High Salt Diet Affects the Reproductive Health in Animals: An Overview
Abstract
Simple summary
Abstract
1. Introduction
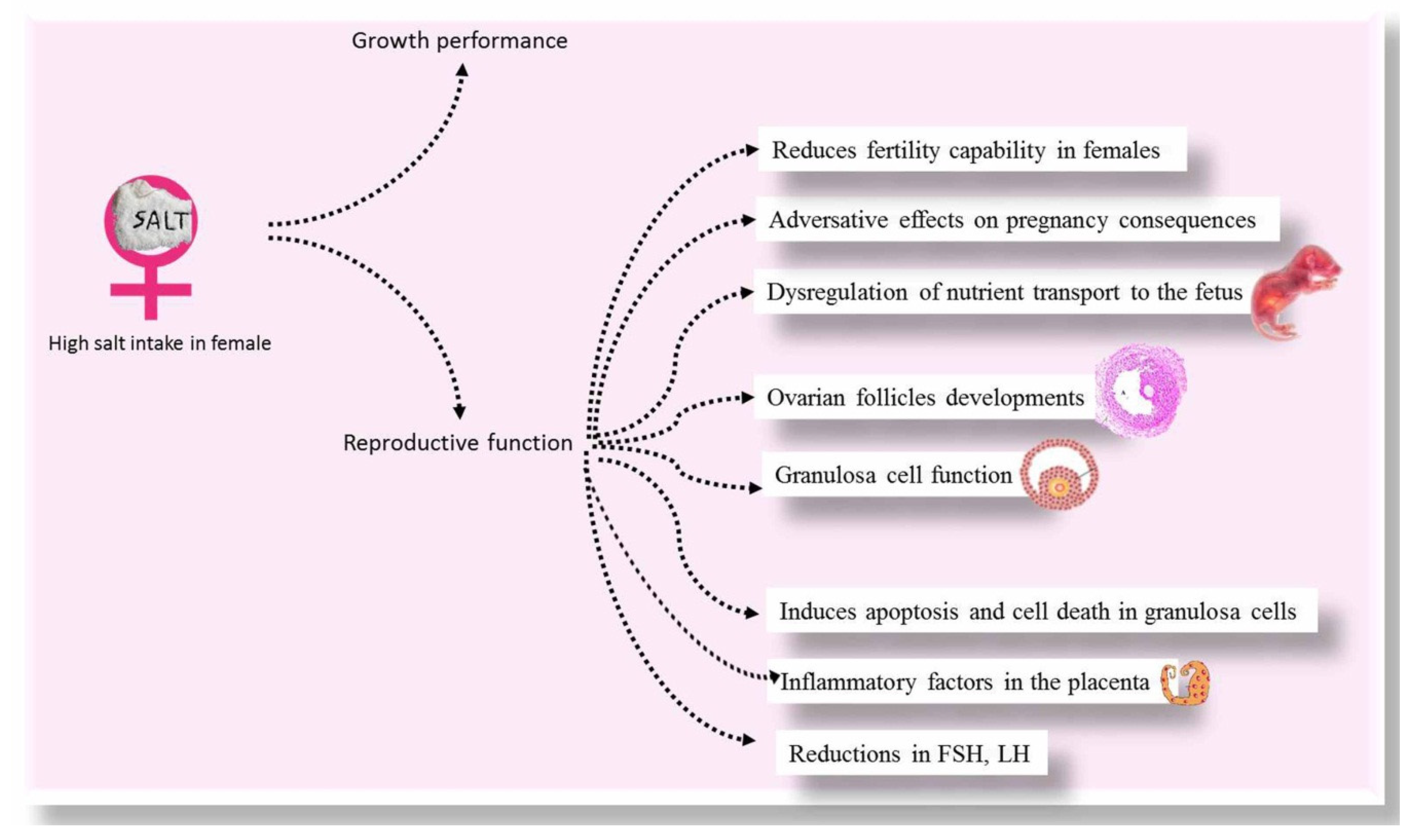
2. Growth Performance
3. Reproductive Variables in Males
3.1. Reproductive Organ Weight
3.2. Sperm Function
3.3. Hormones
3.4. Antioxidant Indices
3.5. Gene Expression
4. Female Reproductive Function
4.1. Ovarian Follicles
4.2. Placental Indices
4.3. Granulosa Cells
4.4. Hormones
5. Consequences of In Vitro Fertilization
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abd El-Hack, M.E.; Alagawany, M.; Arif, M.; Emam, M.; Saeed, M.; Arain, M.A.; Siyal, F.A.; Patra, A.; Elnesr, S.S.; Khan, R.U. The uses of microbial phytase as a feed additive in poultry nutrition—A review. Ann. Anim. Sci. 2018, 18, 639–658. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Samak, D.H.; Noreldin, A.E.; Arif, M.; Yaqoob, H.S.; Swelum, A.A. Towards saving freshwater: Halophytes as unconventional feedstuffs in livestock feed: A review. Environ. Sci. Pollut. Res. 2018, 25, 14397–14406. [Google Scholar] [CrossRef]
- Fang, Y.; Zhong, R.; Sun, X.; Zhou, D. High salt diet decreases reproductive performance in rams and down-regulates gene expression of some components of the renin-angiotensin system in the testis. Theriogenology 2018, 107, 127–133. [Google Scholar] [CrossRef]
- Digby, S.; Masters, D.; Blache, D.; Hynd, P.; Revell, D. Offspring born to ewes fed high salt during pregnancy have altered responses to oral salt loads. Animal 2010, 4, 81–88. [Google Scholar] [CrossRef]
- Ohta, Y.; Tsuchihashi, T.; Kiyohara, K.; Oniki, H. High salt intake promotes a decline in renal function in hypertensive patients: A 10-year observational study. Hypertens. Res. 2013, 36, 172. [Google Scholar] [CrossRef]
- Cohen, A.; Roe, F. Review of risk factors for osteoporosis with particular reference to a possible aetiological role of dietary salt. Food Chem. Toxicol. 2000, 38, 237–253. [Google Scholar] [CrossRef]
- Wang, G.; Yeung, C.-K.; Zhang, J.-L.; Hu, X.-W.; Ye, Y.-X.; Yang, Y.-X.; Li, J.-C.; Lee, K.K.H.; Yang, X.; Wang, L.-J. High salt intake negatively impacts ovarian follicle development. Ann. Anat. Anat. Anz. 2015, 200, 79–87. [Google Scholar] [CrossRef]
- Damasio, P.C.; Amaro, C.R.; Cunha, N.B.; Pichutte, A.C.; Goldberg, J.; Padovani, C.R.; Amaro, J.L. The role of salt abuse on risk for hypercalciuria. Nutr. J. 2011, 10, 3. [Google Scholar]
- Faraco, G.; Brea, D.; Garcia-Bonilla, L.; Wang, G.; Racchumi, G.; Chang, H.; Buendia, I.; Santisteban, M.M.; Segarra, S.G.; Koizumi, K. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 2018, 21, 240. [Google Scholar] [CrossRef]
- Aguiar, S.L.F.; Miranda, M.C.G.; Guimarães, M.A.F.; Santiago, H.C.; Queiroz, C.P.; Cunha, P.d.S.; Cara, D.C.; Foureaux, G.; Ferreira, A.J.; Cardoso, V.N. High-salt diet induces IL-17-dependent gut inflammation and exacerbates colitis in mice. Front. Immunol. 2018, 8, 1969. [Google Scholar] [CrossRef]
- Yi, B.; Titze, J.; Rykova, M.; Feuerecker, M.; Vassilieva, G.; Nichiporuk, I.; Schelling, G.; Morukov, B.; Choukèr, A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: A longitudinal study. Transl. Res. 2015, 166, 103–110. [Google Scholar] [CrossRef]
