The Effect of Heat Stress on Respiratory Alkalosis and Insulin Sensitivity in Cinnamon Supplemented Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diet and Experimental Design
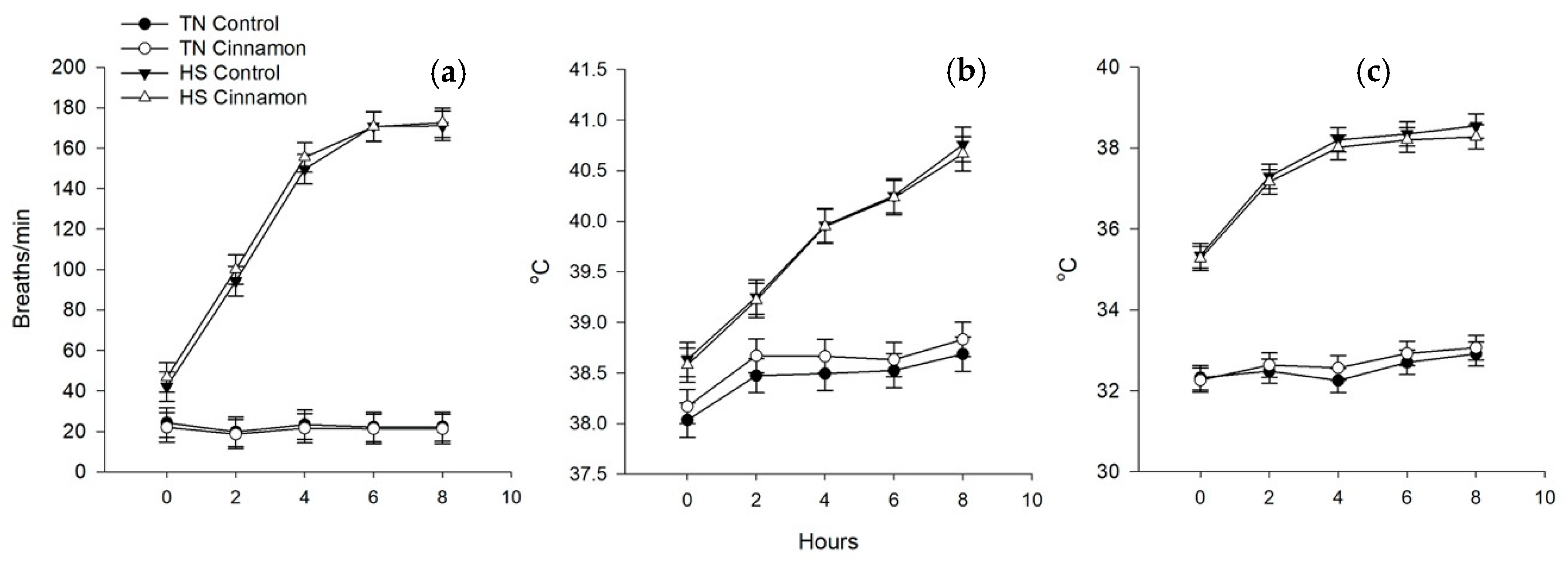
2.2. Indices of Heat Stress
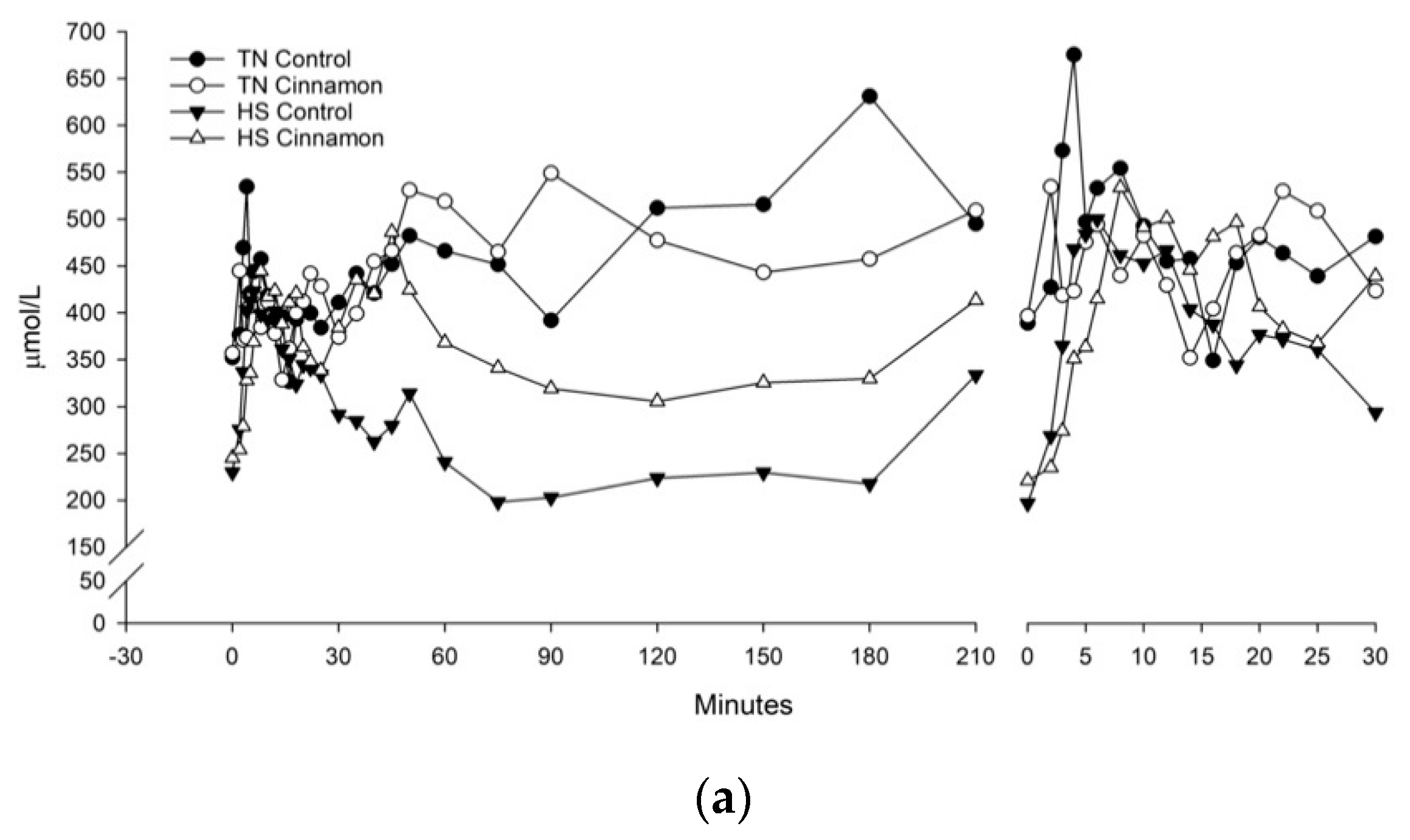
2.3. Intravenous Glucose Tolerance Test
2.4. Plasma and Urine Analysis
2.5. Glucose, NEFA and Insulin IVGTT Modelling
2.6. Ussing Chambers
2.7. Intestinal Gas Composition
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ingram, D. Stimulation of cutaneous glands in the pig. J. Comp. Pathol. 1967, 77, 93–98. [Google Scholar] [CrossRef]
- Cottrell, J.J.; Liu, F.; Hung, A.T.; DiGiacomo, K.; Chauhan, S.S.; Leury, B.J.; Furness, J.B.; Celi, P.; Dunshea, F.R. Nutritional strategies to alleviate heat stress in pigs. Anim. Prod. Sci. 2015, 55, 1391–1402. [Google Scholar] [CrossRef]
- Christon, R. The effect of tropical ambient temperature on growth and metabolism in pigs. J. Anim. Sci. 1988, 66, 3112–3123. [Google Scholar] [CrossRef] [PubMed]
- Stahly, T.S.; Cromwell, G.L.; Aviotti, M.P. The Effect of Environmental Temperature and Dietary Lysine Source and Level on the Performance and Carcass Characteristics of Growing Swine. J. Anim. Sci. 1979, 49, 1242–1251. [Google Scholar] [CrossRef]
- Sanz Fernandez, M.V.; Stoakes, S.K.; Abuajamieh, M.; Seibert, J.T.; Johnson, J.S.; Horst, E.A.; Rhoads, R.P.; Baumgard, L.H. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 2015, 3, e12478. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Ali, I.S.; Currie, R.W. Insulin induces myocardial protection and Hsp70 localization to plasma membranes in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1709–H1721. [Google Scholar] [CrossRef] [Green Version]
- Semenza, J.C.; McCullough, J.E.; Flanders, W.D.; McGeehin, M.A.; Lumpkin, J.R. Excess hospital admissions during the July 1995 heat wave in Chicago. Am. J. Prev. Med. 1999, 16, 269–277. [Google Scholar] [CrossRef]
- Niu, C.-S.; Lin, M.-T.; Liu, I.M.; Cheng, J.-T. Role of striatal glutamate in heat stroke-induced damage in streptozotocin-induced diabetic rats. Neurosci. Lett. 2003, 348, 77–80. [Google Scholar] [CrossRef]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Cinnamon in glycaemic control: Systematic review and meta analysis. Clin. Nutr. 2012, 31, 609–615. [Google Scholar] [CrossRef]
