Inflammasome Activation in Bovine Peripheral Blood-Derived Macrophages Is Associated with Actin Rearrangement
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bovine Monocyte-Derived Macrophages (BMDM)
2.2. Purification of Flagella
2.3. Removal of LPS from Purified Flagella
2.4. Lipo-Transfection of Flagella and Confocal Microscopy
2.5. Western Blot
2.6. Statistical Analysis
3. Results
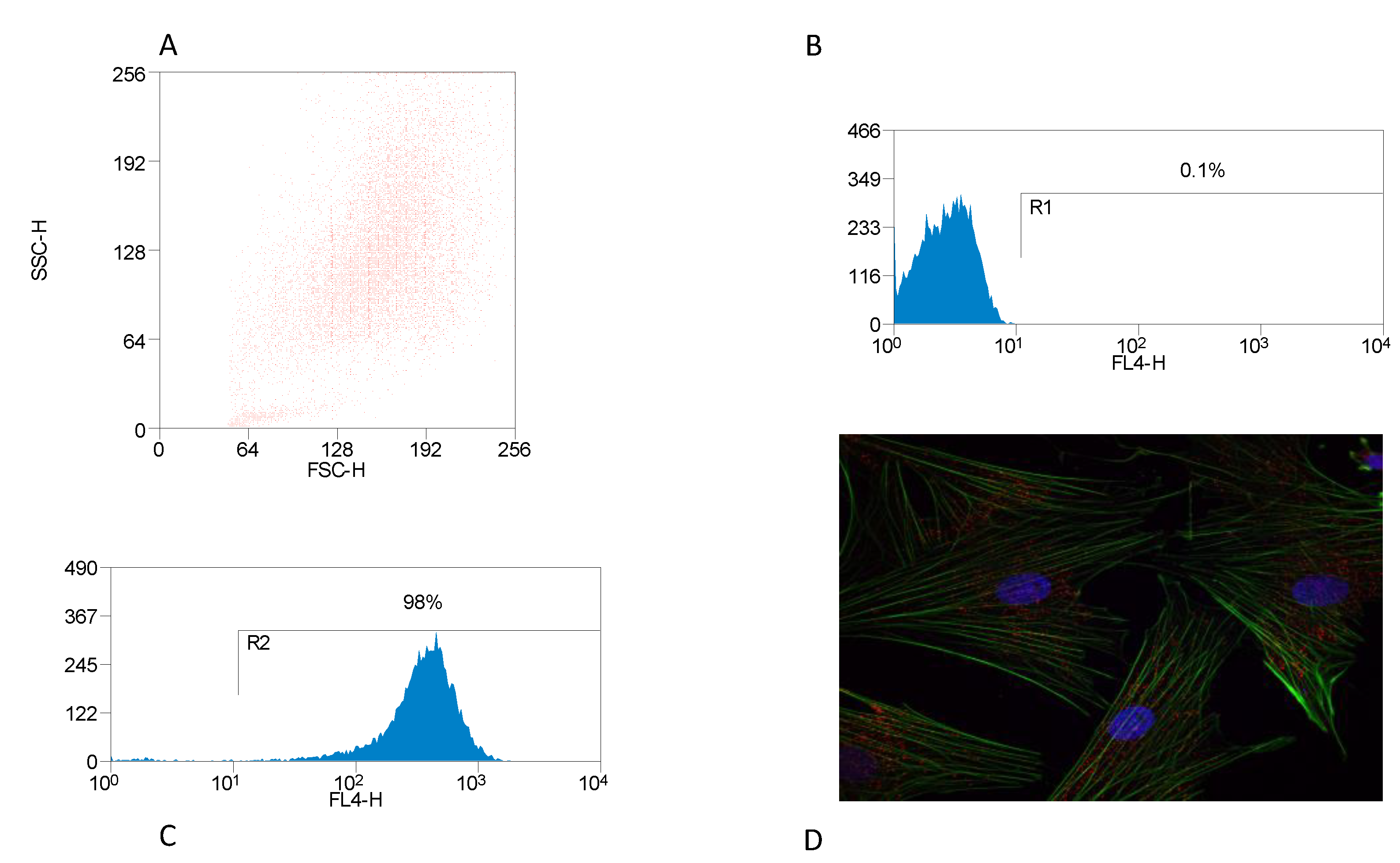
3.1. Identification of BMDM
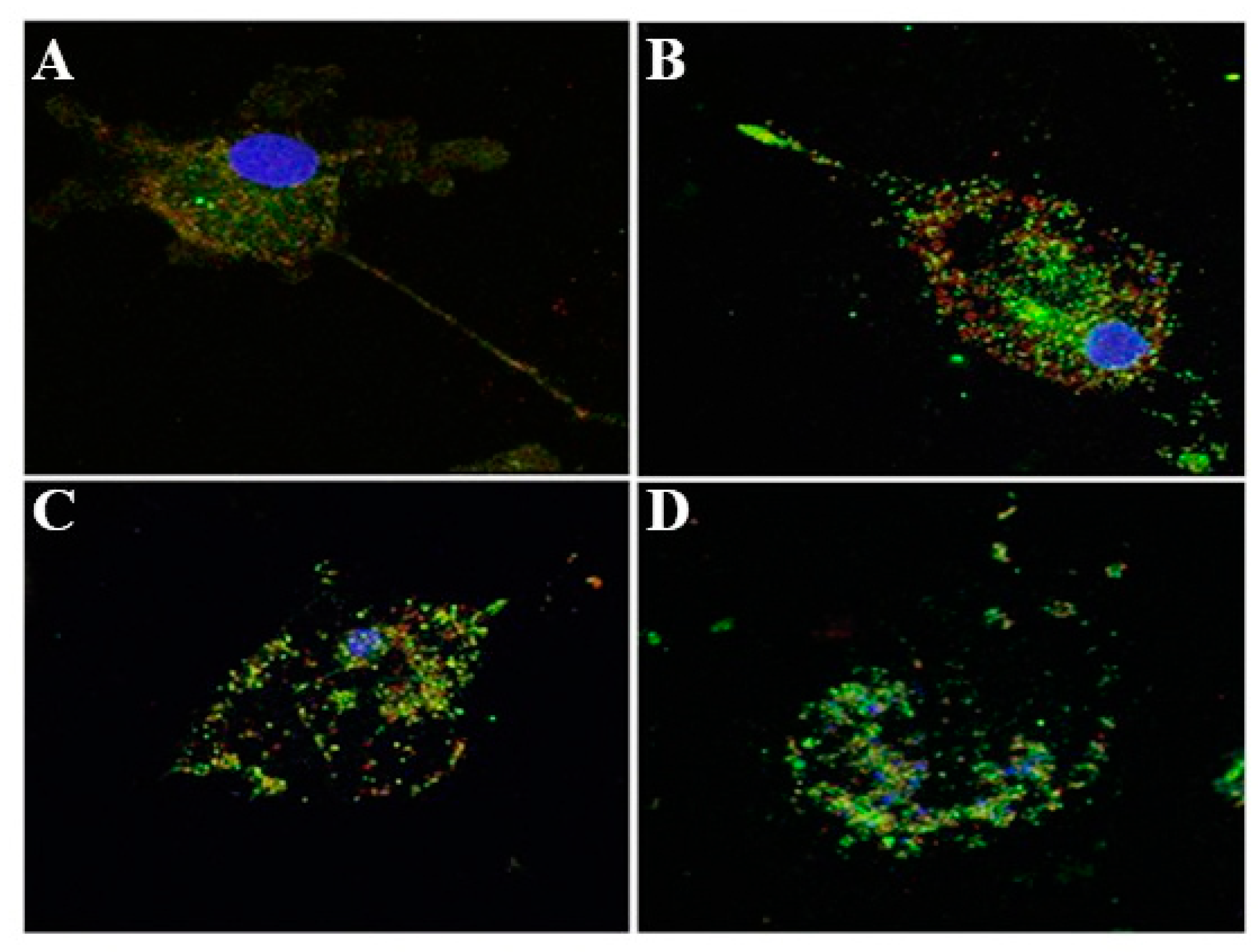
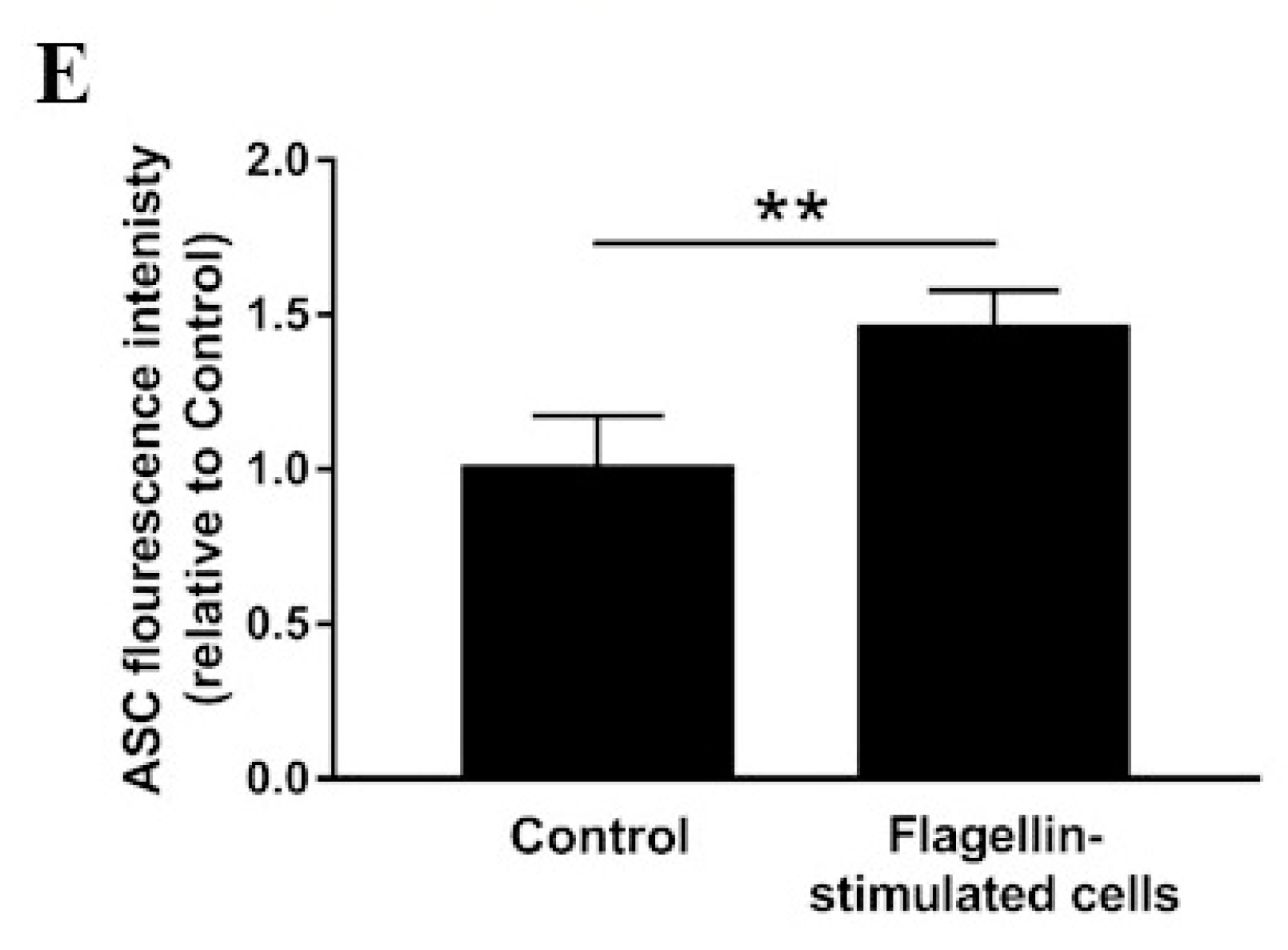
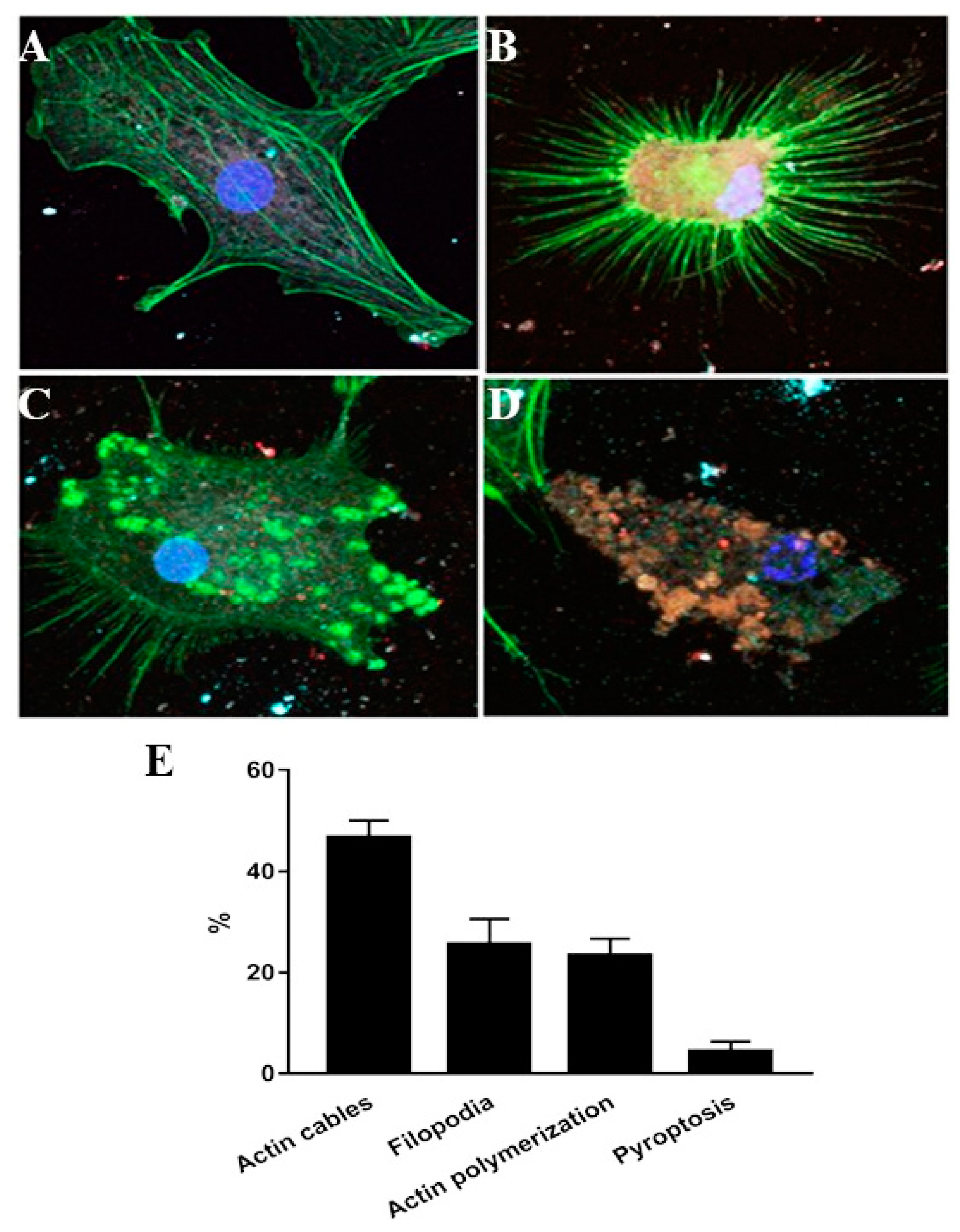
3.2. Inflammasome Activation and Actin Co-Localization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, J.; Auger, M. The Biology of the Macrophage; Burke, B., III, Lewis, C., III, Eds.; The macrophage 1-72; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottazzi, B.; Doni, A.; Garlanda, C.; Mantovani, A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2009, 28, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27. [Google Scholar] [CrossRef] [Green Version]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [Green Version]
- West, T.E.; Myers, N.D.; Chantratita, N.; Chierakul, W.; Limmathurotsakul, D.; Wuthiekanun, V.; Miao, E.A.; Hajjar, A.M.; Peacock, S.J.; Liggitt, H.D. Nlrc4 and tlr5 each contribute to host defense in respiratory melioidosis. PLoS Negl. Trop. Dis. 2014, 8, e3178. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proil-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.