Impact of Prebiotics and Synbiotics Administered in ovo on the Immune Response against Experimental Antigens in Chicken Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In ovo Treatment
2.3. Rearing Conditions
2.4. Collecting the Yolk Sac Content
2.5. Immunization
2.6. Blood Sampling and Serum Preparation
2.7. Evaluation of the IgG Concentration in Chicken’s Serum and Yolk Sac Content
2.8. Determination of the SRBC antibody (against T-dependent antigens)
2.9. Determination of IgG and IgM Dextran Antibodies Using ELISA
2.10. Evaluation of Mitogen-Induced Cutaneous Delayed-Type Hypersensitivity (DTH)
2.11. Statistical Analysis
3. Results
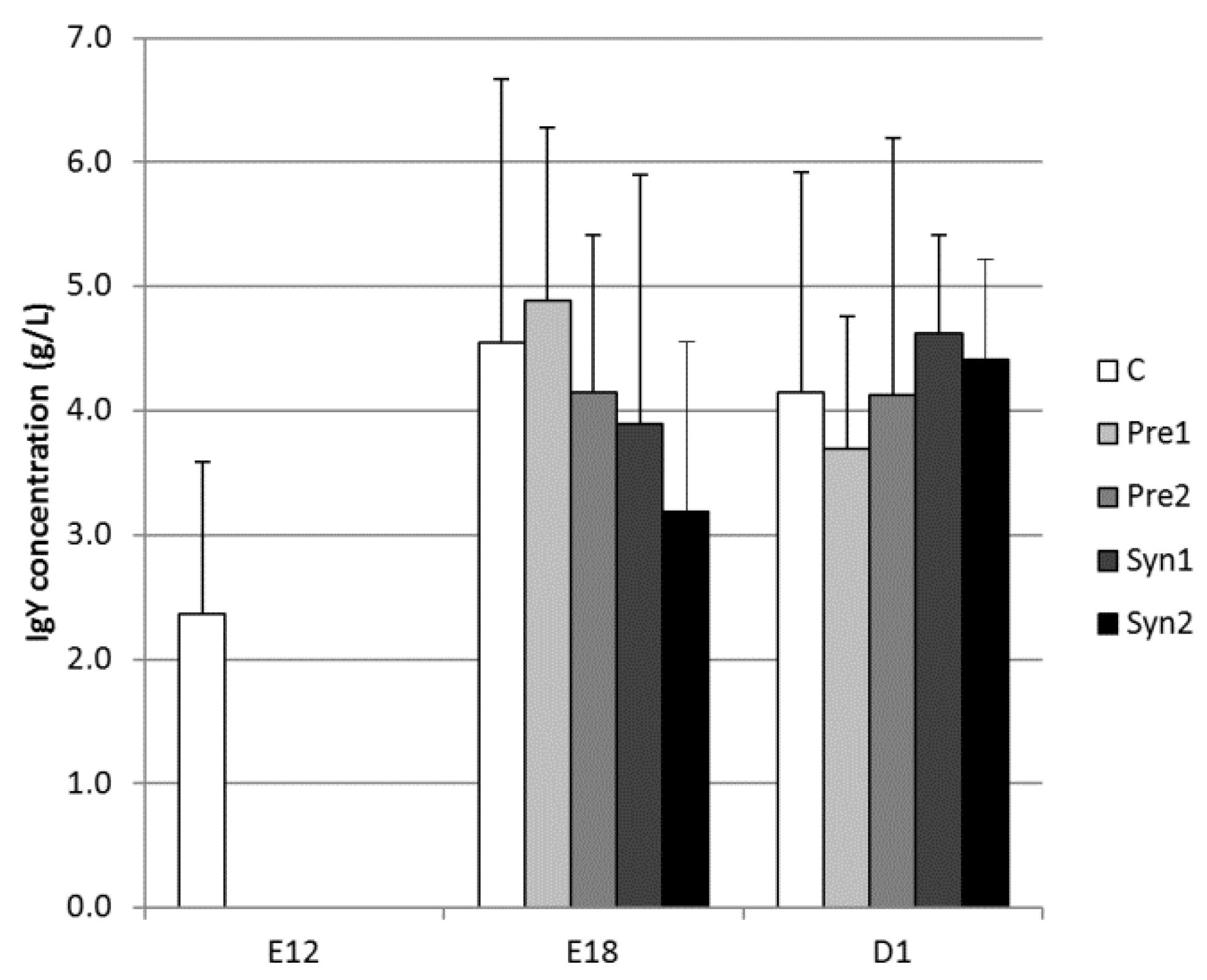
3.1. Yolk Sac IgG (Y) Concentration
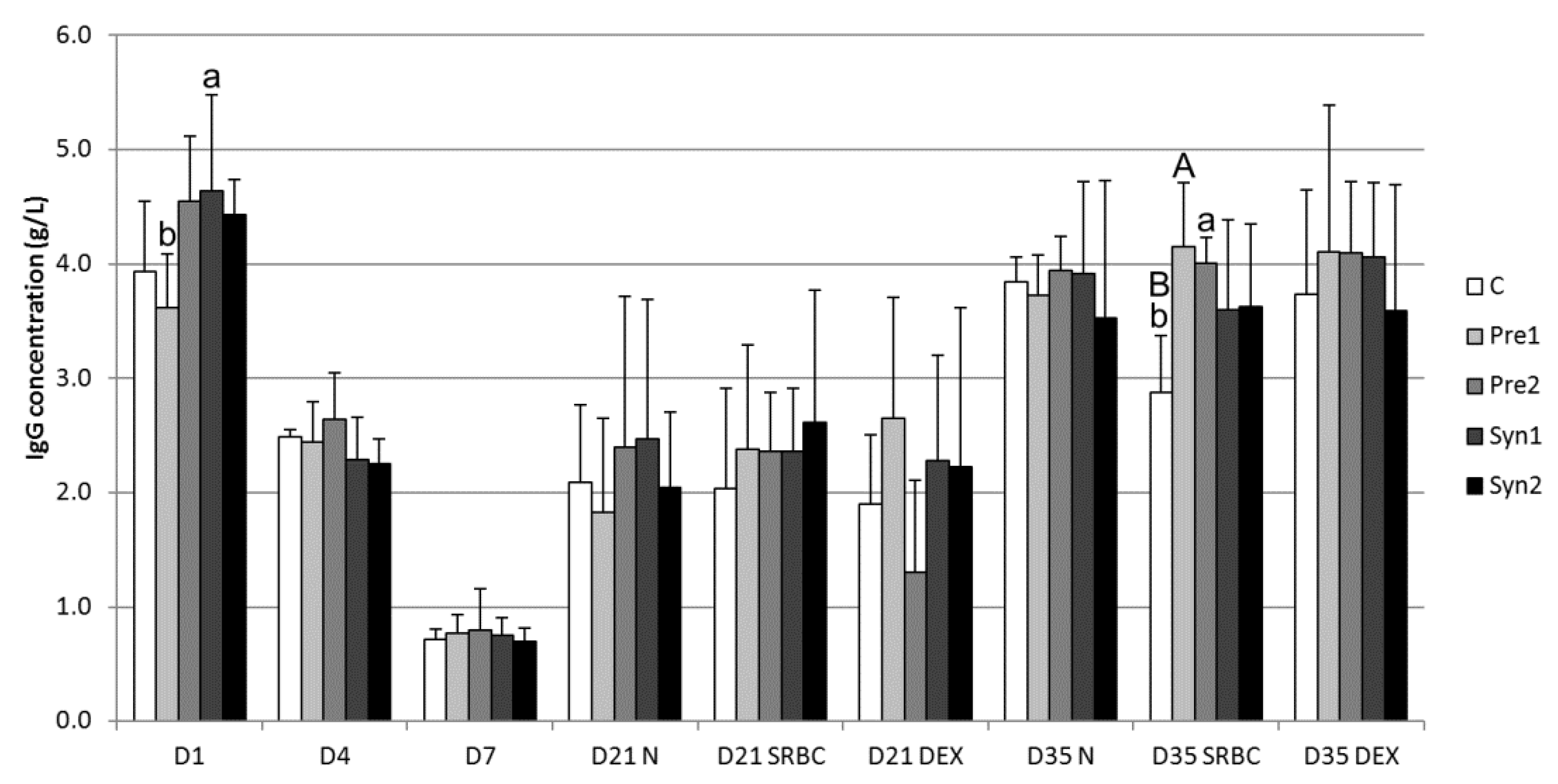
3.2. Serum IgG Concentration
3.3. Anti-SRBC and Anti-Dextran Antibodies
3.4. Delayed-Type Hypersensitivity (DTH)
3.5. Mortality Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Janardhana, V.; Broadway, M.M.; Bruce, M.P.; Lowenthal, J.W.; Geier, M.S.; Hughes, R.J.; Bean, A.G.D. Prebiotics Modulate Immune Responses in the Gut-Associated Lymphoid Tissue of Chickens. J. Nutr. 2009, 139, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Mitsuoka, T.; Hidaka, H.; Eida, T. Effect of fructooligosacharides on intestine microflora. Nahrung 1987, 31, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Ciesiołka, D.; Gulewicz, P.; Martinez-Villaluenga, C.; Pilarski, R.; Bednarczyk, M.; Gulewicz, K. Products and biopreparations from alkaloid-rich lupin in animal nutrition and ecological agriculture. Folia Biologica 2005, 53, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Attia, Y.A.; Hassan, S.S.; Abd-El-Hamid, A.E.; Radwan, M.W. Response of broiler chickens to dietary supplementation of ginger (zingiber officinale) continuously or intermittently in comparison with prebiotics. Egypt Poult. Sci. J. 2017, 37, 523–543. [Google Scholar]
- Tayeri, V.; Seidavi, A.; Asadpour, L.; Phillips, C.J.C. A comparison of the effects of antibiotics, probiotics, synbiotics and prebiotics on the performance and carcass characteristics of broilers. Vet. Res. Commun. 2018, 42, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dong, Z.; Song, D.; Zhou, H.; Wang, W.; Miao, H.; Wang, L.; Li, A. Effects of microencapsulated probiotics and prebiotics on growth performance, antioxidative abilities, immune functions, and caecal microflora in broiler chickens. Food Agric. Immunol. 2018, 29, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Petrovsky, N. Immunomodulation with microbial vaccines to prevent type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2010, 6, 131–138. [Google Scholar] [CrossRef]
