Distribution of Melanin Pigmentation in 33 Organs of Thai Black-Bone Chickens (Gallus gallus domesticus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Histological Study
2.3. Data and Statistical Analysis
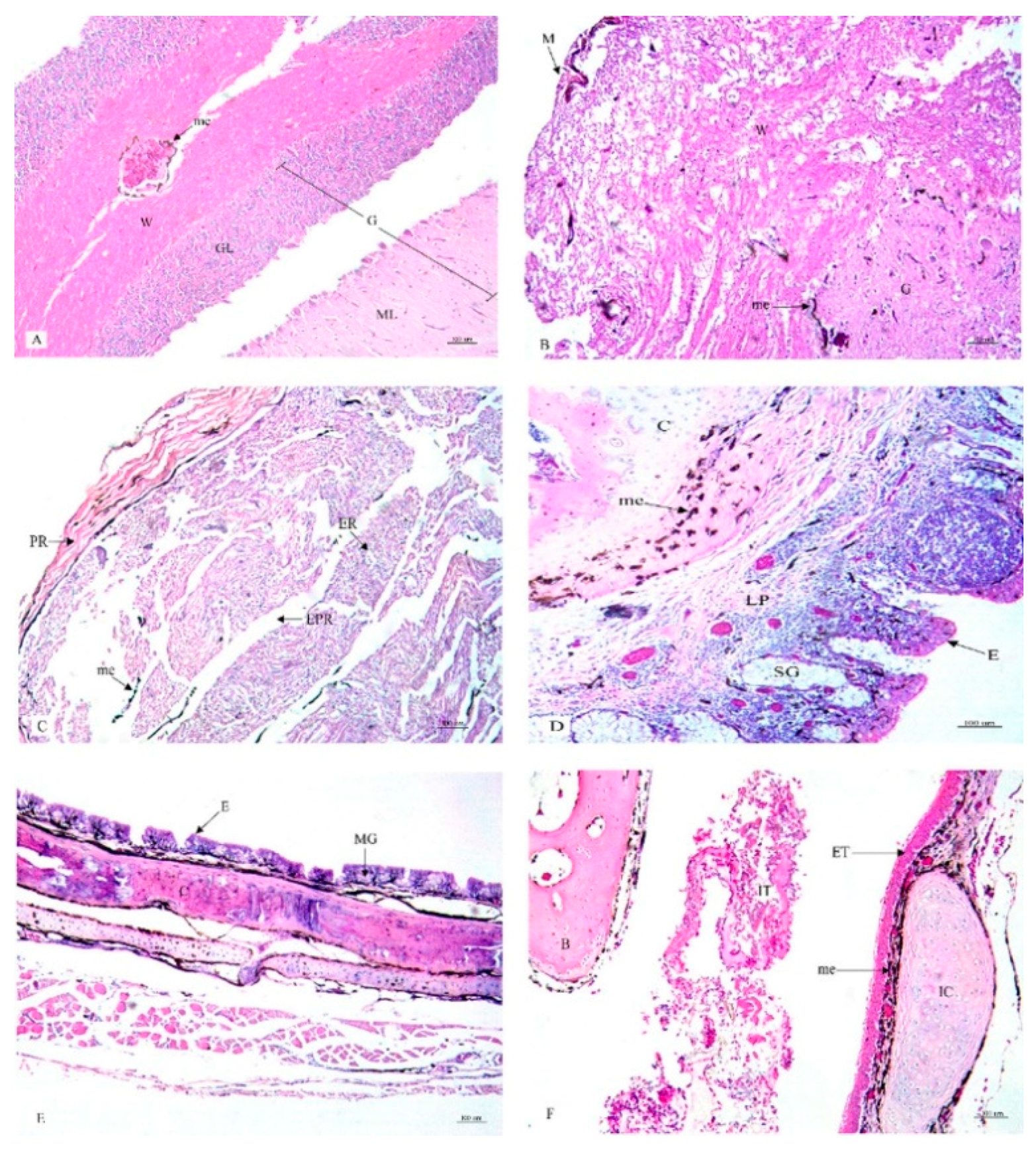
3. Results
4. Discussion
- The liver was only one organ in which the melanin pigment was not found to have accumulated.
