1. Introduction
There is currently an increasing demand for high-quality dry-cured products from traditional pig breeds like the Iberian breed. Such demands are transforming the traditional extensive management of this animal into more intensive systems, by adopting management strategies from modern breeds and selecting lines with higher prolificacy to improve farm profitability.
Traditionally, the Iberian pig is characterized by lower prolificacy than modern commercial breeds, with around 6–7 piglets per litter [
1,
2] due to its small uterus and therefore limited uterine capacity [
3,
4,
5]. Currently, the litter size in some farms has risen to around 9–9.5 piglets, which increases the occurrence of intrauterine growth restriction (IUGR) processes and, subsequently, the incidence of neonates with low birthweight (LBW).
The problems derived from LBW have been widely studied in commercial lean pigs [
6,
7], especially in large litters [
8,
9,
10]. The pork market requires homogeneity in body weight and quality traits of carcasses and meat within batches [
11], and such traits are affected in LBW animals when compared to normal birthweight (NBW) individuals [
12,
13,
14,
15]. Firstly, the appearance of LBW pigs causes a lack of homogeneity within litters and feedlots. Second, LBW piglets show higher morbidity and mortality, and lower growth potential, lower feed efficiency, and lower meat yield than their NBW littermates [
13,
16,
17]. Moreover, LBW piglets may modify their physiology and metabolism via prenatal programming in response to the inadequate intrauterine environment, whether of maternal or placental origin [
15,
18]. After birth, these individuals are predisposed to excess adiposity as an adaptive mechanism for energy storage and survival in the inadequate postnatal environment expected, so carcass yields and meat quality are affected [
13,
15].
In the case of the Iberian pig, there is scarce information about the effects of litter size and birthweight on postnatal development. There have been only a couple of studies using crossbred Iberian × Duroc individuals [
19,
20], which support a influence similar to that of lean breeds of prenatal conditions and birthweight on postnatal development and meat and carcass quality of offspring under farm conditions. Moreover, birthweight is an even more critical subject of study for productive traits in traditional pigs, because the effects increase with offspring age and traditional pigs are characterized by longer cycles than lean pigs. However, there have been no previous studies on purebred Iberian individuals, which are used for the production of the most expensive dry-cured products. The Iberian pig is characterized by a
thrifty genotype, adapted to harsh environments, which includes changes in genes and pathways driving metabolism and energy saving [
21,
22,
23]. Thus, we hypothesized that the energy partitioning between sow and fetuses (more dramatic in large litters) might have some effects on postnatal development independent of those due to the IUGR processes. Hence, the current work reports the results of two studies that aimed to determine: (a) the birthweight-independent effects of the number of piglets in the litter of origin (high vs. low litter size) and (b) the effects of birthweight (LBW vs. NBW in large litters) on postnatal performance and carcass and meat quality of purebred Iberian pigs.
2. Materials and Methods
2.1. Animals, Management, and Ethics Statements
Two experiments, involving 110 purebred Iberian piglets selected from 24 primiparous Iberian sows (a total of 197 piglets born, without stillbirths) from the Retinto strain of this breed, were developed for this study. These sows were genotyped for purity of breed by determining polymorphisms for
LEPR gene (homozygosity for
LEPRc.1987T) by pyrosequencing, as previously described [
24]. All the animals, sows and piglets, with no evidence of health problems and adequate pathogen monitoring reports, were housed and managed at INIA animal facilities in agreement with local, national, and European requirements for scientific procedures and animal establishments according to the EU Directive regarding the protection of animals used for scientific purposes (2010/63/UE).
At the beginning of each trial, all of the females weighed around 130 kg and had a mean back-fat depth of around 40 mm. These females were selected, after pregnancy diagnosis on Day 35 of gestation, from a group of animals that were inseminated with purebred Iberian semen after estrus synchronization with altrenogest (Regumate
®, MSD, Boxmeer, The Netherlands). Sows were allocated to collective outdoor pens with around 7 m
2 of surface area per animal until one week before expected parturition, after which these females were housed in single indoor pens of 5.49 m
2 until piglets were weaned. The collective pens had individual feeders so, during pregnancy, each sow had her own diet consisting of 2 kg of standard grain-based food diet with mean values of 13.0% crude protein, 2.8% fat, and 3.00 Mcal/kg metabolisable energy. From farrowing to weaning, average daily feed intake was increased to 3.5 kg per sow. Further information on diets is provided in
Supplementary File S1. Water was provided ad libitum during all periods.