- Allen, R.W.; Schwartzman, E.; Baker, W.L.; Coleman, C.I.; Phung, O.J. Cinnamon use in type 2 diabetes: An updated systematic review and meta-analysis. Ann. Fam. Med. 2013, 11, 452–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Murali, K.Y.; Tandon, V.; Murthy, P.S.; Chandra, R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.T.; Sabin, M.A.; Leury, B.J.; Dunshea, F.R. Dietary cinnamon improves insulin sensitivity in growning pigs. Australas. Med. J. (Online) 2012, 5, 706. [Google Scholar]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and Vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat stressed pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, T.T.T.; Aarnink, A.J.A.; Verstegen, M.W.A.; Gerrits, W.J.J.; Heetkamp, M.J.W.; Kemp, B.; Canh, T.T. Effects of increasing temperatures on physiological changes in pigs at different relative humidities. J. Anim. Sci. 2005, 83, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 420. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Cottrell, J.; Wijesiriwardana, U.; Kelly, F.; Chauhan, S.; Pustovit, R.; Gonzales-Rivas, P.; DiGiacomo, K.; Leury, B.; Celi, P. Effects of chromium supplementation on physiology, feed intake, and insulin related metabolism in growing pigs subjected to heat stress. Transl. Anim. Sci. 2017, 1, 116–125. [Google Scholar] [CrossRef]
- Niiyama, M.; Yonemichi, H.; Harada, E.; Syuto, B.; Kitagawa, H. A simple catheterization from the ear vein into the jugular vein for sequential blood sampling from unrestrained pigs. Jpn. J. Vet. Res. 1985, 33, 1–9. [Google Scholar]
- Cottrell, J.; Warner, R.; McDonagh, M.; Dunshea, F. Inhibition of endogenous nitric oxide production influences ovine hindlimb metabolism independently of insulin concentrations. J. Anim. Sci. 2004, 82, 2558–2567. [Google Scholar] [CrossRef]
- Clarke, S.D.; Clarke, I.J.; Rao, A.; Cowley, M.A.; Henry, B.A. Sex differences in the metabolic effects of testosterone in sheep. Endocrinology 2012, 153, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Bergman, R.N.; Prager, R.; Volund, A.; Olefsky, J.M. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J. Clin. Investig. 1987, 79, 790–800. [Google Scholar] [CrossRef] [Green Version]