- A uniform pattern of melanin pigment distribution was not observed among all organs of the chickens.
- Gender had no influence on the presence of the melanin pigment.
- The melanin pigment was found to be present in all tissue layers of most organs, while in some organs the melanin pigment was only found in specific layers.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaturasitha, S.; Srikanchai, T.; Kreuzer, M.; Wicke, M. Differences in Carcass and Meat Characteristics Between Chicken Indigenous to Northern Thailand (Black-Boned and Thai Native) and Imported Extensive Breeds (Bresse and Rhode Island Red). Poult. Sci. 2008, 87, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bubphapant, J. Factors influencing consumer purchase intension of Ku Phupan Black-cone chicken production in Sakon Nakhon province, Thailand: A study based on the theory of planned behavior (TBR). Int. J. Manag. Sustain. 2017, 6, 23–32. [Google Scholar]
- Tian, Y.; Xie, M.; Wang, W.; Wu, H.; Fu, Z.; Lin, L. Determination of carnosine in Black-Bone Silky Fowl (Gallus gallus domesticus Brisson) and common chicken by HPLC. Eur. Food Res. Technol. 2007, 226, 311–314. [Google Scholar] [CrossRef]
- Tu, Y.-G.; Sun, Y.-Z.; Tian, Y.; Xie, M.-Y.; Chen, J. Physicochemical characterisation and antioxidant activity of melanin from the muscles of Taihe Black-bone silky fowl (Gallus gallus domesticus Brisson). Food Chem. 2009, 114, 1345–1350. [Google Scholar] [CrossRef]
- Rózanowska, M.; Sarna, T.; Land, E.J.; Truscott, T.G. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free. Radic. Boil. Med. 1999, 26, 518–525. [Google Scholar]
- Serbedzija, G.N.; Bronner-Fraser, M.; Fraser, S.E. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Dev. 1989, 106, 809–816. [Google Scholar]
- Slominski, A.T.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Boissy, R.E. Melanosome transfer to and translocation in the keratinocyte. Exp. Dermatol. 2003, 12, 5–12. [Google Scholar] [CrossRef]
- Erickson, C.A.; Reedy, M.V. Neural crest development: The interplay between morphogenesis and cell differentiation. Curr. Top. Dev. Biol. 1998, 40, 177–209. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.A.; Goins, T.L. Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development 1995, 121, 915–924. [Google Scholar] [PubMed]
- Ortolani-Machado, C.; Freitas, P.; Faraco, C.; Ortolani-Machado, C. Melanogenesis in dermal melanocytes of Japanese Silky chicken embryos. Tissue Cell 2009, 41, 239–248. [Google Scholar] [CrossRef]
- Reedy, M.V.; Faraco, C.D.; Erickson, C.A. Specification and migration of melanoblasts at the vagal level and in hyperpigmented silkie chickens. Dev. Dyn. 1998, 213, 476–485. [Google Scholar] [CrossRef]
- Faraco, C.D.; Erickson, C.A. Hyperpigmentation in the Silkie fowl correlates with abnormal migration of fate-restricted melanoblasts and loss of environmental barrier molecules. Dev. Dyn. 2001, 220, 212–225. [Google Scholar] [CrossRef]
