Effects of Dietary Quebracho Tannin on Performance Traits and Parasite Load in an Italian Slow-Growing Chicken (White Livorno Breed)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Diets
2.2. Growth Performance and Feed Conversion Ratio
2.3. Blood Sampling
2.4. Excreta Sampling, Dry Matter and Parasite Load
2.5. Weight of Major Body Parts and Internal Organs
2.6. Statistical Analysis
3. Results
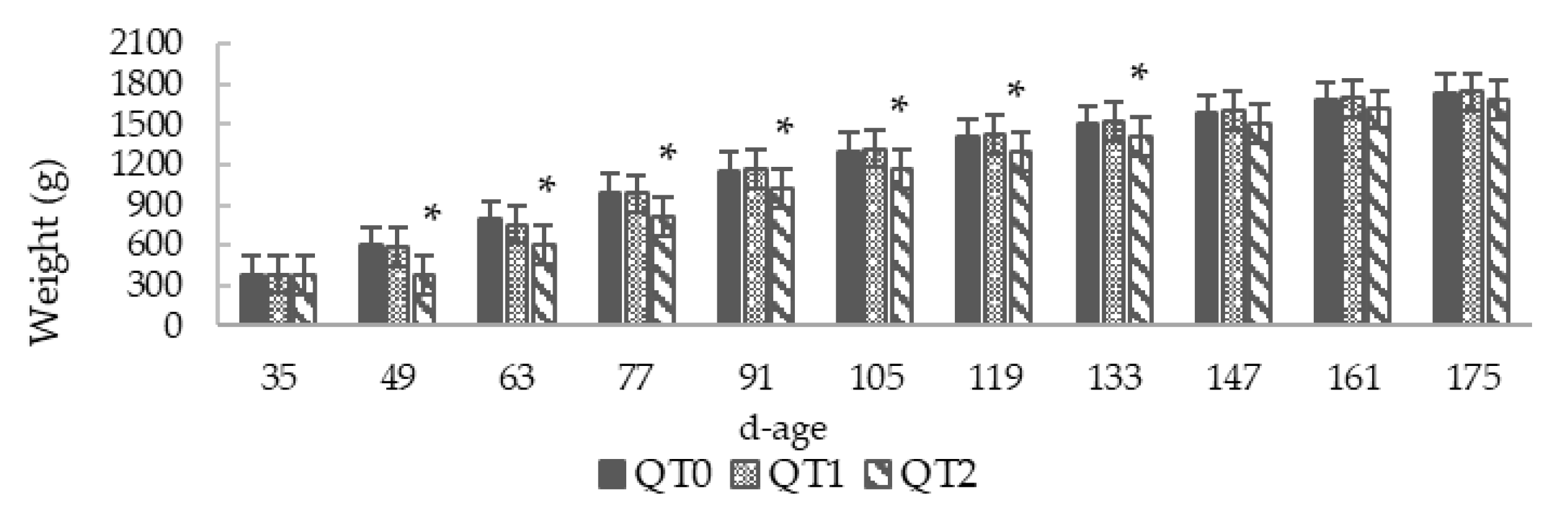
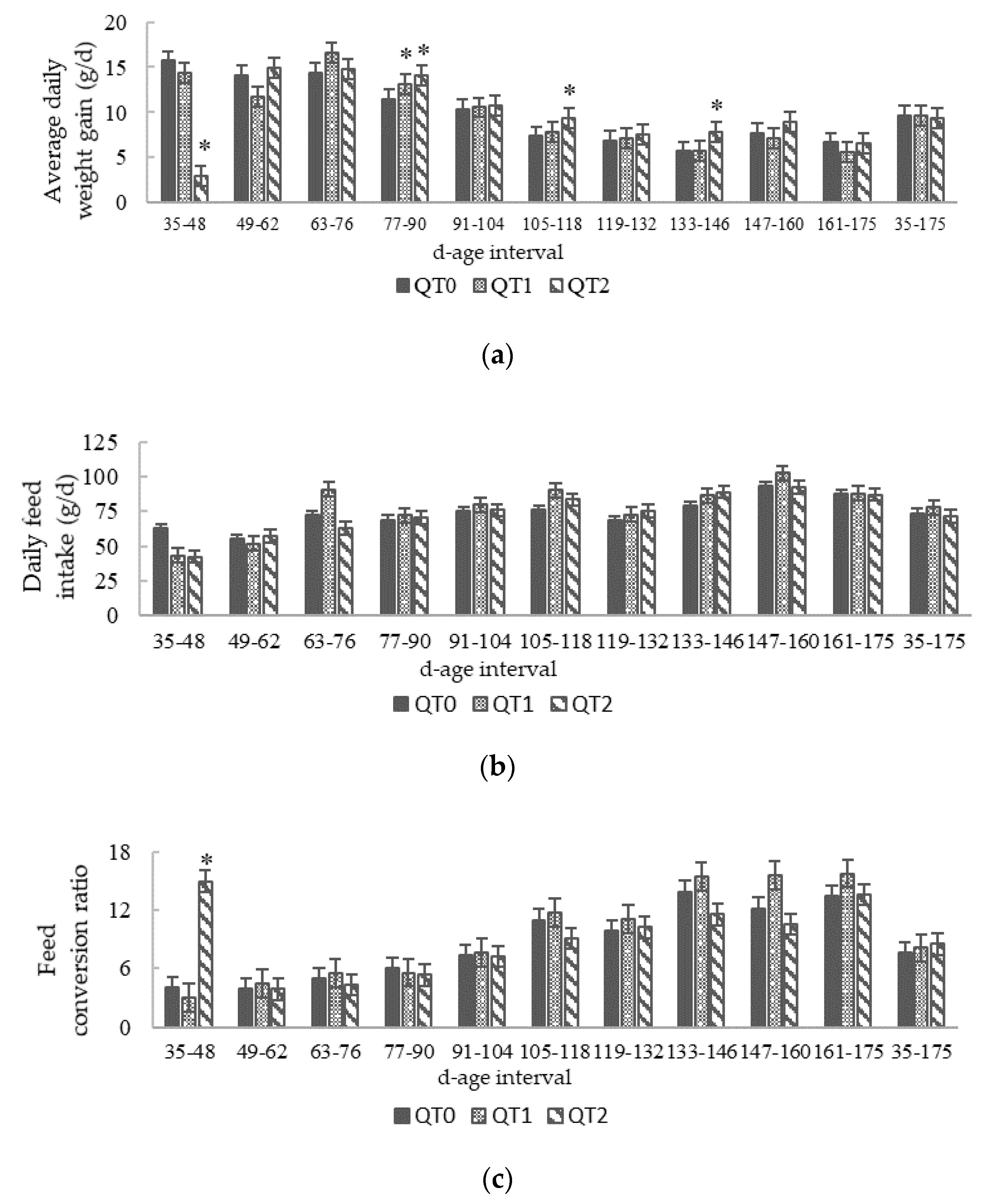
3.1. Growth Performance and Feed Conversion Ratio
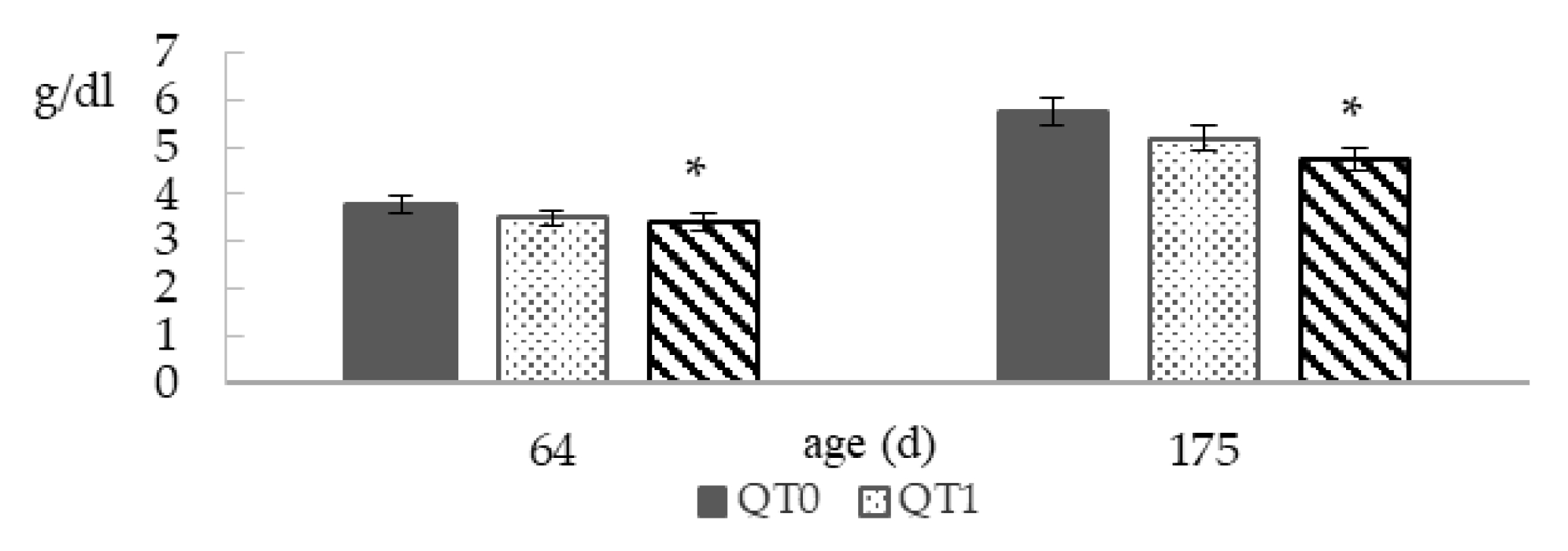
3.2. Total Blood Proteins
3.3. Excreta Dry Matter Evaluation
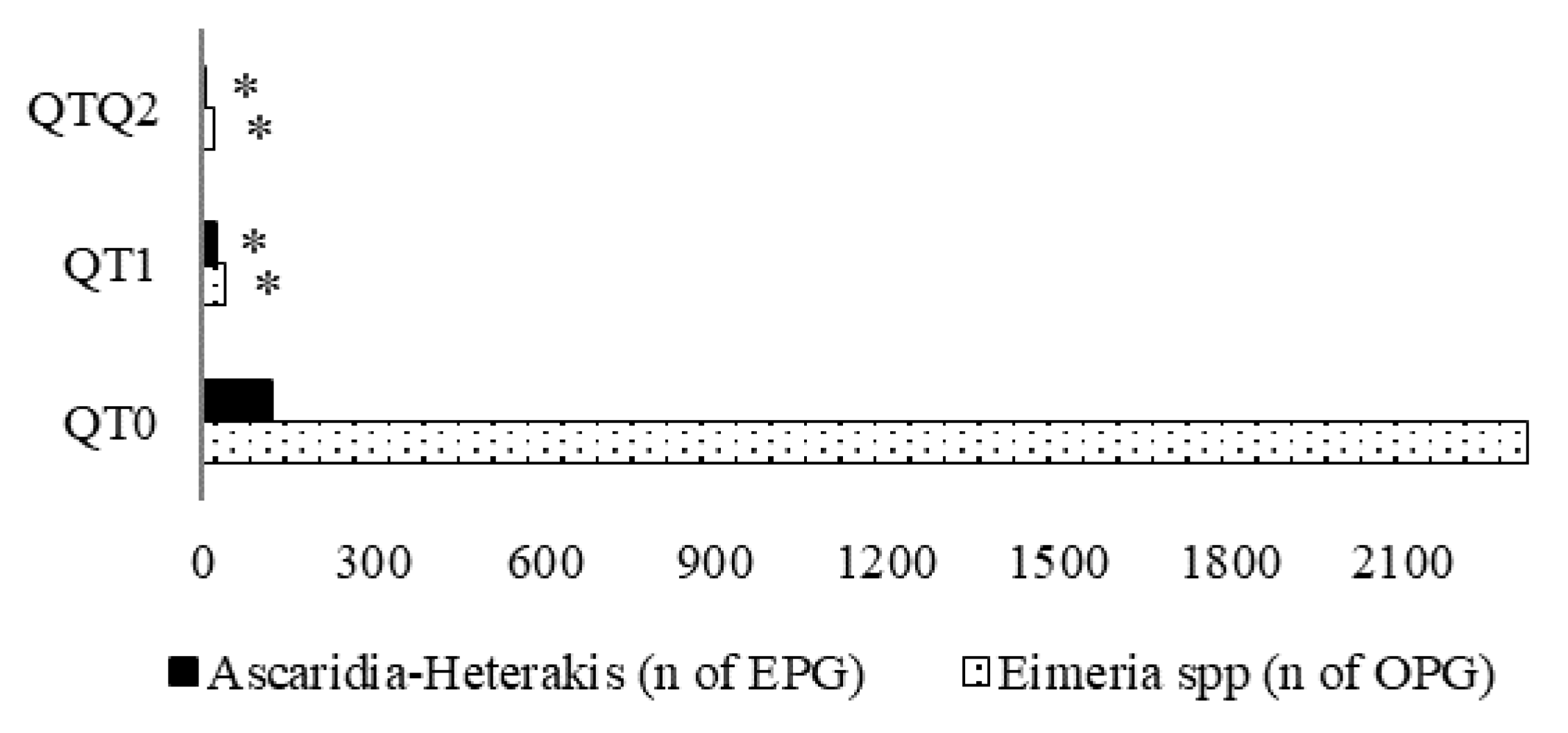
3.4. Excreta Parasite Load
3.5. Weight of Major Body Parts and Internal Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugiharto, S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agr. Sci. 2016, 15, 99–111. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Mikkelsen, L.L.; Sims, I.; Iji, P.A.; Choct, M. Phytobiotics: Alternatives to antibiotic growth promoters in monogastric animal feeds. Recent Adv. Anim. Nutr. Aust. 2005, 15, 131–144. [Google Scholar]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Panda, K.; Rao, S.V.R.; Raju, M.V.L.N. Natural growth promoters have potential in poultry feeding systems. Feed Tech. 2006, 10, 23–35. [Google Scholar]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Kroismayr, A. The Effects of Phytobiotics on Performance and Gut Function in Monogastrics. Phytobiotics on Performance and Gut Function in Monogastrics. 2007. Available online: https://en.engormix.com/feed-machinery/articles/phytobiotics-on-performance-gut-function-in-monogastrics-t33528.htm (accessed on 13 February 2020).
- Adedokun, S.A.; Olojede, O.C. Optimizing gastrointestinal integrity in poultry: The role of nutrients and feed additives. Front. Vet. Sci. 2019, 5. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Liu, X.L.; Zhao, G.Q.; Hu, T.M.; Wang, Y.X. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Marzoni, M.; Chiarini, R.; Castillo, A.; Romboli, I.; De Marco, M.; Schiavone, A. Effects of dietary natural antioxidant supplementation on broiler chicken and Muscovy duck meat quality. Anim. Sci. Pap. 2014, 32, 359–368. [Google Scholar]