-D.; Franchi, L.; Núñez, G. Caspase-1 inflammasomes in infection and inflammation. J. Leukoc. Biol. 2007, 82, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, L.; Guo, H.; David, C.m.N.; Brickey, W.J.; Jha, S.; Ting, J.P.-Y. Nlr members nlrc4 and nlrp3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 2017, 214, 1351–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Chang, H.Y.; Baltimore, D. Autoproteolytic activation of pro-caspases by oligomerization. Mol. Cell 1998, 1, 319–325. [Google Scholar] [CrossRef]
- Perregaux, D.G.; McNiff, P.; Laliberte, R.; Conklyn, M.; Gabel, C.A. Atp acts as an agonist to promote stimulus-induced secretion of il-1β and il-18 in human blood. J. Immunol. 2000, 165, 4615–4623. [Google Scholar] [CrossRef] [Green Version]
- Howard, A.D.; Kostura, M.J.; Thornberry, N.; Ding, G.; Limjuco, G.; Weidner, J.; Salley, J.; Hogquist, K.; Chaplin, D.; Mumford, R. Il-1-converting enzyme requires aspartic acid residues for processing of the il-1 beta precursor at two distinct sites and does not cleave 31-kda il-1 alpha. J. Immunol. 1991, 147, 2964–2969. [Google Scholar]
- Gu, Y.; Kuida, K.; Tsutsui, H.; Ku, G.; Hsiao, K.; Fleming, M.A.; Hayashi, N.; Higashino, K.; Okamura, H.; Nakanishi, K. Activation of interferon-خ³ inducing factor mediated by interleukin-1خ² converting enzyme. Science 1997, 275, 206–209. [Google Scholar] [CrossRef]