- Prota, G. The role of peroxidase in melanogenesis revisited. Pigment. Cell Res. 1992, 3, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K. Current Update and Trends in Melanin Pigmentation and Melanin Biology. Keio J. Med. 1995, 44, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Nofsinger, J.B.; Weinert, E.E.; Simon, J.D. Establishing structure-function relationships for eumelanin. Biopolymers 2002, 67, 302–305. [Google Scholar] [CrossRef]
- Mohagheghpour, N.; Waleh, N.; Garger, S.J.; Dousman, L.; Grill, L.K.; Tuse, D. Synthetic Melanin Suppresses Production of Proinflammatory Cytokines. Cell. Immunol. 2000, 199, 25–36. [Google Scholar] [CrossRef]
- Le Poole, I.; Wijngaard, R.V.D.; Westerhof, W.; Verkruisen, R.; Dutrieux, R.; Dingemans, K.; Das, P. Phagocytosis by Normal Human Melanocytes in Vitro. Exp. Cell Res. 1993, 205, 388–395. [Google Scholar] [CrossRef]
- Han, D.; Wang, S.; Hu, Y.; Zhang, Y.; Dong, X.; Yang, Z.; Wang, J.; Li, J.; Deng, X. Hyperpigmentation Results in Aberrant Immune Development in Silky Fowl (Gallus gallus domesticus Brisson). PLoS ONE 2015, 10, e0125686. [Google Scholar] [CrossRef]
- Kuklenski, J. Over occurrence and the distribution of the pigment in the organs and tissues of Japanese Silky chickens. Arch. Mikrosk. Anat. 1915, 87, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Le Douarin, N.M.; Teillet, M.A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J. Embryol. Exp. Morphol. 1973, 30, 31–48. [Google Scholar] [PubMed]
- Thitaram, C.; Matchimakul, P.; Pongkan, W.; Tangphokhanon, W.; Maktrirat, R.; Khonmee, J.; Sathanawongs, A.; Kongtueng, P.; Nganvongpanit, K. Histology of 24 organs from Asian elephant calves (Elephas maximus). PeerJ 2018, 6, e4947. [Google Scholar] [CrossRef] [PubMed]
- Engle, S.; Whalen, S.; Joshi, A.; Pollard, K.S. Unboxing cluster heatmaps. BMC Bioinform. 2017, 18, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, M.C.; Stucky, M.; Wakefield, C.; Melott, J.M.; Akbani, R.; Weinstein, J.N.; Broom, B.M. Interactive Clustered Heat Map Builder: An easy web-based tool for creating sophisticated clustered heat maps. F1000Research 2020, 8, 1750. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.D. A Review of Hierarchical Classification. J. R. Stat. Soc. Ser. A (General) 1987, 150, 119. [Google Scholar] [CrossRef]
- Kogan, J.; Nicholas, C.; Teboulle, M. Grouping Multidimensional Data-Recent Advances in Clustering; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Anonymous. The R Project for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 13 February 2012).
- Supakankul, P.; Kumchoo, T.; Mekchay, S. Identification and characterization of novel single nucleotide polymorphism markers for fat deposition in muscle tissue of pigs using amplified fragment length polymorphism. Asian-Australas. J. Anim. Sci. 2016, 30, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Mills, J.C.; Shivdasani, R.A. Gastric epithelial stem cells. Gastroenterology 2010, 140, 412–424. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.A.; DeLaForest, A.; Battle, M.A. Patterning the gastrointestinal epithelium to confer regional-specific functions. Dev. Boil. 2018, 435, 97–108. [Google Scholar] [CrossRef]