- Yang, C.B.; Chowdhury, M.A.K.; Hou, Y.Q.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Dollahite, J.W.; Pigeon, R.F.; Camp, B.J. The toxicity of gallic acid, pyrogallol, tannic acid, and Quercus havardi in the rabbit. Am. J. Vet. Res. 1962, 23, 1264–1267. [Google Scholar]
- Mcleod, M.N. Plant tannins-Their role in forage quality. Nutr. Abstr. Rev. 1974, 44, 803–812. [Google Scholar]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Tech. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Schiavone, A.; Guo, K.; Tassone, S.; Gasco, L.; Hernandez, E.; Denti, R.; Zoccarato, I. Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poult. Sci. 2008, 87, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Zotte, A.D.; Cossu, M.E. Dietary inclusion of tannin extract from red quebracho trees (Schinopsis spp.) in the rabbit meat production. Ital. J. Anim. Sci. 2009, 8, 784–786. [Google Scholar] [CrossRef]
- Biagi, G.; Cipollini, I.; Paulicks, B.R.; Roth, F.X. Effect of tannins on growth performance and intestinal ecosystem in weaned piglets. Arch. Anim. Nutr. 2010, 64, 121–135. [Google Scholar] [CrossRef]
- Brus, M.; Dolinsek, J.; CenCic, A.; Skorjanc, D. Effect of chestnut (Castanea sativa Mill.) wood tannins and organic acids on growth performance and fecal microbiota of pigs from 23 to 127 days of age. Bulg. J. Agric. Sci. 2013, 19, 841–847. [Google Scholar]
- Starcevic, K.; Krstulovic, L.; Brozic, D.; Mauric, M.; Stojevic, Z.; Mikulec, Z.; Bajic, M.; Masek, T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015, 95, 1172–1178. [Google Scholar] [CrossRef]
- Marzoni, M.; Castillo, A.; Romboli, I. Dietary inclusion of Quebracho (Schinopsis lorentzii) tannins on productive performances of growing pheasant females. Ital. J. Anim. Sci. 2005, 4, 507–509. [Google Scholar] [CrossRef]