After weaning, all the piglets were housed, with males and females separated, in collective outdoor pens and fed with one of two standards diets adapted to age intervals (26–60 and 60–180 days old). From the first month after weaning, the piglets were fed a standard diet with mean values of 18% crude protein, 4.5% fat, and 3.35 Mcal/kg of metabolizable energy; average daily feed intake was 0.5 kg. From 60 to 120 days of age, the piglets were fed a diet containing mean values of 15.1% crude protein, 2.8% fat and 3.08 Mcal/kg of metabolizable energy; the amount of food offered and therefore average daily feed intake were recalculated with age, from 1 to 2.5 kg, to fulfil daily maintenance requirements. Further information on diets is provided in
Supplementary File S1. Water was provided
ad libitum during all periods.
The experimental procedures were assessed and approved by the INIA Committee of Ethics in Animal Research and subsequently by the competent regional authority (report PROEX114/16), according to the Spanish Policy for Animal Protection (RD 53/2013), which meets the European Union Directive 2010/63/UE on the protection of research animals.
2.2. Experimental Design
The selection of the piglets for each experiment was done by prioritizing the LITTER-SIZE experiment (first trial) in order to exclude NBW pigs with extreme birthweight and size. Second, LBW and NBW piglets were selected from the same litters for the BIRTH-WEIGHT experiment (second trial). The remaining piglets were only maintained until weaning to avoid differences in the productive condition due to litter size during lactation.
The first experiment (litter size) aimed to study the effects of the number of piglets in the litter of origin (high vs. low litter size), independently of birthweight, and included 54 piglets from small (<8 piglets/litter; 12 females and 12 males) and large litters (≥8 piglets/litter; 15 females and 15 males). In order to avoid effects due to birthweight, the 54 piglets selected were to be similar in weight between both groups and representative of the average weight and size of the breed (i.e., all of them were NBW), and, to avoid effects of milk availability during lactation, underwent within-group fostering in order to equalize the number of piglets among sows.
The second experiment (birthweight) aimed to study the effects of birthweight and included 18 LBW piglets (9 females and 9 males) and 38 NBW piglets (19 females and 19 males) from large litters (≥8 piglets/litter). The threshold for LBW was a birthweight more than one standard deviation below the average birthweight, adjusted by sex [
19,
25,
26]. In all the piglets of both experiments, sex and number of littermates were recorded at birth. The same data and laboratory analyses, described below, were recorded and carried out in both experiments. At weaning, a total of 44 piglets representative of mean body weight and size for their groups were sampled to determine characteristics of the carcass and the meat (four females and four males from small litters plus six females and six males from large litters in the LITTER-SIZE trial, and four LBW females and four LBW males plus eight NBW females and eight NBW males in the BIRTH-WEIGHT trial).
The remaining 66 piglets were used to determine the effects of litter size and birthweight on juvenile development (patterns of growth and fattening and metabolic traits) and quality of carcass and meat at 180 days old (8 females and 8 males from small litters plus 9 females and 9 males from large litters in the LITTER-SIZE trial, and 5 LBW females and 5 LBW males plus 11 NBW females and 11 NBW males in the birthweight trial). However, one male from a large litter and one female from a small litter, and two NBW females died due to accidents not related to the experimental design.
2.3. Assessment of Patterns of Growth and Fattening during the Juvenile Period
Body weight and size (trunk length from the nape of the neck to the tail, and abdominal and thoracic circumferences) were measured at birth and again at 15 and 25 days old (when weaning was performed) to characterize the early postnatal performance. Subsequently, values for body weight and size, subcutaneous back-fat depth, and loin diameter were obtained twice monthly for the first three months after weaning (45, 60, 75, and 90 days old) and monthly after that (120, 150 and 180 days old) to characterize the postnatal performance. Body weights were used to calculate intermediate and total average daily weight gain (ADWG) and fractional growth rates (weight gained per day per starting weight; defined by Hansen and co-workers [
27]) during the period of study. Subcutaneous back-fat depth (divided into outer and inner layers) and loin diameter were measured using a SonoSite S-Series ultrasound machine with a multifrequency linear array probe (5–8 MHz; SonoSite Inc., Bothell, WA, USA) at the P2 point of the last rib head.
