wilkins: Baltimore, NY, USA, 1994. [Google Scholar]
- ISO 6888-2:1999. International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and other species) e Part 2: Technique Using Rabbit Plasma Fibrinogen Agar Medium; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- ISO 6579:2002. International Organization for Standardization. Horizontal Method for the Detection of Salmonella spp. Microbiology of Food and Animal Feeding Stuffs; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Lee, M.; Arp, L. Colibacillosis. In A Laboratory Manual for the Isolation and Identification of Avian Pathogens, 4th ed.; Swayne, D.E., Ed.; American Association of Avian Pathologists: Jacksonville, FL, USA, 1998; pp. 14–16. [Google Scholar]
- Soumet, C.; Ermel, G.; Rose, V.; Rose, N.; Drouin, P.; Salvat, G.; Colin, P. Identification by a multiplex PCR-based assay of Salmonella typhimurium and Salmonella enteritidis strains from environmental swabs of poultry houses. Lett. Appl. Microbiol. 1999, 29, 1–6. [Google Scholar] [CrossRef]
- Ahmed, S.; Marai, I.F.M. Milk yield and litter traits of rabbits as affected by breed, parity, number of nipples, and aminazine injection, under Egyptian sub-tropical conditions. In Proceedings of the First International Conference for Animal Production and Health in Semi-Arid Area, El-Arish, Egypt, 5–9 December 1998. [Google Scholar]
- Karu, P.; Muthusamy, P.; Gopi, H.; Balasubramanyam, D.; Babu, M. Survivability in New Zealand White breed of rabbits under farming condition in Tamilnadu. Int. Sci. Environ. Technol. 2014, 3, 1772–1777. [Google Scholar]
- Mohammed, H.A.; Eid, A.A.M.; El-Bakrey, R.M.M. A review of rabbit diseases in Egypt. Wartazoa 2013, 23, 185–194. [Google Scholar] [CrossRef]
- Drummond, H.; Vázquez, E.; Sánchez–Colón, S.; Martinez–Gómez, M.; Hudson, R. Competition for milk in the domestic rabbit: Survivors benefit from littermate deaths. Ethology 2000, 106, 511–526. [Google Scholar] [CrossRef]
- Poigner, J.; Szendrộ, Z.; Lèvai, A.; Radnai, I.; Birớ-Nemeth, E. Effect of birth weight and litter size at suckling age on reproductive performance in does as adults. World Rabbit Sci. 2000, 8, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Castellini, C.; Dal Bosco, A.; Mugnai, C. Comparison of different reproduction protocol for rabbit does: Effect of rabbit production in tropical and sub-tropical agricultural system. J. Amin. Sci. 2003, 63, 1581. [Google Scholar]
- Bautista, A.; Mendoza-Degante, M.; Coureaud, G.; Martinez-Gómez, M.; Hudson, R. Scramble competition in newborn domestic rabbits for an unusually restricted milk supply. Animal Behav. 2005, 70, 1011–1021. [Google Scholar] [CrossRef]
- Hudson, R.; Trillmich, F. Sibling competition and cooperation in mammals: Challenges, developments and prospects. Behav. Ecol. Sociobiol. 2008, 62, 299–307. [Google Scholar] [CrossRef]
- Gotz, A.A.; Wolf, M.; Stefanski, V. Psychosocial maternal stress during pregnancy: Effects on reproduction for F0 and F1 generation laboratory rats. Physiol. Behav. 2008, 93, 1055–1060. [Google Scholar] [CrossRef]