- Marzoni, M.; Castillo, A.; Romboli, I. Performances of growing Muscovy ducks fed on diets supplemented with Quebracho tannin powder. In Proceedings of the XVII the European Symposium on the Quality of Poultry Meat, Doorwerth, The Netherlands, 23–26 May 2005; pp. 336–341. [Google Scholar]
- Giavarini, I. Le Razze dei Polli. In Le Razze dei Polli; Edagricole: Bologna, Italy, 1983; pp. 3–14. [Google Scholar]
- AOAC. International Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academic Press: Washington, DC, USA, 1994. [Google Scholar]
- Salamano, G.; Mellia, E.; Tarantola, M.; Gennero, M.S.; Doglione, L.; Schiavone, A. Acute phase proteins and heterophil-lymphocyte ratio in laying hens in different housing systems. Vet. Rec. 2010, 167, 749–751. [Google Scholar] [CrossRef]
- Dabbou, S.; Schiavone, A.; Gai, F.; Martinez, S.; Madrid, J.; Hernandez, F.; Martínez Marín, A.L.; Soglia, D.; Sartore, S.; Kalmar, I.D.; et al. Effect of dietary globin, a natural emulsifier, on the growth performance and digestive efficiency of broiler chickens. Ital. J. Anim. Sci. 2019, 18, 530–537. [Google Scholar] [CrossRef]
- Long, P.L.; Millard, B.J.; Joyner, L.P.; Norton, C.C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976, 6, 201–217. [Google Scholar] [PubMed]
- Schmidt, U. Parasitologische Kotuntersuchung durch ein neues Verdünnungsverfahren. Tieraerztl. Umsch. 1971, 26, 229–230. [Google Scholar]
- SAS Institute Inc. JMP Statistical Discovery, 5.0.1.; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Liukkonen-Anttila, T.; Kentala, A.; Hissa, R. Tannins-a dietary problem for hand-reared grey partridge Perdix perdix after release? Comp. Biochem. Phys. C 2001, 130, 237–248. [Google Scholar] [CrossRef]
- Grashorn, M.A. Use of phytobiotics in broiler nutrition-an alternative to infeed antibiotics? J. Anim. Feed Sci. 2010, 19, 338–347. [Google Scholar] [CrossRef]