- Ghayur, T.; Banerjee, S.; Hugunin, M.; Butler, D.; Herzog, L.; Carter, A.; Quintal, L.; Sekut, L.; Talanian, R.; Paskind, M. Caspase-1 processes ifn-خ³-inducing factor and regulates lps-induced ifn-خ³ production. Nature 1997, 386, 619. [Google Scholar] [CrossRef]
- Wen, H.; Miao, E.A.; Ting, J.P.-Y. Mechanisms of nod-like receptor-associated inflammasome activation. Immunity 2013, 39, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Vanaja, S.K.; Rathinam, V.A.; Fitzgerald, K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015, 25, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vance, R.E. The naip/nlrc4 inflammasomes. Curr. Opin. Immunol. 2015, 32, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Boyden, E.D.; Dietrich, W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 2006, 38, 240. [Google Scholar] [CrossRef] [PubMed]
- Lightfield, K.L.; Persson, J.; Brubaker, S.W.; Witte, C.E.; Von Moltke, J.; Dunipace, E.A.; Henry, T.; Sun, Y.-H.; Cado, D.; Dietrich, W.F. Critical function for naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 2008, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Storek, K.M.; Monack, D.M. Bacterial recognition pathways that lead to inflammasome activation. Immunol. Rev. 2015, 265, 112–129. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Vrentas, C.E.; Boggiatto, P.M.; Olsen, S.C.; Leppla, S.H.; Moayeri, M. Characterization of the nlrp1 inflammasome response in bovine species. Innate Immun. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, P.; Zhang, N.; Li, L.; Chen, S.; Jia, L.; Liu, X.; Li, J.; Zhang, X. Inflammasome activation restrains the intracellular neospora caninum proliferation in bovine macrophages. Vet. Parasitol. 2019, 268, 16–20. [Google Scholar] [CrossRef]