- Dobson, A.; Cotter, P.D.; Paul Ross, R.; Hill, C. Bacteriocin production: A probiotic trait? Appl. Environ. Microbiol. 2012, 78, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, H.R.; Gong, J.; Gyles, C.L.; Hayes, M.A.; Zhou, H.; Sanei, B.; Chambers, J.R.; Sharif, S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006, 13, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Brisbin, J.T.; Gong, J.; Orouji, S.; Esufali, J.; Mallick, A.I.; Parvizi, P.; Shewen, P.E.; Sharif, S. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccine Immunol. 2011, 18, 1447–1455. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal Structure, Function, and Histology of Mucosa-Associated Lymphoid Tissue. Toxicol. Pathol. 2006, 34, 599–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torshizi, M.A.K.; Moghaddam, A.R.; Rahimi, S.; Mojgani, N. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Br. Poult. Sci. 2010, 51, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, M.; Urbanowski, M.; Gulewicz, P.; Kasperczyk, K.; Maiorano, G.; Szwaczkowski, T. Field and in vitro study on prebiotic effect of raffinose family oligosaccharides in chickens. Bull Vet Inst Pulawy 2011, 55, 465–469. [Google Scholar]
- Cheled-Shoval, S.L.; Amit-Romach, E.; Barbakov, M.; Uni, Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre- and posthatch periods in chickens. Poult. Sci. 2011, 90, 2301–2310. [Google Scholar] [CrossRef]
- Villaluenga, C.M.; Wardeńska, M.; Pilarski, R.; Bednarczyk, M.; Gulewicz, K. Utilization of the chicken embryo model for assessment of biological activity of different oligosaccharides. Folia Biol. (Praha). 2004, 52, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Siwek, M.; Slawinska, A.; Stadnicka, K.; Bogucka, J.; Dunislawska, A.; Bednarczyk, M. Prebiotics and synbiotics - in ovo delivery for improved lifespan condition in chicken. BMC Vet Res. 2018, 14, e402. [Google Scholar] [CrossRef]
- Pilarski, R.; Bednarczyk, M.; Lisowski, M.; Rutkowski, A.; Bernacki, Z.; Wardeńska, M.; Gulewicz, K. Assessment of the effect of α-galactosides injected during embryogenesis on selected chicken traits. Folia Biol. (Praha). 2005, 53, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Dunislawska, A.; Slawinska, A.; Stadnicka, K.; Bednarczyk, M.; Gulewicz, P.; Jozefiak, D.; Siwek, M. Synbiotics for broiler chickens-In vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS ONE 2017, 12, e0168587. [Google Scholar] [CrossRef] [Green Version]
- Madej, J.P.; Stefaniak, T.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chickens. Poult. Sci. 2015, 94, 1209–1219. [Google Scholar] [CrossRef]
- Madej, J.P.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on the morphology and specific immune cell composition in the gut-associated lymphoid tissue. Poult. Sci. 2016, 95, 19–29. [Google Scholar] [CrossRef]