- Hornick, J.L.; Van Eenaeme, C.; Gérard, O.; Dufrasne, I.; Istasse, L. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrin. 2000, 19, 121–132. [Google Scholar] [CrossRef]
- Rahimi, S.; Seidavi, A.; Sahraei, M.; Blanco, F.P.; Schiavone, A.; Marin, A.L.M. Effects of feed restriction and diet nutrient density during re-alimentation on growth performance, carcass traits, organ weight, blood parameters and the immune response of broilers. Ital. J. Anim. Sci. 2015, 14, 583–590. [Google Scholar] [CrossRef]
- Marzoni, M.; Castillo, A.; Chiarini, R.; Romboli, I. Indagine preliminare sulle prestazioni produttive di una razza avicola autoctona: La razza Livorno. In Proceedings of the Parliamo di …Allevamenti Alternativi e Valorizzazione del Territorio, Cuneo, Italy, 23–25 September 2003. [Google Scholar]
- Butler, L.G.; Rogler, J.C. Biochemical mechanisms of the antinutritional effects of tannins. Acts Symp. Series 1992, 506, 298–304. [Google Scholar] [CrossRef]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Miyakawa, M.E.F. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Buchsbaum, R.; Valiela, I.; Swain, T. The role of phenolic compounds and other plant constituents in feeding by Canada geese in a coastal marsh. Oecology 1984, 63, 343–349. [Google Scholar] [CrossRef]
- Werner, L.L.; Reavill, D.R. The diagnostic utility of serum protein electrophoresis. Vet. Clin. N. Am. Exotic. 1999, 2, 651–662. [Google Scholar] [CrossRef]
- Filipović, N.; Stojević, Z.; Milinković-Tur, S.; Beer Ljubić, B.; Zdelar-Tuk, M. Changes in concentration and fractions of blood serum proteins of chickens during fattening. Vet. Arhiv. 2007, 77, 319–326. [Google Scholar]
- Piotrowska, A.; Burlikowska, K.; Szymeczko, R. Changes in blood chemistry in broiler chickens during the fattening period. Folia Biol. 2011, 59, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Jones, M. The biochemical profile. In Proceedings of the Avian Clinical Pathology, Washington, DC, USA, 1 April 2008. [Google Scholar]
- Scanes, C.G. Protein metabolism. In Sturkie’s Avian Physiolog; Scanes, C., Ed.; Elsevier Inc.: Waltham, MA, USA, 2015; pp. 455–468. [Google Scholar]