- Rodel, H.G.; Prager, G.; Stefanski, V.; von Holst, D.; Hudson, R. Separating maternal and litter size effects on early postnatal growth in two species of altricial mammals. Physiol. Behav. 2008, 93, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.S.; Sharma, S.R.; Singh, U.; Kumar, D.; Bhasin, V. Effect of different season on the performance of grey giant rabbits under sub-temperate Himalayan conditions. Asian-Australian J. Anim. Sci. 2002, 15, 812–820. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, U.; Bhatt, R.S.; Risam, K.S. Reproductive efficiency of female German Angola under Indian sub- temperate climatic conditions. World Rabbit Sci. 2005, 13, 113–122. [Google Scholar]
- Rodel, H.G.; Starkloff, A.; Bautista, A.; Friedrich, A.C.; von Holst, D. Infanticide and maternal offspring defence in European rabbits under natural breeding conditions. Ethology 2008, 114, 22–31. [Google Scholar] [CrossRef]
- Rashwan, A.A.; Marai, I.F.M. Mortality in young rabbits: A review. World Rabbit Sci. 2000, 8, 111–124. [Google Scholar]
- Rashed, E.A.O. Survey on Parasitic Diseases of Rabbits. Master Thesis, Zagazig University, Zagazig, Egypt, 1993. [Google Scholar]
- Dagleish, M.P.; Ali, Q.; Powell, R.K.; Butz, D.; Woodford, M.H. Fetal Sarcoptes scabiei infection of blue sheep (Pseudoisnayaur) in Pakistan. J. Wildl. Dis. 2007, 43, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonanno, A.; Mazza, F.; Di Grigoli, A.; Alicata, M.L. Body condition score and related productive responses in rabbit does. In Proceedings of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008. [Google Scholar]
- Rodel, H.G.; Starkloff, A.; Seltmann, M.W.; Prager, G.; von Holst, D. Causes and predictors of nest mortality in a European rabbit population. Mamm. Biol. 2009, 74, 198–209. [Google Scholar] [CrossRef]
- EL-Zemity, S.R.; Rezk, H.A.; Zaitoon, A.A. Acaricidal activity of some essential oils and their monoterpenoidal constituents against the parasitic Bee mites Varroa destructor (Acari: Varroidae). J. Appl. Sci. Res. 2006, 2, 1032–1036. [Google Scholar]
- Rosell, J.M.; de la Fuente, L.F. Culling and mortality in breeding rabbits. Prev. Vet. Med. 2009, 88, 120–127. [Google Scholar] [CrossRef]
- Sánchez, J.P.; de la Fuente, L.F.; Rosell, J.M. Health and body condition of lactating females on rabbit farms. J. Anim. Sci. 2012, 90, 2353–2361. [Google Scholar] [CrossRef]
- Eid, A.A.M.; Ibraheem, O.K. Sudden death among rabbits in Sharkia Province, Egypt. Zagazig Vet. J. 2006, 34, 108–119. [Google Scholar]
- Peeters, J.E. Recent advances in intestinal pathology of rabbits and further prespectives. In Proceedings of the 4th World Rabbit Congress, Toulouse, France, 9–12 July 1996. [Google Scholar]
- Shahin, A.M.; Lebdah, M.A.; Ali, G.R.M. Escherichia coli as an etiological agent of mucoid enteropathy in rabbits. Researcher 2011, 3, 8–16. [Google Scholar]
- Habeeb, A.A.M.; Marai, I.F.M.; El-Maghawry, A.M.; Gad, A.A. Growth rabbits as effected by salinity in drinking water under winter and hot summer conditions of Egypt. Egyptian J. Rabbit Sci. 1997, 7, 81–94. [Google Scholar]
- Shehata, A.S.; Sarhan, M.A.; El-Gendy, K.M. Digestibility, thyroid function and growth performance of New Zealand White rabbits as affected by season of the year and age. Egyptian J. Rabbit Sci. 1998, 8, 141–156. [Google Scholar]
- Fortun-Lamothe, L. Energy balance and reproductive performance in rabbit does. Anim. Reprod. Sci. 2006, 93, 1–15. [Google Scholar] [CrossRef] [PubMed]


| Causes of Kit’s Death | Mortality Related to Mothers | Mortality Related to Kits | SEM | p * |
|---|---|---|---|---|