- Pruszynska-Oszmalek, E.; Kolodziejski, P.A.; Stadnicka, K.; Sassek, M.; Chalupka, D.; Kuston, B.; Nogowski, L.; Mackowiak, P.; Maiorano, G.; Jankowski, J.; et al. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 2015, 94, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, E.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Tavaniello, S.; Maiorano, G.; Slawinska, A. Splenic Gene Expression Signatures in Slow-Growing Chickens Stimulated in Ovo with Galactooligosaccharides and Challenged with Heat. Animals 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sławinska, A.; Siwek, M.Z.; Bednarczyk, M.F. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014, 75, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, A.; Siwek, M.; Zylińska, J.; Bardowski, J.; Brzezińska, J.; Gulewicz, K.A.; Nowak, M.; Urbanowski, M.; Płowiec, A.; Bednarczyk, M. Influence of synbiotics delivered in ovo on immune organs development and structure. Folia Biol. 2014, 62, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Płowiec, A.; Sławinska, A.; Siwek, M.Z.; Bednarczyk, M.F. Effect of in ovo administration of inulin and lactococcus lactis on immune-related gene expression in broiler chickens. Am. J. Vet. Res. 2015, 76, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, T.; Madej, J.P.; Graczyk, S.; Siwek, M.; Łukaszewicz, E.; Kowalczyk, A.; Sieńczyk, M.; Bednarczyk, M. Selected prebiotics and synbiotics administered in ovo can modify innate immunity in chicken broilers. BMC Vet. Res. 2019, 15, e105. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Lakota, P.; Zylinska, J.; Chmielewska, M.; Bardowski, J.; Dankowiakowska, A.; Maiorano, G. In vitro and in vivo selection of bioactives enabling the stimulation of chicken microbiome. Ital. J. Anim. Sci. 2013, 12, e132. [Google Scholar]
- Sławińska, A.; Siwek, M.; Bednarczyk, M. In vitro screening of immunomodulatory properties of synbiotics in chicken DT40 cell line. Anim. Sci. Pap. Rep. 2016, 34, 81–94. [Google Scholar]
- Gąsowska, A.; Stefaniak, T. Evaluation of the effects of egg yolk immunoglobulin (IgY) oral administration to calves during the period of macromolecules absorption from the intestine. Folia Univ. Agric. Stetin. 2003, 45, 87–92. [Google Scholar]
- Hebishima, T.; Matsumoto, Y.; Soma, G.-I.; Kohchi, C.; Watabenabe, G.; Taya, K.; Hayashi, Y.; Hirota, Y. Immune Recovery Effects of Immunopotentiator from Pantoea agglomerans 1 (IP-PA1) on Low Antibody Productions in Response to Salmonella Enteritidis Vaccine and Sheep Red Blood Cells in Dexamethasone-Treated Stressed Chicken Models. J. Vet. Med. Sci. 2010, 72, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, C.; Möller, G. Immunological unresponsiveness to native dextran B512 in young animals of dextran high responder strains is due to lack of Ig receptors expression. Evidence for a nonrandom expression of V-genes. J. Exp. Med. 1978, 