- Jansman, A. Tannins in feedstuffs for simple-stomached animals. Nutr. Res. Rev. 1993, 6, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Cattley, R.C.; Cullen, J.M. Liver and Gall Bladder. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1509–1566. [Google Scholar] [CrossRef]
- Cejas, E.; Pinto, S.; Prosdocimo, F.; Batalle, M.; Barrios, H.; Tellez, G.; De Franceschi, M. Evaluation of Quebracho red wood (Schinopsis lorentzii) polyphenolic vegetable extract for the reduction of coccidiosis in broiler chicks. Int. J. Poult. Sci. 2011, 10, 344–349. [Google Scholar]
- Molan, A.L.; Waghorn, G.C.; Min, B.R.; McNabb, W.C. The effect of condensed tannins from seven herbages on Trichostrongylus colubriformis larval migration in vitro. Folia Parasit. 2000, 47, 39–44. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: In vitro and in vivo studies. Vet. Parasitol. 2001, 99, 205–219. [Google Scholar] [CrossRef]
- Brunet, S.; Martinez Ortiz de Montellano, C.; Torres Acosta, J.F.J.; Sandoval Castro, C.A.; Aguilar Caballero, A.J.; Capetillo Leal, C.M.; Hoste, H. Effect of the consumption of Lysiloma latisiliquum on the larval establishment of gastrointestinal nematodes in goats. Vet. Parasitol. 2008, 157, 81–88. [Google Scholar] [CrossRef]
- Hoste, H.; Martinez Ortiz de Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda Robertos, N.; Fourquaux, I.; Torres Acosta, J.F.; Sandoval Castro, C.A. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet. Parasitol. 2012, 186, 18–27. [Google Scholar] [CrossRef]
- Min, B.R.; Hart, S.P. Tannins for suppression of internal parasites. J. Anim. Sci. 2003, 81, E102–E109. [Google Scholar] [CrossRef]
- Pathak, A.K.; Dutta, N.; Banerjee, P.S.; Goswami, T.K.; Sharma, K. Effect of condensed tannins supplementation through leaf meal mixture on voluntary feed intake, immune response and worm burden in Haemonchus contortus infected sheep. J. Parasit. Dis. 2016, 40, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Allen, P.C.; Danforth, H.D.; Augustine, P.C. Dietary modulation of avian coccidiosis. Int. J. Parasitol. 1998, 28, 1131–1140. [Google Scholar] [CrossRef]