2.4. Assessment of Metabolic Traits
Indexes of lipids and glucose metabolism were determined for piglets sampled at weaning (25 days old) and then at 120, 150, and 180 days old. Blood samples were drawn between 9:00 and 10:00 a.m. after a fasting period of around 18 hours, as previously described [
28], using heparin vacuum tubes (Vacutainer
® Systems Europe, Becton Dickinson, Meylan, France) from either the external jugular vein at 120 and 150 days old, or from the orbital sinus at 180 days old for animal welfare, as fattening of the neck made it difficult to get samples from the jugular. Samples were immediately centrifuged at 1500×
g for 10 min, and the plasma was separated and stored at −20 °C until it was assayed. Plasma concentrations of parameters indicative of lipids (total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and triglycerides) and glucose metabolism (glucose and fructosamine) were measured using a clinical chemistry analyzer (Saturno 300 plus, Crony Instruments SRL, Rome, Italy), according to the manufacturer’s instructions.
2.5. Assessment of Body Composition and Tissue Sampling at 25 and 180 Days Old
At 25 and 180 days old, pigs were sequentially euthanized by stunning and exsanguination in compliance with RD53/2013 standard procedures. Immediately, weights of head, carcass, and total and individual viscerae (adrenal glands, brain, heart, intestine, kidneys, liver, lungs, pancreas, and spleen) were determined. The ratios of carcass and viscera weight to body weight were also calculated. Afterwards, samples of brain, liver (the right lateral lobe), muscle (gluteus medius (GM) and longissimus dorsi (LD) at the level of the last rib), and subcutaneous back-fat were obtained and stored at −20 °C to determine fat percentage and fatty acid (FA) composition. A second sample of LD muscle was used on the day of sampling to assess drip-loss capacity. The carcass ratio and data from muscles were used to evaluate carcass and meat quality.
2.6. Assessment of Fatty Acid Composition in Diet and Pigs at 25 and 180 Days Old
Fatty acids in the feed were identified, after extraction and methylation, using a gas chromatograph (Hewlett Packard HP-6890, Palo Alto, CA, USA) with a capillary column (HP-Innowax, 30 m × 0.32 mm i.d., and 0.25 µm polyethylene glycol-film thickness and a flame ionization detector [
29]). Data are detailed in
Supplementary File S1.
The lipids from brain, liver, and muscle (intramuscular fat; IMF) were extracted (expressed as a dry matter percentage [
30] and fractionated into the main lipid fractions: neutral lipids (triglycerides) and polar lipids (phospholipids) [
31]). Finally, FAs were methylated and identified [
32]. Fatty acids of subcutaneous back-fat were also separately analyzed in outer and inner layers. From individual FA values, proportions of saturated, monounsaturated, and polyunsaturated FA (SFA, MUFA, and PUFA), the unsaturated index (UI), and the sum of total n−3 FA (∑n−3) and ∑n−6 FA and its ratio (∑n−6/∑n−3) were calculated [
33]. Moreover, the activity of stearoyl-CoA desaturase enzyme 1 (SCD1) was estimated using the desaturation indexes, ratios of C18:1/C18:0 and MUFA/SFA [
34].
2.7. Statistical Analyses
Data were analyzed using the SAS version 9.4 (SAS Institute Inc., Carry, NC, USA). Dependent variables at 180 and 25 days old (weight, ADWG, body, back-fat and loin measures, FA profile, body composition, and metabolism indexes) were assessed using two-way ANOVA in a general linear model in each independent trial. The LITTER-SIZE trial included sex (female/male) and litter size (<8/≥8 piglets/litter) effects and interactions. The BIRTH-WEIGHT trial included sex and birthweight (LBW/NBW) effects and interactions. Variables with changes over time (all weights, ADWGs, metabolism indexes, and body, back-fat, and loin measures) were also assessed using a repeated-measures ANOVA with the Greenhouse–Geisser correction in each independent trial, using the same fixed factors described for the previous analyses plus time and its interactions. When the interaction was significant, litter size or birthweight were studied within sex. The pig was the experimental unit, and statistical significance was accepted from
p < 0.05. All the significant results are expressed as mean ±SD in the manuscript and
supplementary tables.
4. Discussion
The results of the present study indicated that the effect of a restricted prenatal environment on offspring phenotype and performance goes beyond occurrence of intrauterine growth restriction (IUGR) and subsequent lowBW (LBW) with its inherent complications. Piglets with normal BW (NBW) but born within large litters (larger than eight piglets), which implies a lower availability of uterine space and therefore of nutrients and oxygen, had differences in developmental patterns, fat deposition, and FA composition when compared to NBW piglets from small litters. These data support the notion that the prenatal environment, even when the individual may cope with it, inescapably affects postnatal life.