147, 645–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kean, R.P.; Lamond, S.J. Effect of injection site on cutaneous basophil hypersensitivity response to phytohemagglutinin. Poult. Sci. 1994, 73, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, S.; Wieliczko, A.; Pliszczak-Król, A.; Janaczyk, B. Humoral and cellular response of pheasants vaccinated against Newcastle disease and haemorrhagic enteritis. Acta Vet. Brno 2006, 75, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Davison, F.; Magor, K.E.; Kaspers, B. Structure and evolution of avian immunoglobulins. Avian Immunol. 2008, 1, 107–127. [Google Scholar]
- Sugimoto, M.; Yasuda, T.; Egashira, Y. Development of the embryonic chicken thymus. I. Characteristic synchronous morphogenesis of lymphocytes accompanied by the appearance of an embryonic thymus-specific antigen. Dev. Biol. 1977, 56, 281–292. [Google Scholar] [CrossRef]
- Lawrence, E.C.; Arnaud-Battandier, F.; Grayson, J.; Koski, I.R.; Dooley, N.J.; Muchmore, A.V.; Blaese, R.M. Ontogeny of humoral immune function in normal chickens: A comparison of immunoglobulin-secreting cells in bone marrow, spleen, lungs and intestine. Clin. Exp. Immunol. 1981, 43, 450–457. [Google Scholar]
- Kaspers, B.; Bondl, H.; Göbel, T. Transfer of IgA from albumen into the yolk sac during embryonic development in the chicken. Zentralbl Vet. A. 1996, 43, 225–231. [Google Scholar] [CrossRef]
- Chrząstek, K.; Wieliczko, A. Influence of enrofloxacine, florfenicol, and ceftiofur on IgY in the yolk sac and serum in chicks. Med. Weter. 2015, 71, 369–372. [Google Scholar]
- Leslie, G.A. Ontogeny of the chicken humoral immune mechanism. Am. J. Vet. Res. 1975, 36, 482–485. [Google Scholar]
- Kramer, T.T.; Cho, H.C. Transfer of immunoglobulins and antibodies in the hen’s egg. Immunology 1970, 19, 157–167. [Google Scholar]
- Kowalczyk, K.; Daiss, J.; Halpern, J.; Roth, T.F. Quantitation of maternal-fetal IgG transport in the chicken. Immunology 1985, 54, 755–762. [Google Scholar] [PubMed]
- West, A.P.; Herr, A.B.; Bjorkman, P.J. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity 2004, 20, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Khattab, M.S.; Craig, J.V. Ontogeny of allotype synthesis and decay of maternal allotype in young chickens. Poult. Sci. 1970, 49, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Kaspers, B.; Schranner, I.; Lösch, U. Distribution of Immunoglobulins During Embryogenesis in the Chicken. J. Vet. Med. Ser. A 1991, 38, 73–79. [Google Scholar] [CrossRef]
- Radziwill-Bienkowska, J.M.; Le, D.T.L.; Szczesny, P.; Duviau, M.P.; Aleksandrzak-Piekarczyk, T.; Loubière, P.; Mercier-Bonin, M.; Bardowski, J.K.; Kowalczyk, M. Adhesion of the genome-sequenced Lactococcus lactis subsp. cremoris IBB477 strain is mediated by specific molecular determinants. Appl. Microbiol. Biotechnol. 2016, 100, 9605–9617. [Google Scholar] [CrossRef] [Green Version]