- Perseghin, G.; Caumo, A.; Caloni, M.; Testolin, G.; Luzi, L. Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J. Clin. Endocrinol. Metab. 2001, 86, 4776–4781. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, L.J.; Callaghan, B.; Rivera, L.R.; Lieu, T.; Poole, D.P.; Cho, H.J.; Bravo, D.M.; Furness, J.B. Effects of Food Components That Activate TRPA1 Receptors on Mucosal Ion Transport in the Mouse Intestine. Nutrients 2016, 8, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, M.; Ohkubo, R.; Hayashi, M. Lipopolysaccharide transport system across colonic epithelial cells in normal and infective rat. Drug Metab. Pharmacokinet. 2004, 19, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, M.D.; Rhoads, R.P.; Sanders, S.R.; Duff, G.C.; Baumgard, L.H. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 2010, 38, 86–94. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; Vanbaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Wheelock, J.B.; Sanders, S.R.; Moore, C.E.; Green, H.B.; Waldron, M.R.; Rhoads, R.P. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows. J. Dairy Sci. 2011, 94, 5620–5633. [Google Scholar] [CrossRef]
- Pearce, S.C.; Gabler, N.K.; Ross, J.W.; Escobar, J.; Patience, J.F.; Rhoads, R.P.; Baumgard, L.H. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 2013, 91, 2108–2118. [Google Scholar] [CrossRef] [Green Version]
- Sanz Fernandez, M.V.; Johnson, J.S.; Abuajamieh, M.; Stoakes, S.K.; Seibert, J.T.; Cox, L.; Kahl, S.; Elsasser, T.H.; Ross, J.W.; Isom, S.C.; et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 2015, 3, e12315. [Google Scholar] [CrossRef]
- Scott, D.; McIntosh, G.H. Changes in blood composition and in urinary mineral excretion in the pig in response to acute acid-base disturbance. Q. J. Exp. Physiol. Cogn. Med. Sci. 1975, 60, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Beatty, D.T.; Barnes, A.; Taylor, E.; Pethick, D.; McCarthy, M.; Maloney, S.K. Physiological responses of Bos taurus and Bos indicus cattle to prolonged, continuous heat and humidity. J. Anim. Sci. 2006, 84, 972–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, M.; Martz, F.A.; Stanley, R.W.; Johnson, H.D.; Campbell, J.R.; Hilderbrand, E. Effect of Diet and Ambient Temperature-Humidity on Ruminal