The current study included two different trials aiming to determine, in animals of the same breed maintained under same environment and management conditions, the relative weight of the effects of either BIRTH-WEIGHT (by comparing NBW and LBW piglets) or the LITTER-SIZE (availability of uterine space and resources) independently of the birthweight (by comparing NBW piglets from large and small litters).
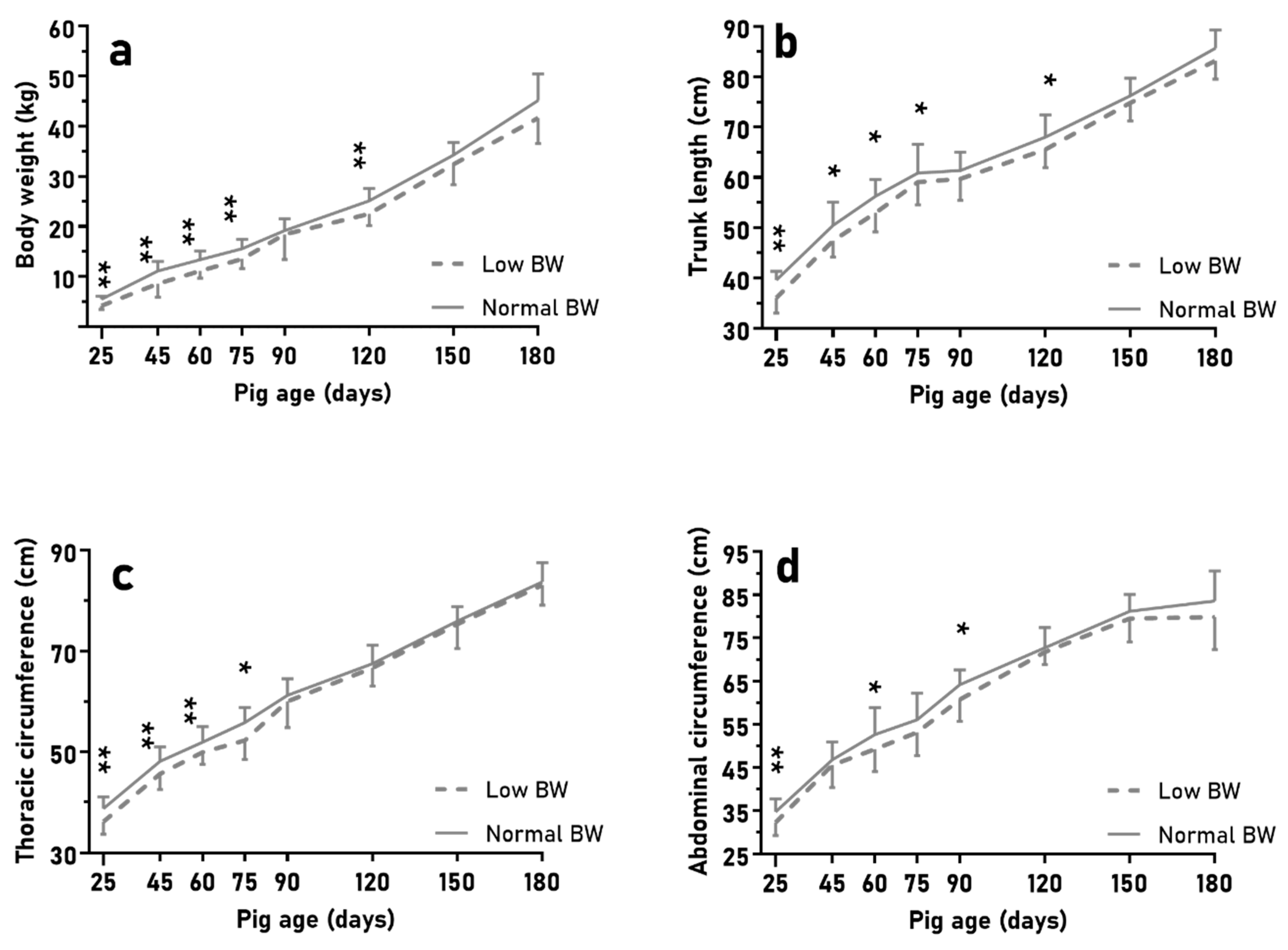
The results of the experiment aiming to determine the effects of BIRTH-WEIGHT showed, as expected, similar results to previous studies on both commercial lean strains [
9,
13] and traditional fatty pigs [
19,
20,
35]. In brief, in agreement with the experimental design, the piglets selected as representative of LBW showed, at birth, significantly lower body weight and size (trunk length and abdominal and thoracic circumferences) than the piglets selected as NBW. These differences were maintained or even increased during the early postnatal period, because NBW piglets showed higher ADWG and better muscle development, in agreement with results previously reported for lean breeds [
36]. Hence, LBW piglets remained smaller and lighter at weaning, showing higher relative weights of head, heart, liver, kidneys, adrenal glands, and pancreas than NBW piglets (i.e., evidenced asymmetrical growth patterns), which are all signs of IUGR [
37].