- Parmentier, H.K.; Lammers, A.; Hoekman, J.J.; De Vries Reilingh, G.; Zaanen, I.T.A.; Savelkoul, H.F.J. Different levels of natural antibodies in chickens divergently selected for specific antibody responses. Dev. Comp. Immunol. 2004, 28, 39–49. [Google Scholar] [CrossRef]
- Siwek, M.; Knol, E.F. Genetic aspects of biological processes underlying the defense system in the neonate. Folia Biol. 2005, 53, 39–43. [Google Scholar] [CrossRef]
- Kozłowska, I.; Marć-Pieńkowska, J.; Bednarczyk, M. Beneficial Aspects of Inulin Supplementation as a Fructooligosaccharide Prebiotic in Monogastric Animal Nutrition-A Review. Ann. Anim. Sci. 2016, 16, 315–331. [Google Scholar] [CrossRef] [Green Version]
- Attia, Y.A.; Hamed, R.S.; Abd El-Hamid, A.E.; Shahba, H.A.; Bovera, F. Effect of inulin and mannan-oligosaccharides compared with zinc-bacitracin on growing performance, nutrient digestibility and hematological profiles of growing rabbits. Anim. Prod. Sci. 2015, 55, 80–86. [Google Scholar] [CrossRef]
- Attia, Y.; Bovera, F.; El-Tahawy, W.; El-Hanoun, A.; Al-Harthi, M.; Habiba, H.I. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit Sci. 2015, 23, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Buddington, K.K.; Donahoo, J.B.; Buddington, R.K. Dietary oligofructose and inulin protect mice from enteric and systemic pathogens and tumor inducers. J. Nutr. 2002, 132, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roller, M.; Rechkemmer, G.; Watzl, B. Nutritional Immunology—Research Communication Prebiotic Inulin Enriched with Oligofructose in Combination with the Probiotics Lactobacillus rhamnosus and Bifidobacterium lactis Modulates Intestinal Immune Functions in Rats. J. Nutr. 2004, 134, 153–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, S.; Watzl, B. Inulin and Oligofructose: Review of Experimental Data on Immune Modulation. J. Nutr. 2007, 137, 2563–2567. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.G.; Cooper, P.D.; Petrovsky, N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol. Cell Biol. 2004, 82, 611–616. [Google Scholar] [CrossRef] [PubMed]



| Time | Groups and Number of Embryos/Chickens | Treatment | ||||
|---|---|---|---|---|---|---|
| Pre1 | Pre2 | Syn1 | Syn2 | C | ||
| 900 | incubated eggs | |||||
| E12 | −7 | randomly selected embryos sacrificed and used before treatment for IgG (Y) estimation | ||||
| E12 | 160 | 160 | 160 | 160 | 160 | embryos given to experimental groups and treated in ovo |
| E18 | −7 | −7 | −7 | −7 | −7 | randomly selected embryos from each group sacrificed and used for IgG (Y) estimation |
| D1 | 145 | 137 | 114 | 145 | 130 | hatched chickens |