- Kreis, T.E.; Birchmeier, W. Stress fiber sarcomeres of fibroblasts are contractile. Cell 1980, 22, 555–561. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers–assembly, dynamics and biological roles. J Cell Sci 2012, 125, 1855–1864. [Google Scholar] [CrossRef] [Green Version]
- Matsudaira, P. Modular organization of actin crosslinking proteins. Trends Biol. Sci. 1991, 16, 87–92. [Google Scholar] [CrossRef]
- Honda, K.; Yamada, T.; Endo, R.; Ino, Y.; Gotoh, M.; Tsuda, H.; Yamada, Y.; Chiba, H.; Hirohashi, S. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J. Cell Biol. 1998, 140, 1383–1393. [Google Scholar] [CrossRef]
- Jensen, K.; Anderson, J.A.; Glass, E.J. Comparison of small interfering rna (sirna) delivery into bovine monocyte-derived macrophages by transfection and electroporation. Vet. Immunol. Immunopathol. 2014, 158, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Tahoun, A.; Jensen, K.; Corripio-Miyar, Y.; McAteer, S.P.; Corbishley, A.; Mahajan, A.; Brown, H.; Frew, D.; Aumeunier, A.; Smith, D.G. Functional analysis of bovine tlr5 and association with iga responses of cattle following systemic immunisation with h7 flagella. Vet. Res. 2015, 46, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearon, D.T.; Locksley, R.M. The instructive role of innate immunity in the acquired immune response. Science 1996, 272, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Lipoldová, M.; Demant, P. Genetic susceptibility to infectious disease: Lessons from mouse models of leishmaniasis. Nat. Rev. Genet. 2006, 7, 294. [Google Scholar] [CrossRef] [PubMed]