pH, Oxidation Reduction Potential, Ammonia and Lactic Acid in Lactating Cows2. J. Anim. Sci. 1970, 30, 1023–1028. [Google Scholar] [CrossRef]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Schneider, P.L.; Beede, D.K.; Wilcox, C.J. Nycterohemeral Patterns of Acid-Base Status, Mineral Concentrations and Digestive Function of Lactating Cows in Natural or Chamber Heat Stress Environments1,2. J. Anim. Sci. 1988, 66, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Rivas, P.; DiGiacomo, K.; Giraldo, P.; Leury, B.; Cottrell, J.; Dunshea, F. Reducing rumen starch fermentation of wheat with three percent sodium hydroxide has the potential to ameliorate the effect of heat stress in grain-fed wethers. J. Anim. Sci. 2017, 95, 5547–5562. [Google Scholar] [CrossRef] [Green Version]
- Teeter, R.G.; Smith, M.O.; Owens, F.N.; Arp, S.C.; Sangiah, S.; Breazile, J.E. Chronic Heat Stress and Respiratory Alkalosis: Occurrence and Treatment in Broiler Chicks. Poult. Sci 1985, 64, 1060–1064. [Google Scholar] [CrossRef]
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.Z.; Cottrell, J.J.; Ha, N.; Pillai, N.; Yao, C.K.; Berean, K.J.; Ward, S.A.; Grando, D.; Muir, J.G.; Harrison, C.J.; et al. Potential of in vivo real-time gastric gas profiling: A pilot evaluation of heat-stress and modulating dietary cinnamon effect in an animal model. Sci. Rep. 2016, 6, 33387. [Google Scholar] [CrossRef]
- Jensen, B.B.; Jørgensen, H. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl. Environ. Microb. 1994, 60, 1897–1904. [Google Scholar] [CrossRef] [Green Version]

| Ingredient | % |
|---|---|
| Wheat | 54.0 |
| Lupin kernels 33% | 7.25 |
| Mill mix 1 | 13.5 |
| Cinnamon 2 | 1.5 |
| Canola meal 37% | 12.0 |
| Meat meal 57% | 4.50 |
| Blood meal | 1.00 |
| Water | 1.00 |
| Phosphorous pre-mix 3 | 0.010 |
| Xylanase 4 | 0.020 |
| Tallow-mixer | 3.00 |
| Limestone | 1.35 |
| DL-Methionine | 0.060 |
| Copper proteinate | 0.050 |
| Vitamin premix | 0.150 |
| Lysine | 0.350 |
| Threonine | 0.105 |
| Salt bin micro | 0.200 |
| Calculated values | |
| Digestible energy (MJ/kg) | 14 |
| Crude Protein (%) | 18.3 |
| Lysine (%) | 1.40 |
| Leucine (%) | 1.33 |
| Calcium (%) | 1.07 |
| Total phosphorous (%) | 0.642 |