- Eroschenko, V.P. Atlas of Histology with Fuctional Correlations, 8th ed.; Eroschenko, V.P., Ed.; Williams & Wikins: Media, PA, USA, 1996; p. 361. [Google Scholar]
- Hirano, S. Observations on pigment granules in the bones of silky fowls. Arch. Histol. Cytol. 1990, 53, 89–93. [Google Scholar] [CrossRef] [Green Version]






| System | Organs | Layers | Male | Female |
|---|---|---|---|---|
| Nervous system | Brain | Meninges | 5/5 | 5/5 |
| Gray matter | 4/5 | 4/5 | ||
| White matter | 0/5 | 2/5 | ||
| Spinal cord | Meninges | 3/3 | 3/3 | |
| Gray matter | 3/3 | 3/3 | ||
| White matter | 2/3 | 2/3 | ||
| Sciatic nerve | Epineurium | 5/5 | 4/4 | |
| Perineurium | 5/5 | 4/4 | ||
| Endoneurium | 5/5 | 4/4 | ||
| Respiratory system | Larynx | Epithelium | 0/5 | 2/5 |
| Lamina propria | 5/5 | 5/5 | ||
| Elastic cartilage | 5/5 | 5/5 | ||
| Trachea | Hyaline cartilage | 3/3 | 4/4 | |
| Epithelium | 0/3 | 0/4 | ||
| Mucous gland | 3/3 | 4/4 | ||
| Syrinx | External tympanic membrane | 2/2 | 3/3 | |
| Internal tympanic membrane | 2/2 | 3/3 | ||
| Intermediate syringeal cartilage | 2/2 | 3/3 | ||
| Bony pessulus | 2/2 | 3/3 | ||
| Lung | Parabronchial wall | 5/5 | 5/5 | |
| Blood vessel | 5/5 | 5/5 | ||
| Circulatory system | Heart | Epicardium | 1/5 | 3/5 |
| Myocardium | 1/5 | 1/5 | ||
| Endocardium | 1/5 | 0/5 | ||
| Pericardium | Fibrous pericardium | 4/4 | 3/3 | |
| Serous pericardium | 4/4 | 3/3 | ||
| Aorta | Tunica adventitia | 4/4 | 4/4 | |
| Tunica media | 4/4 | 4/4 | ||
| Tunica intima | 4/4 | 4/4 | ||
| Brachial vein | Tunica adventitia | 3/3 | 5/5 | |
| Tunica media | 3/3 | 5/5 | ||
| Tunica intima | 0/3 | 3/5 | ||
| Urinary system | Kidney | Capsule | 2/5 | 1/5 |
| Cortex | 1/5 | 0/5 | ||
| Medulla | 4/5 | 2/5 | ||
| Cloaca | Epithelium | 0/4 | 0/4 | |
| Connective tissue | 4/4 | 4/4 | ||
| Muscular layer | 4/4 | 4/4 | ||
| Reproductive system | Oviduct | Tunica serosa | 0/0 | 3/3 |
| Tunica muscularis | 0/0 | 3/3 | ||
| Tunica mucosa | 0/0 | 3/3 | ||
| Epithelium | 0/0 | 0/3 | ||
| Testis | Tunica albuginia | 4/4 | 0/0 | |
| Leydig cells | 4/4 | 0/0 | ||
| Smooth muscle | 4/4 | 0/0 | ||
| Sperm | 0/0 | 0/0 | ||
| Skeleton system | Gastrocnemius muscle | Epimysium | 5/5 | 5/5 |
| Perimysium | 5/5 | 4/5 | ||
| Endomysium | 5/5 | 5/5 | ||
| Femur | Periosteum | 4/4 | 5/5 | |
| Compact bone | 4/4 | 5/5 | ||
| Medullary cavity | 2/4 | 3/5 | ||
| Digestive system | Tongue | Epithelium | 4/4 | 5/5 |
| Lamina propria | 4/4 | 5/5 | ||
| Muscular layer | 4/4 | 5/5 | ||
| Esophagus | Tunica serosa | 5/5 | 4/4 | |
| Tunica muscularis | 0/5 | 0/4 | ||
| Tunica submucosa | 0/5 | 0/4 | ||
| Tunica mucosa | 0/5 | 0/4 | ||
| Crop | Tunica adventitia | 5/5 | 5/5 | |
| Tunica muscularis | 5/5 | 5/5 | ||