- Hawley, D.M.; Altizer, S.M. Disease ecology meets ecological immunology: Understanding the links between organismal immunity and infection dynamics in natural populations. Funct. Ecol. 2011, 25, 48–60. [Google Scholar] [CrossRef]
- Ulevitch, R.; Tobias, P. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 1995, 13, 437–457. [Google Scholar] [CrossRef]
- Fearon, D.T.; Austen, K.F. The alternative pathway of complement a system for host resistance to microbial infection. N. Engl. J. Med. 1980, 303, 259–263. [Google Scholar] [CrossRef]
- Ding, A.H.; Nathan, C.F.; Stuehr, D. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar]
- Freimer, N.; Ögmundsdóttir, H.M.; Blackwell, C.; Sutherland, I.; Graham, L.; Weir, D. The role of cell wall carbohydrates in binding of microorganisms to mouse peritoneal exudate macrophages. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. 1978, 86, 53–58. [Google Scholar] [CrossRef]
- Ogmundsdottir, H.; Weir, D.; Marmion, B. Binding of microorganisms to the macrophage plasma membrane; effects of enzymes and periodate. Br. J. Exp. Pathol. 1978, 59, 1. [Google Scholar]
- Sallusto, F.; Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, E.A.; Alpuche-Aranda, C.M.; Dors, M.; Clark, A.E.; Bader, M.W.; Miller, S.I.; Aderem, A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via ipaf. Nat. Immunol. 2006, 7, 569. [Google Scholar] [CrossRef]