- Sugiura, Y.; Tanaka, R.; Katsuzaki, H.; Imai, K.; Matsushita, T. The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J. Funct. Foods 2013, 5, 2019–2023. [Google Scholar] [CrossRef]
- Park, M.; Cho, H.; Jung, H.; Lee, H.; Hwang, K.T. Antioxidant and anti-Inflammatory activities of tannin fraction of the extract from black raspberry seeds compared to grape seeds. J. Food Biochem. 2014, 38, 259–270. [Google Scholar] [CrossRef]
- Souza, S.M.C.; Aquino, L.C.M.; Milach, A.C.; Bandeira, M.A.M.; Nobre, M.E.P.; Viana, G.S.B. Anti-inflammatory and antiulcer properties of tannins from Myracrodruon urundeuva Allemao (Anacardiaceae) in rodents. Phytother. Res. 2007, 21, 220–225. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Kim, D.K.; Bravo, D.M.; Lee, S.H. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. BMC Proc. 2011, 5, S34. [Google Scholar] [CrossRef]
- Putaala, A.; Hissa, R. Effects of hand-rearing on physiology and anatomy in the grey partridge. Wildl. Biol. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Liukkonen-Anttila, T.; Saartoala, R.; Hissa, R. Impact of hand-rearing on morphology and physiology of the capercaillie (Tetrao urogallus). Comp. Biochem. Phys. A. 2000, 125, 211–221. [Google Scholar] [CrossRef]

| Ingredient (%) | 0–49 Days | 50–175 Days |
|---|---|---|
| Corn | 52.0 | 46.0 |
| Full fat soybean | 30.5 | 12.7 |
| Wheat | - | 20.00 |
| Soybean meal | 9.0 | 14.0 |
| Alfalfa meal | 2.8 | 2.8 |
| Gluten feed | 3.0 | 2.0 |
| Dicalcium phosphate | 1.0 | 1.0 |
| Sodium bicarbonate | 0.5 | 0.5 |
| Sodium chloride | 0.2 | 0.2 |
| Vitamin-mineral premix 1 | 0.5 | 0.5 |
| DL-Met | 0.35 | 0.2 |
| L-Lys | 0.15 | 0.1 |
| Chemical composition (% of DM) | ||
| Dry matter | 90.89 | 90.80 |
| Crude protein | 22.30 | 18.05 |
| Ether extract | 7.95 | 4.98 |
| Crude fiber | 4.67 | 4.01 |