- Reynolds, C.M.; Vickers, M.H.; Harrison, C.J.; Segovia, S.A.; Gray, C. Maternal high fat and/or salt consumption induces sex-specific inflammatory and nutrient transport in the rat placenta. Physiol. Rep. 2015, 3, e12399. [Google Scholar] [CrossRef]
- Farquhar, W.B.; Edwards, D.G.; Jurkovitz, C.T.; Weintraub, W.S. Dietary sodium and health: More than just blood pressure. J. Am. Coll. Cardiol. 2015, 65, 1042–1050. [Google Scholar] [CrossRef]
- Frisoli, T.M.; Schmieder, R.E.; Grodzicki, T.; Messerli, F.H. Salt and hypertension: Is salt dietary reduction worth the effort? Am. J. Med. 2012, 125, 433–439. [Google Scholar] [CrossRef]
- Appel, L.J.; Frohlich, E.D.; Hall, J.E.; Pearson, T.A.; Sacco, R.L.; Seals, D.R.; Sacks, F.M.; Smith, S.C., Jr.; Vafiadis, D.K.; Van Horn, L.V. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: A call to action from the American Heart Association. Circulation 2011, 123, 1138–1143. [Google Scholar] [CrossRef]
- Boon, C.S.; Taylor, C.L.; Henney, J.E. Strategies to Reduce Sodium Intake in the United States; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Kotchen, T.A.; Cowley, A.W., Jr.; Frohlich, E.D. Salt in health and disease—A delicate balance. N. Engl. J. Med. 2013, 368, 1229–1237. [Google Scholar] [CrossRef]
- Nicholls, M.G. Population-wide dietary sodium restriction: A cautious view. Curr. Hypertens. Rep. 2011, 13, 325. [Google Scholar] [CrossRef]
- Oh, Y.S.; Appel, L.J.; Galis, Z.S.; Hafler, D.A.; He, J.; Hernandez, A.L.; Joe, B.; Karumanchi, S.A.; Maric-Bilkan, C.; Mattson, D. National Heart, Lung, and Blood Institute Working Group report on salt in human health and sickness: Building on the current scientific evidence. Hypertension 2016, 68, 281–288. [Google Scholar] [CrossRef]
- Masters, D.G.; Rintoul, A.J.; Dynes, R.A.; Pearce, K.L.; Norman, H.C. Feed intake and production in sheep fed diets high in sodium and potassium. Aust. J. Agric. Res. 2005, 56, 427–434. [Google Scholar] [CrossRef]
- Chadwick, M.; Williams, I.; Vercoe, P.; Revell, D. Feeding pregnant ewes a high-salt diet or saltbush suppresses their offspring’s postnatal renin activity. Animal 2009, 3, 972–979. [Google Scholar] [CrossRef]
- Digby, S.; Masters, D.; Blache, D.; Blackberry, M.; Hynd, P.; Revell, D. Reproductive capacity of Merino ewes fed a high-salt diet. Animal 2008, 2, 1353–1360. [Google Scholar] [CrossRef]
- López, A.; Arroquy, J.I.; Juárez Sequeira, A.; García, M.; Nazareno, M.; Coria, H.; Distel, R. Effect of protein supplementation on tropical grass hay utilization by beef steers drinking saline water. J. Anim. Sci. 2014, 92, 2152–2160. [Google Scholar] [CrossRef]
- Adekunbi, D.; Ogunsola, O.; Oyelowo, O.; Aluko, E.; Popoola, A.; Akinboboye, O. Consumption of high sucrose and/or high salt diet alters sperm function in male Sprague–Dawley rats. Egypt. J. Basic Appl. Sci. 2016, 3, 194–201. [Google Scholar]