- Franchi, L.; Amer, A.; Body-Malapel, M.; Kanneganti, T.D.; Ozören, N.; Jagirdar, R.; Inohara, N.; Vandenabeele, P.; Bertin, J.; Coyle, A.; et al. Cytosolic flagellin requires ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 2006, 7, 576–582. [Google Scholar] [CrossRef]
- Miao, E.A.; Andersen-Nissen, E.; Warren, S.E.; Aderem, A. Tlr5 and ipaf: Dual sensors of bacterial flagellin in the innate immune system. Semin. Immunopathol. 2007, 29, 275–288. [Google Scholar] [CrossRef]
- Tahoun, A.; Jensen, K.; Corripio-Miyar, Y.; McAteer, S.; Smith, D.G.; McNeilly, T.N.; Gally, D.L.; Glass, E.J. Host species adaptation of tlr5 signalling and flagellin recognition. Sci. Rep. 2017, 7, 17677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, W.E.; Jones, G.E.; Pollard, J.W.; Ridley, A.J. Rho, rac and cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 1997, 110, 707–720. [Google Scholar] [PubMed]
- Guasch, R.M.; Scambler, P.; Jones, G.E.; Ridley, A.J. Rhoe regulates actin cytoskeleton organization and cell migration. Mol. Cell. Biol. 1998, 18, 4761–4771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373. [Google Scholar] [CrossRef]
- Kofoed, E.M.; Vance, R.E. Innate immune recognition of bacterial ligands by naips determines inflammasome specificity. Nature 2011, 477, 592. [Google Scholar] [CrossRef]