| Ash | 5.76 | 5.59 |
| Metabolizable energy 2 (MJ/kg) | 12.54 | 12.98 |
| Item 2 | Diet 1 | |||
|---|---|---|---|---|
| QT0 | QT1 | QT2 | ||
| Birds 3 | n | 12 | 12 | 12 |
| LBW | g | 1729 ± 23 | 1733 ± 26 | 1723 ± 46 |
| Breast | % of RCC | 22.6 ± 0.52 | 21.9 ± 0.97 | 22.3 ± 0.87 |
| RCC | % of LBW | 61.5 ± 1.39 | 62.7 ± 1.57 | 61.2 ± 0.88 |
| Head-Neck | % of LBW | 6.79 ± 0.29 | 6.95 ± 0.26 | 6.89 ± 0.16 |
| Feet | % of LBW | 2.78 ± 0.08 | 2.85 ± 0.12 | 2.82 ± 0.05 |
| Liver | % of LBW | 1.52 ± 0.06 | 1.57 ± 0.07 | 1.76 ± 0.07 |
| Gizzard | % of LBW | 2.25 ± 0.09 | 2.10 ± 0.13 | 2.46 ± 0.12 |
| S. Int. L. | cm/100gLBW | 6.96 ± 0.26 | 7.03 ± 0.35 | 7.65 ± 0.30 |
| Caeca L. | cm/100gLBW | 1.92 ± 0.07 | 2.01 ± 0.07 | 2.01 ± 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzoni, M.; Castillo, A.; Franzoni, A.; Nery, J.; Fortina, R.; Romboli, I.; Schiavone, A. Effects of Dietary Quebracho Tannin on Performance Traits and Parasite Load in an Italian Slow-Growing Chicken (White Livorno Breed). Animals 2020, 10, 684. https://doi.org/10.3390/ani10040684
Marzoni M, Castillo A, Franzoni A, Nery J, Fortina R, Romboli I, Schiavone A. Effects of Dietary Quebracho Tannin on Performance Traits and Parasite Load in an Italian Slow-Growing Chicken (White Livorno Breed). Animals. 2020; 10(4):684. https://doi.org/10.3390/ani10040684
Chicago/Turabian StyleMarzoni, Margherita, Annelisse Castillo, Alessandro Franzoni, Joana Nery, Riccardo Fortina, Isabella Romboli, and Achille Schiavone. 2020. "Effects of Dietary Quebracho Tannin on Performance Traits and Parasite Load in an Italian Slow-Growing Chicken (White Livorno Breed)" Animals 10, no. 4: 684. https://doi.org/10.3390/ani10040684
APA StyleMarzoni, M., Castillo, A., Franzoni, A., Nery, J., Fortina, R., Romboli, I., & Schiavone, A. (2020). Effects of Dietary Quebracho Tannin on Performance Traits and Parasite Load in an Italian Slow-Growing Chicken (White Livorno Breed). Animals, 10(4), 684. https://doi.org/10.3390/ani10040684