| Insufficient milk supply | 561(37.37%) | 0 | 1.109 | 0.000 |
| Infanticidal does (cannibalism) | 396 (26.38%) | 0 | ||
| Mange infested does | 333 (22.20%) | 0 | ||
| Mastitis | 64 (4.30%) | 0 | ||
| Lack of mothering | 75 (5.00%) | 0 | ||
| Bronchopneumonia in litters | 0 | 32 (2.13%) | ||
| Enteritis in litters with diarrhea | 0 | 27 (1.80%) | ||
| Sudden death in litters | 0 | 13 (0.90%) | ||
| Total | 1429 (95.23%) | 72 (4.83%) |
| Causes of Kit’s Death. | Total No. of Dead Kits | Autumn | % | Winter | % | Spring | % | SEM | p * |
|---|---|---|---|---|---|---|---|---|---|
| Insufficient milk supply | 561 | 149 | 26.6 | 254 | 45.28 | 158 | 28.16 | 0.751 | 0.000 |
| Mange infested does | 333 | 213 | 63.96 | 48 | 14.41 | 72 | 21.62 | ||
| Infanticidal does | 396 | 47 | 11.87 | 201 | 50.76 | 148 | 37.37 | ||
| Mastitis in lactating females | 64 | - | 0 | 18 | 28.1 | 46 | 71.9 | ||
| Lack of mothering | 75 | 65 | 86.7 | - | 0 | 10 | 13.3 | ||
| Bronchopneumonia | 32 | 5 | 15.6 | 15 | 46.9 | 12 | 37.5 | ||
| Enteritis | 27 | 7 | 25.9 | 6 | 22.2 | 14 | 51.9 | ||
| Sudden death | 13 | 3 | 23 | 6 | 46.15 | 4 | 30.8 | ||
| Total | 1501 | 489 | 32.6 | 548 | 36.5 | 464 | 30.9 |
| Causes of Death | Number of Dead Kits/737 | % | SEM | p * |
|---|---|---|---|---|
| Sudden death with Septicemia | 103 | 13.80 | 0.725 | 0.000 |
| Diarrhea (enteritis) | 71 | 9.63 | ||
| Bronchopneumonia | 40 | 5.43 | ||
| Mange infestation | 15 | 2.04 | ||
| Malnutrition | 6 | 0.81 | ||
| Total | 235 | 31.90 |
| Pathogenic Agents | Postweaning Mortality (n = 229) | Prevalence % | SEM | p * |
|---|---|---|---|---|
| Eimeria spp. Oocysts | 198 | 86.50 | 1.26 | 0.000 |
| Cittotaenia spp. Eggs | 10 | 4.37 | ||
| Sarcoptes scabiei | 15 | 6.55 | ||
| E. coli | 229 | 100 | ||
| Salmonella | 100 | 43.67 | ||
| Eimeria spp. + E. coli | 198 | 86.50 | ||
| Eimeria spp. + Salmonella | 100 | 43.67 |
| Season | Postweaning Mortality | % | SEM | p * |
|---|---|---|---|---|
| Autumn | 31 | 13.2 | 0.638 | 0.008 |
| Winter | 89 | 37.9 | ||
| Spring | 115 | 48.9 | ||
| Total | 235 | 31.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ashram, S.; Aboelhadid, S.M.; Abdel-Kafy, E.-S.M.; Hashem, S.A.; Mahrous, L.N.; Farghly, E.M.; Kamel, A.A. Investigation of Pre- and Post-Weaning Mortalities in Rabbits Bred in Egypt, with Reference to Parasitic and Bacterial Causes. Animals 2020, 10, 537. https://doi.org/10.3390/ani10030537
El-Ashram S, Aboelhadid SM, Abdel-Kafy E-SM, Hashem SA, Mahrous LN, Farghly EM, Kamel AA. Investigation of Pre- and Post-Weaning Mortalities in Rabbits Bred in Egypt, with Reference to Parasitic and Bacterial Causes. Animals. 2020; 10(3):537. https://doi.org/10.3390/ani10030537
Chicago/Turabian StyleEl-Ashram, Saeed, Shawky M. Aboelhadid, El-Sayed M. Abdel-Kafy, Shymaa A. Hashem, Lilian N. Mahrous, Eman M. Farghly, and Asmaa A. Kamel. 2020. "Investigation of Pre- and Post-Weaning Mortalities in Rabbits Bred in Egypt, with Reference to Parasitic and Bacterial Causes" Animals 10, no. 3: 537. https://doi.org/10.3390/ani10030537
APA StyleEl-Ashram, S., Aboelhadid, S. M., Abdel-Kafy, E.-S. M., Hashem, S. A., Mahrous, L. N., Farghly, E. M., & Kamel, A. A. (2020). Investigation of Pre- and Post-Weaning Mortalities in Rabbits Bred in Egypt, with Reference to Parasitic and Bacterial Causes. Animals, 10(3), 537. https://doi.org/10.3390/ani10030537