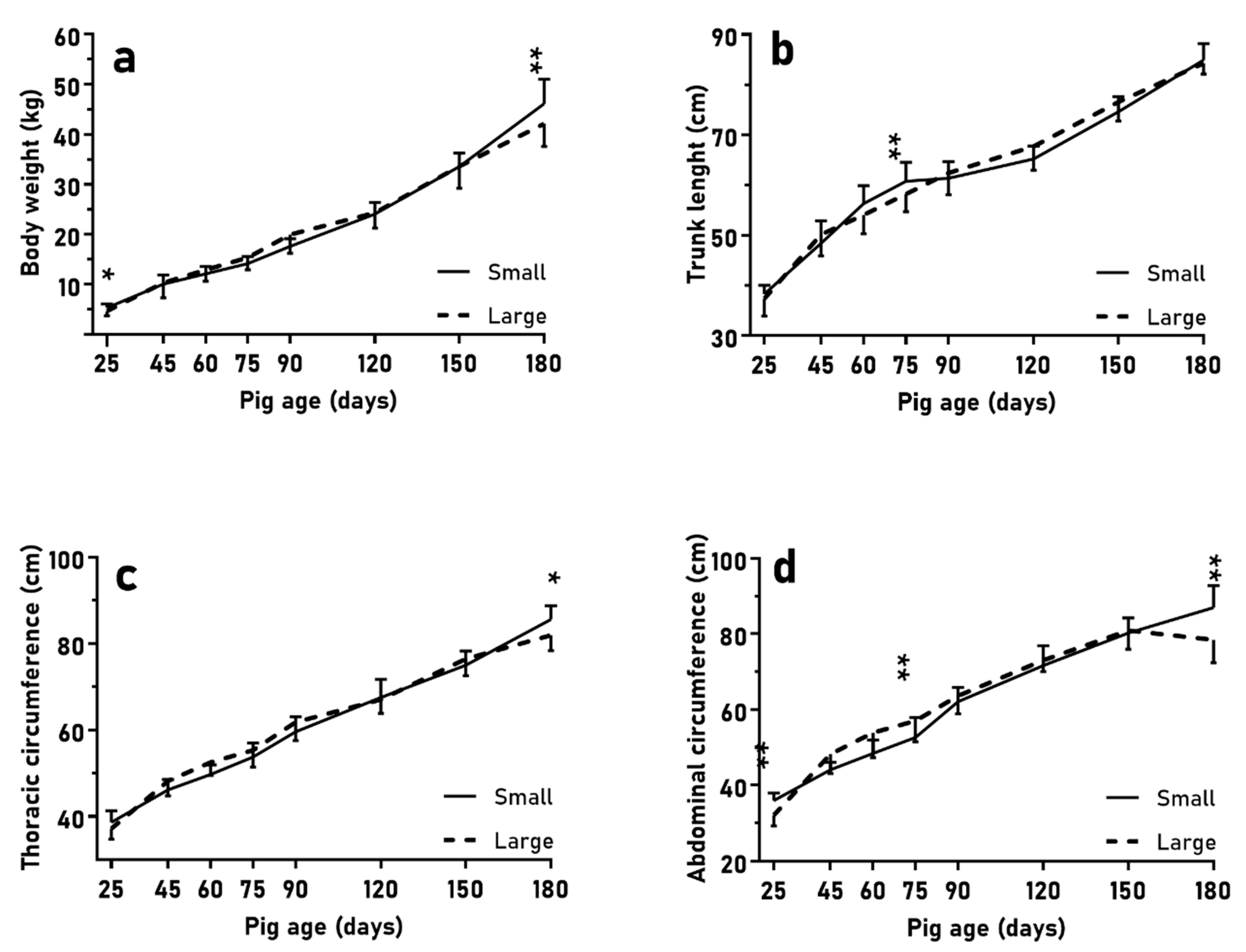
After weaning, during the juvenile period, NBW pigs remained significantly heavier and larger until 75 days old (i.e., during the post-weaning phase and before the late-growing phase). Afterwards, differences in body weight and corpulence between NBW and LBW pigs were not statistically significant and the relative sizes of carcass and viscerae were ultimately similar in both groups, except for intestine and kidneys, with lower relative weights in LBW than in NBW at 180 days old, these being two of the systems more affected in case of IUGR [
38,
39]. We also have to note that NBW pigs maintained better muscle development while LBW pigs showed increased intramuscular fat content, despite no differences in subcutaneous or visceral adiposity. These results are in agreement with previous studies in both lean [
40,
41] and fatty pigs [
42,
43] and are thought to be consequent to alterations in adipogenesis during prenatal stages [
44].