- Wang, C.; Huang, Z.; Yu, K.; Ding, R.; Ye, K.; Dai, C.; Xu, X.; Zhou, G.; Li, C. High-salt diet has a certain impact on protein digestion and gut microbiota: A sequencing and proteome combined study. Front. Microbiol. 2017, 8, 1838. [Google Scholar]
- Lins, T.; Menezes, V.; Barberino, R.; Costa, S.; Santos, N.; Nascimento, T.; Queiroz, M.; Cordeiro, M.; Ribeiro, L.; Araujo, G. Sperm quality, and morphology and apoptosis of germinal epithelium cells of ram lambs receiving water of different salinities. Anim. Prod. Sci. 2018, 58, 1608–1614. [Google Scholar]
- Ogihara, T.; Asano, T.; Ando, K.; Chiba, Y.; Sekine, N.; Sakoda, H.; Anai, M.; Onishi, Y.; Fujishiro, M.; Ono, H. Insulin resistance with enhanced insulin signaling in high-salt diet–fed rats. Diabetes 2001, 50, 573–583. [Google Scholar] [CrossRef]
- Norman, H.C.; Freind, C.; Masters, D.G.; Rintoul, A.J.; Dynes, R.A.; Williams, I.H. Variation within and between two saltbush species in plant composition and subsequent selection by sheep. Aust. J. Agric. Res. 2004, 55, 999–1007. [Google Scholar] [CrossRef]
- Obih, P.O.; Oyekan, A. Proteomic Analysis of Salt-Induced Changes in Protein Expression in PPARα Null Mice. Pharmacol. Pharm. 2014, 5, 996. [Google Scholar] [CrossRef][Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295. [Google Scholar]
- Wube, T.; Haim, A.; Fares, F. Effect of increased dietary salinity on the reproductive status and energy intake of xeric and mesic populations of the spiny mouse, Acomys. Physiol. Behav. 2009, 96, 122–127. [Google Scholar] [CrossRef]
- Sellers, R.S.; Mortan, D.; Michael, B.; Roome, N.; Johnson, J.K.; Yano, B.L.; Perry, R.; Schafer, K. Society of Toxicologic Pathology position paper: Organ weight recommendations for toxicology studies. Toxicol. Pathol. 2007, 35, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F. Function of seminal vesicles and their role on male fertility. Asian J. Androl. 2001, 3, 251–258. [Google Scholar]
- Mukerjee, B.; Rajan, T. Morphometric study of seminal vesicles of rat in normal health and stress conditions. J. Anat. Soc. India 2006, 55, 33–36. [Google Scholar]
- Basnet, P.; Hansen, S.A.; Olaussen, I.K.; Hentemann, M.A.; Acharya, G. Changes in the semen quality among 5739 men seeking infertility treatment in Northern Norway over past 20 years (1993–2012). J. Reprod. Biotechnol. Fertil. 2016, 5, 2058915816633539. [Google Scholar] [CrossRef]
- Iranloye, B.O.; Oludare, G.O.; Morakinyo, A.O.; Esume, N.A.; Ekeh, L.C. Reproductive parameters and oxidative stress status of male rats fed with low and high salt diet. J. Hum. Reprod. Sci. 2013, 6, 267. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.-S.; Pang, M.-G. Calcium influx and male fertility in the context of the sperm proteome: An update. BioMed Res. Int. 2014, 2014, 841615. [Google Scholar] [CrossRef]
- Burg, M.B.; Ferraris, J.D.; Dmitrieva, N.I. Cellular response to hyperosmotic stresses. Physiol. Rev. 2007, 87, 1441–1474. [Google Scholar] [CrossRef]
- Burnaugh, L.; Ball, B.; Sabeur, K.; Thomas, A.; Meyers, S.A. Osmotic stress stimulates generation of superoxide anion by spermatozoa in horses. Anim. Reprod. Sci. 2010, 117, 249–260. [Google Scholar] [CrossRef]