- Schumann, R.R.; Belka, C.; Reuter, D.; Lamping, N.; Kirschning, C.J.; Weber, J.R.; Pfeil, D. Lipopolysaccharide activates caspase-1 (interleukin-1–converting enzyme) in cultured monocytic and endothelial cells. Blood 1998, 91, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Pastrana, J.L.; Li, X.; Huang, X. Inflammasomes: Sensors of metabolic stresses for vascular inflammation. Front. Biosci. A J. Virtual Libr. 2013, 18, 638. [Google Scholar]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhter, A.; Caution, K.; Khweek, A.A.; Tazi, M.; Abdulrahman, B.A.; Abdelaziz, D.H.; Voss, O.H.; Doseff, A.I.; Hassan, H.; Azad, A.K. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 2012, 37, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahoun, A.; Jensen, K.; El-Sharkawy, H.; Gally, D.; Rizk, A.M.; Ajarem, J.; Allam, A.; Mahmoud, A.M. Inflammasome Activation in Bovine Peripheral Blood-Derived Macrophages Is Associated with Actin Rearrangement. Animals 2020, 10, 655. https://doi.org/10.3390/ani10040655
Tahoun A, Jensen K, El-Sharkawy H, Gally D, Rizk AM, Ajarem J, Allam A, Mahmoud AM. Inflammasome Activation in Bovine Peripheral Blood-Derived Macrophages Is Associated with Actin Rearrangement. Animals. 2020; 10(4):655. https://doi.org/10.3390/ani10040655
Chicago/Turabian StyleTahoun, Amin, Kirsty Jensen, Hanem El-Sharkawy, David Gally, Amira M. Rizk, Jamaan Ajarem, Ahmed Allam, and Ayman M. Mahmoud. 2020. "Inflammasome Activation in Bovine Peripheral Blood-Derived Macrophages Is Associated with Actin Rearrangement" Animals 10, no. 4: 655. https://doi.org/10.3390/ani10040655