| Parameter | Thermoneutral | Heat Stress | SED | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Cinnamon | Control | Cinnamon | Diet | Temp. | D × T | ||
| Glucose | ||||||||
| AUC (mmol/L h) | 19.2 | 19.2 | 21.5 | 18.7 | 1.62 | 0.24 | 0.44 | 0.23 |
| Gb (mmol/L) | 5.65 | 5.79 | 6.24 | 5.65 | 0.293 | 0.28 | 0.28 | 0.088 |
| Sg (min−1) | 0.36 | 0.061 | 0.040 | 0.045 | 0.0153 | 0.18 | 0.61 | 0.39 |
| Insulin | ||||||||
| AUC (mU/L h) | 21.6 a | 13.1 ab | 12.5 ab | 8.4 b | 4.58 | 0.062 | 0.043 | 0.49 |
| Ib (mU/L) | 5.59 | 4.38 | 3.15 | 2.84 | 1.38 | 0.44 | 0.052 | 0.64 |
| Si (mU/L min) | 53 ab | 10 a | 165 ab | 403 b | 128 | 0.29 | 0.011 | 0.13 |
| AIRg (mU/L min) | 142 a | 133 a | 80 b | 109 ab | 14.1 | 0.33 | <0.001 | 0.068 |
| -cell (mU/mM) | −35.1 | −27.6 | −20.4 | −17.9 | 8.65 | 0.42 | 0.058 | 0.68 |
| Insulin resistance (mM mU L−2) | 0.079 | 0.063 | 0.057 | 0.040 | 0.020 | 0.27 | 0.14 | 0.96 |
| DI | 5730 | 5004 | 12,623 | 38,851 | 13,338 | 0.19 | 0.043 | 0.17 |
| NEFA | ||||||||
| Fasting (nmol/L) | 382 | 356 | 187 | 245 | 73.3 | 0.75 | 0.006 | 0.42 |
| AUC (nmol/L h) | 1720 | 1576 | 763 | 1227 | 299 | 0.46 | 0.004 | 0.16 |
| QUICKI | 0.439 | 0.469 | 0.467 | 0.548 | 0.033 | 0.026 | 0.029 | 0.28 |
| mQUICKI | 0.445 a | 0.547 ab | 0.619 b | 0.574 ab | 0.053 | 0.22 | 0.033 | 0.90 |
| Glycogen (µmol/g) | ||||||||
| Liver | 101 | 118 | 128 | 128 | 16.4 | 0.63 | 0.16 | 0.46 |
| Muscle | 152 | 157 | 194 | 171 | 27.1 | 0.50 | 0.14 | 0.49 |
| Parameter | Thermoneutral | Heat Stress | SED | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Cinnamon | Control | Cinnamon | Diet | Temp. | D × T | ||
| Blood | ||||||||
| pH | 7.42 | 7.42 | 7.43 | 7.44 | 0.010 | 0.51 | 0.087 | 0.70 |
| pO2 (mmHg) | 31.7 | 32.1 | 37.0 | 40.4 | 3.20 | 0.42 | 0.008 | 0.51 |
| pCO2 (mmHg) | 53.5 | 49.3 | 49.3 | 45.4 | 1.28 | 0.024 | <0.001 | 0.094 |
| HCO3 (mmol/L) | 34.5 | 34.3 | 32.5 | 30.6 | 1.09 | 0.18 | 0.002 | 0.27 |
| Haematocrit (%) | 31.2 | 31.5 | 26.2 | 26.0 | 1.38 | 0.95 | <0.001 | 0.81 |
| Base excess [b] (mmol/L) | 8.60 | 8.45 | 7.27 | 5.70 | 1.07 | 0.27 | 0.014 | 0.36 |
| Na+ (mmol/L) | 141 | 138 | 140 | 141 | 1.33 | 0.34 | 0.27 | 0.079 |
| K+ (mmol/L) | 4.00 | 3.88 | 4.30 | 4.07 | 0.151 | 0.12 | 0.036 | 0.59 |
| Ca++ (mmol/L) | 1.28 | 1.31 | 1.33 | 1.31 | 0.025 | 0.76 | 0.22 | 0.16 |
| Urine | ||||||||