- Ofem, O.; Ani, E.; Okoi, O.; Effiang, A.; Eno, A.; Ibu, J. Effect of Viscum album (mistletoe) extract on some serum electrolytes, organ weight and cytoarchitecture of the heart, kidney and blood vessels in high salt fed rats. Internet J. Nutr. Wellness 2006, 4, 1–10. [Google Scholar]
- Morita, H.; Kurihara, H.; Kurihara, Y.; Kuwaki, T.; Shindo, T.; Oh-hashi, Y.; Kumada, M.; Yazaki, Y. Responses of blood pressure and catecholamine metabolism to high salt loading in endothelin-1 knockout mice. Hypertens. Res. 1999, 22, 11–16. [Google Scholar] [CrossRef][Green Version]
- Zieba, D.A.; Amstalden, M.; Williams, G. Regulatory roles of leptin in reproduction and metabolism: A comparative review. Domest. Anim. Endocrinol. 2005, 29, 166–185. [Google Scholar] [CrossRef]
- Blache, D.; Grandison, M.J.; Masters, D.G.; Dynes, R.A.; Blackberry, M.A.; Martin, G.B. Relationships between metabolic endocrine systems and voluntary feed intake in Merino sheep fed a high salt diet. Aust. J. Exp. Agric. 2007, 47, 544–550. [Google Scholar] [CrossRef]
- Evenson, D.; Jost, L. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci. 2000, 22, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Campino, C.; Carvajal, C.; Olivieri, O.; Guidi, G.; Faccini, G.; Vöhringer, P.; Cerda, J.; Owen, G.; Kalergis, A. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin. Endocrinol. 2014, 80, 677–684. [Google Scholar] [CrossRef]
- Usukura, M.; Zhu, A.; Yoneda, T.; Karashima, S.; Yagi, K.; Yamagishi, M.; Takeda, Y. Effects of a high-salt diet on adipocyte glucocorticoid receptor and 11-β hydroxysteroid dehydrogenase 1 in salt-sensitive hypertensive rats. Steroids 2009, 74, 978–982. [Google Scholar] [CrossRef]
- Ziegler, T.E.; Scheffler, G.; Snowdon, C.T. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm. Behav. 1995, 29, 407–424. [Google Scholar] [CrossRef]
- Mostafa, T.; Anis, T.; Imam, H.; El‐Nashar, A.; Osman, I. Seminal reactive oxygen species‐antioxidant relationship in fertile males with and without varicocele. Andrologia 2009, 41, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.; El-Neweshy, M.; Hassan, M.; Noreldin, A. Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: Possible mechanisms are involved. Life Sci. 2019, 230, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.; Sernia, C. The renin-angiotensin system and male reproduction: New functions for old hormones. J. Mol. Endocrinol. 2003, 30, 263–270. [Google Scholar] [CrossRef]
- Herr, D.; Bekes, I.; Wulff, C. Local renin-angiotensin system in the reproductive system. Front. Endocrinol. 2013, 4, 150. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.; Carlsson, P. Tissue renin-angiotensin system: Its expression, localization, regulation and potential role in the pancreas. J. Mol. Endocrinol. 2001, 26, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.F.; Barth, A.D.; Rawlings, N.C.; Wilde, R.E.; Crews, D.H., Jr.; Mir, P.S.; Kastelic, J.P. Effect of nutrition during calfhood and peripubertal period on serum metabolic hormones, gonadotropins and testosterone concentrations, and on sexual development in bulls. Domest. Anim. Endocrinol. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Young, S.L.; Grattan, D.R.; Jasoni, C.L. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol. Reprod. 2014, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.M.; Vickers, M.H.; Harrison, C.J.; Segovia, S.A.; Gray, C. High fat and/or high salt intake during pregnancy alters maternal meta-inflammation and offspring growth and metabolic profiles. Physiol. Rep. 2014, 2, e12110. [Google Scholar] [CrossRef] [PubMed]
- Lager, S.; Samulesson, A.M.; Taylor, P.D.; Poston, L.; Powell, T.L.; Jansson, T. Diet-induced obesity in mice reduces placental efficiency and inhibits placental mTOR signaling. Physiol. Rep. 2014, 2, e00242. [Google Scholar] [CrossRef]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef]
- Tatone, C.; Amicarelli, F.; Carbone, M.C.; Monteleone, P.; Caserta, D.; Marci, R.; Artini, P.G.; Piomboni, P.; Focarelli, R. Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update 2008, 14, 131–142. [Google Scholar] [CrossRef]

| Items | Species | Dose | Main Findings | References |
|---|---|---|---|---|
| 1- Growth performance | Sheep | (13% NaCl) diet during prenatal life |
| [4] |
| Sheep | 80 g/kg DM |
| [20] | |
| Merino ewes | (14% NaCl) diet during prenatal life |
| [21] | |
| C57BL/6J mice | 3.15% NaCl |
| [25] | |
| Merino sheep | (20% NaCl of dry matter) |
| [44] | |
| Endothelin-1 knockout mice. | 8% NaCl diet |
| [42] | |
| 2- Reproductive functions of male | Merino rams | 12% NaCl diet |
| [3] |
| Rats | 8% salt diet |
| [24] | |
| Rats | 8% salt diet |
| [37] | |
| Golden spiny mice | 5% |
| [32] | |
| Common spiny mice | 3.5% |
| [32] | |
| 3- Reproductive functions of female | Mouse | 4% NaCl water |
| [7] |
| Merino ewes | (NaCl 13% of dry matter) |
| [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelnour, S.A.; Abd El-Hack, M.E.; Noreldin, A.E.; Batiha, G.E.; Beshbishy, A.M.; Ohran, H.; Khafaga, A.F.; Othman, S.I.; Allam, A.A.; Swelum, A.A. High Salt Diet Affects the Reproductive Health in Animals: An Overview. Animals 2020, 10, 590. https://doi.org/10.3390/ani10040590
Abdelnour SA, Abd El-Hack ME, Noreldin AE, Batiha GE, Beshbishy AM, Ohran H, Khafaga AF, Othman SI, Allam AA, Swelum AA. High Salt Diet Affects the Reproductive Health in Animals: An Overview. Animals. 2020; 10(4):590. https://doi.org/10.3390/ani10040590
Chicago/Turabian StyleAbdelnour, Sameh A., Mohamed E. Abd El-Hack, Ahmed E. Noreldin, Gaber Elsaber Batiha, Amani Magdy Beshbishy, Husein Ohran, Asmaa F. Khafaga, Sarah I. Othman, Ahmed A. Allam, and Ayman A. Swelum. 2020. "High Salt Diet Affects the Reproductive Health in Animals: An Overview" Animals 10, no. 4: 590. https://doi.org/10.3390/ani10040590
APA StyleAbdelnour, S. A., Abd El-Hack, M. E., Noreldin, A. E., Batiha, G. E., Beshbishy, A. M., Ohran, H., Khafaga, A. F., Othman, S. I., Allam, A. A., & Swelum, A. A. (2020). High Salt Diet Affects the Reproductive Health in Animals: An Overview. Animals, 10(4), 590. https://doi.org/10.3390/ani10040590