| D1 | −7 | −7 | −7 | −7 | −7 | randomly selected chickens sacrificed and used for IgG (Y) and IgG estimation |
| D4 | −7 | −7 | −7 | −7 | −7 | randomly selected chickens sacrificed and used for IgG estimation |
| D7 | −7 | −7 | −7 | −7 | −7 | randomly selected chickens sacrificed and used for IgG, SRBC, DEX (IgM), and DEX (IgG) antibody estimation |
| Groups destined for an evaluation of the serum IgG concentration and DTH test. | ||||||
| Pre1(N) | Pre2(N) | Syn1(N) | Syn2(N) | C(N) | ||
| D7 | 45 | 42 | 37 | 43 | 32 | chickens randomly selected for N groups |
| D21 | −7 | −7 | −7 | −7 | −7 | randomly selected chickens sacrificed and used for IgG estimation |
| −8 | −8 | −8 | −8 | −8 | randomly selected chickens used for DTH | |
| D35 | −7 | −7 | −7 | −7 | −7 | randomly selected chickens sacrificed and used for IgG estimation |
| −8 | −8 | −8 | −8 | −8 | randomly selected chickens used for DTH | |
| Time | Groups and Number of Embryos/Chickens | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C SRBC | C DEX | Pre1 SRBC | Pre1 DEX | Pre2 SRBC | Pre2 DEX | Syn1 SRBC | Syn1 DEX | Syn2 SRBC | Syn2 DEX | ||
| D7 | 25 | 25 | 35 | 35 | 30 | 30 | 25 | 25 | 35 | 35 | 1st immunization |
| D21 | −7 | −7 | −7 | −7 | −7 | −7 | −7 | −7 | −7 | −7 | randomly selected chickens sacrificed and used for IgG, SRBC (RAT), DEX (IgM), and DEX (IgG) |
| D21 | 18 | 18 | 28 | 28 | 23 | 23 | 18 | 18 | 28 | 28 | 2nd immunization |
| D35 | −7 | −7 | −7 | −7 | −7 | −7 | −7 | −7 | −7 | −7 | randomly selected chickens sacrificed and used for IgG, SRBC (RAT), DEX (IgM), and DEX (IgG) |
| Groups | Number of Chickens at the Start of the Experiment | Mortality Cases | Mortality Rate (%) |
|---|---|---|---|
| C | 130 | 11 | 8.5 a |
| Pre1 | 145 | 3 | 2.1 b |
| Pre2 | 137 | 8 | 5.8 ab |
| Syn1 | 114 | 6 | 5.3 ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefaniak, T.; Madej, J.P.; Graczyk, S.; Siwek, M.; Łukaszewicz, E.; Kowalczyk, A.; Sieńczyk, M.; Maiorano, G.; Bednarczyk, M. Impact of Prebiotics and Synbiotics Administered in ovo on the Immune Response against Experimental Antigens in Chicken Broilers. Animals 2020, 10, 643. https://doi.org/10.3390/ani10040643
Stefaniak T, Madej JP, Graczyk S, Siwek M, Łukaszewicz E, Kowalczyk A, Sieńczyk M, Maiorano G, Bednarczyk M. Impact of Prebiotics and Synbiotics Administered in ovo on the Immune Response against Experimental Antigens in Chicken Broilers. Animals. 2020; 10(4):643. https://doi.org/10.3390/ani10040643
Chicago/Turabian StyleStefaniak, Tadeusz, Jan P. Madej, Stanisław Graczyk, Maria Siwek, Ewa Łukaszewicz, Artur Kowalczyk, Marcin Sieńczyk, Giuseppe Maiorano, and Marek Bednarczyk. 2020. "Impact of Prebiotics and Synbiotics Administered in ovo on the Immune Response against Experimental Antigens in Chicken Broilers" Animals 10, no. 4: 643. https://doi.org/10.3390/ani10040643