| pH | 6.38 | 6.39 | 5.39 | 5.83 | 0.297 | 0.29 | 0.001 | 0.32 |
| Osmolarity (mOsm) | 296 | 243 | 308 | 290 | 121.8 | 0.69 | 0.74 | 0.84 |
| Parameter | Thermoneutral | Heat Stress | SED | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Cinnamon | Control | Cinnamon | Diet | Temp. | D × T | ||
| TER (Ω cm2) | 80.3 a | 79.8 a | 78.8 a | 101.8 b | 6.19 | 0.016 | 0.027 | 0.012 |
| Papp (×10−4 cm/sec) | 174 | 89 | 136 | 124 | 21.5 | 0.13 | 0.96 | 0.25 |
| Parameter | Thermoneutral | Heat Stress | SED | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Cinnamon | Control | Cinnamon | Diet | Temp. | D × T | |||
| Stomach | CO2 | 5397 | 3918 | 4084 | 4513 | 908 | 0.34 | 0.54 | 0.15 |
| CH4 | 156 | 163 | 174 | 140 | 25.8 | 0.56 | 0.94 | 0.26 | |
| Jejunum | CO2 | 9374 | 4329 | 3386 | 5811 | 3100 | 0.68 | 0.23 | 0.10 |
| CH4 | 160 | 133 | 179 | 144 | 24.3 | 0.084 | 0.38 | 0.83 | |
| Ileum | CO2 | 3180 | 8709 | 2828 | 4694 | 2622 | 0.066 | 0.26 | 0.34 |
| CH4 | 149 | 130 | 161 | 165 | 14.9 | 0.57 | 0.037 | 0.30 | |
| Proximal | CO2 | 8094 | 8891 | 7011 | 6812 | 1329 | 0.85 | 0.12 | 0.60 |
| Colon | CH4 | 6430 | 6740 | 7581 | 5747 | 1626 | 0.47 | 0.92 | 0.36 |
| Distal | CO2 | 5578 | 4844 | 5839 | 5992 | 767 | 0.59 | 0.22 | 0.42 |
| Colon | CH4 | 7510 | 7233 | 8420 | 8920 | 1370 | 0.90 | 0.20 | 0.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cottrell, J.J.; Furness, J.B.; Wijesiriwardana, U.A.; Ringuet, M.; Liu, F.; DiGiacomo, K.; Leury, B.J.; Clarke, I.J.; Dunshea, F.R. The Effect of Heat Stress on Respiratory Alkalosis and Insulin Sensitivity in Cinnamon Supplemented Pigs. Animals 2020, 10, 690. https://doi.org/10.3390/ani10040690
Cottrell JJ, Furness JB, Wijesiriwardana UA, Ringuet M, Liu F, DiGiacomo K, Leury BJ, Clarke IJ, Dunshea FR. The Effect of Heat Stress on Respiratory Alkalosis and Insulin Sensitivity in Cinnamon Supplemented Pigs. Animals. 2020; 10(4):690. https://doi.org/10.3390/ani10040690
Chicago/Turabian StyleCottrell, Jeremy J., John B. Furness, Udani A. Wijesiriwardana, Mitchell Ringuet, Fan Liu, Kristy DiGiacomo, Brian J. Leury, Iain J. Clarke, and Frank R. Dunshea. 2020. "The Effect of Heat Stress on Respiratory Alkalosis and Insulin Sensitivity in Cinnamon Supplemented Pigs" Animals 10, no. 4: 690. https://doi.org/10.3390/ani10040690