| Tunica submucosa | 5/5 | 5/5 | ||
| Tunica mucosa | 0/5 | 0/5 | ||
| Proventriculus | Tunica serosa | 5/5 | 5/5 | |
| Tunica muscularis | 5/5 | 5/5 | ||
| Tunica submucosa | 5/5 | 5/5 | ||
| Tunica mucosa | 5/5 | 5/5 | ||
| Gizzard | Connective tissue | 3/5 | 5/5 | |
| Muscular layer | 4/5 | 5/5 | ||
| Tunica submucosa | 2/5 | 2/5 | ||
| Gland in mucous membrane | 1/5 | 1/5 | ||
| Duodenum | Tunica serosa | 5/5 | 5/5 | |
| Tunica muscularis | 1/5 | 2/5 | ||
| Tunica submucosa | 0/5 | 1/5 | ||
| Tunica mucosa | 0/5 | 1/5 | ||
| Jejunum | Tunica serosa | 5/5 | 4/4 | |
| Tunica muscularis | 2/5 | 2/4 | ||
| Tunica submucosa | 0/5 | 0/5 | ||
| Tunica mucosa | 0/5 | 0/5 | ||
| Ileum | Tunica serosa | 5/5 | 5/5 | |
| Outer longitudinal muscle | 4/5 | 5/5 | ||
| Inner circular muscle | 2/5 | 3/5 | ||
| Tunica submucosa | 1/5 | 1/5 | ||
| Tunica mucosa | 0/5 | 0/5 | ||
| Cecum | Tunica serosa | 5/5 | 5/5 | |
| Tunica muscularis | 5/5 | 5/5 | ||
| Tunica submucosa | 2/5 | 3/5 | ||
| Tunica mucosa | 0/5 | 0/5 | ||
| Pancreas | Exocrine | 0/5 | 0/5 | |
| Endocrine | 0/5 | 0/5 | ||
| Connective tissue | 5/5 | 5/5 | ||
| Liver | Capsule | 0/5 | 0/5 | |
| Hepatic lobules | 0/5 | 0/5 | ||
| Portal tracts | 0/5 | 0/5 | ||
| Gall bladder | Tunica adventitia | 3/3 | 1/1 | |
| Tunica muscularis | 1/3 | 0/1 | ||
| Tunica submucosa | 0/3 | 0/1 | ||
| Lamina propria | 0/3 | 0/1 | ||
| Epithelium | 0/3 | 0/1 | ||
| Omentum | Connective tissue | 5/5 | 5/5 | |
| Cytoplasm | 5/5 | 5/5 | ||
| Abdominal fat | Cytoplasm | 0/0 | 3/3 | |
| Lymphatic system | Spleen | Capsule | 5/5 | 4/5 |
| Red pulp | 0/5 | 0/5 | ||
| White pulp | 0/5 | 0/5 | ||
| Central artery | 0/5 | 0/5 | ||
| Integumentary System | Skin | Epidermis | 0/5 | 1/5 |
| Dermis | 5/5 | 5/5 | ||
| Subcutis | 5/5 | 5/5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nganvongpanit, K.; Kaewkumpai, P.; Kochagul, V.; Pringproa, K.; Punyapornwithaya, V.; Mekchay, S. Distribution of Melanin Pigmentation in 33 Organs of Thai Black-Bone Chickens (Gallus gallus domesticus). Animals 2020, 10, 777. https://doi.org/10.3390/ani10050777
Nganvongpanit K, Kaewkumpai P, Kochagul V, Pringproa K, Punyapornwithaya V, Mekchay S. Distribution of Melanin Pigmentation in 33 Organs of Thai Black-Bone Chickens (Gallus gallus domesticus). Animals. 2020; 10(5):777. https://doi.org/10.3390/ani10050777
Chicago/Turabian StyleNganvongpanit, Korakot, Piyatida Kaewkumpai, Varankpicha Kochagul, Kidsadagon Pringproa, Veerasak Punyapornwithaya, and Supamit Mekchay. 2020. "Distribution of Melanin Pigmentation in 33 Organs of Thai Black-Bone Chickens (Gallus gallus domesticus)" Animals 10, no. 5: 777. https://doi.org/10.3390/ani10050777
APA StyleNganvongpanit, K., Kaewkumpai, P., Kochagul, V., Pringproa, K., Punyapornwithaya, V., & Mekchay, S. (2020). Distribution of Melanin Pigmentation in 33 Organs of Thai Black-Bone Chickens (Gallus gallus domesticus). Animals, 10(5), 777. https://doi.org/10.3390/ani10050777