The results of the experiment aiming to determine the influence of LITTER-SIZE showed that piglets from large litters, similarly to LBW piglets in the BIRTH-WEIGHT trial, showed the worst ADWG during lactation and, therefore, the lowest body weight and size at weaning. However, in contrast to the LBW piglets of the BIRTH-WEIGHT trial, piglets from large litters showed higher adiposity than piglets from small litters at weaning,without evidence of asymmetrical growth patterns between groups except in the pancreas. It was not possible to elucidate the metabolic implications of differences in the size of the pancreas under the design of the current study; however, it is known that this organ is one of the most affected in case of prenatal programming, with changes having a prominent role in metabolic disturbances [
45,
46].
Subsequently, during the postnatal period, piglets from large and small reached similar values of body weight and size in spite of very different patterns of ADWG. Pigs from large litters had a more precocious weight gain, with higher fractional growth rate, during the post-weaning phase (25–75 days old), and even higher adiposity and larger abdominal circumference at 75 days old (abdominal circumference is predictive of carcass and visceral fat, as validated by quantitative dissection [
47]). Afterwards, pigs from small litters showed a higher weight gain, with higher ADWG and fractional growth rate, during the late-growing phase (75–180 days old) and reached higher body weight, body size, and adiposity (including higher intramuscular fat content) than piglets from small litters at 180 days old.
The comparison of growth patterns in LBW piglets in the BIRTH-WEIGHT trial and NBW piglets from large litters in the LITTER-SIZE experiment suggested two very different developmental patterns. In brief, LBW piglets were still affected by growth restriction during the post-weaning period (25–75 days old), in spite of developing a higher fractional growth rate than NBW counterparts. They also showed compensatory catch-up growth only during the late-growing period (75–180 days old), as already observed in previous studies in LBW Iberian crossbred pigs [
20]. On the other hand, piglets from large litters showed a very early catch-up growth during the post-weaning period (25–75 days old) which slowed down during the late-growing period, when pigs from small litters were initiating their fattening period. There were no major effects of BIRTH-WEIGHT or LITTER-SIZE on metabolic features during postnatal development, except at weaning. At that moment, there were higher concentrations of total and LDL cholesterol in LBW piglets and higher fructosamine concentrations in piglets from large litters. Fructosamine is indicative of precedent glucose availability, so such data may indicate some degree of insulin resistance for attaining compensatory growth [
48].
The assessment of FA composition at weaning showed, besides similarities in the developmental pattern during lactation, analogous changes in LBW piglets from the BIRTH-WEIGHT trial and piglets from large litters in the LITTER-SIZE experiment when compared to their respective NBW and small-litter counterparts. In brief, there were no differences in the FA profile of the brain among piglets in both trials. On the other hand, the assessment of the polar lipid fraction of the liver showed a decrease in SFA levels with increases in Σn−3 and Σn−6 FA in both LBW and large-litter piglets; piglets from large litters also showed a decrease in MUFA values. The assessment of the neutral fraction of the liver showed no changes in piglets from large litters, but LBW piglets showed increases in MUFA content and desaturation index, which it is a feature commonly related to metabolic disorders such as obesity and insulin resistance in both humans [
34,
49] and pigs [
50,
51]. Similarities at the muscle level were less evident, with only a decrease in the desaturation index of both lipid fractions in LBW and large-litter piglets. Piglets from large litters also showed a decrease in MUFA values and increases in PUFA concentrations in the polar lipid fraction. LBW piglets showed an increase of the Σn−6/Σn−3 FA ratio in the polar fraction, which may confirm previous evidence of metabolic disorders and insulin resistance [
52]. Conversely, the assessment of FA composition at 180 days old showed completely different profiles between LBW and large-litter piglets when compared to their NBW and small-litter counterparts. Thus, these groups had different evolutions in their FA composition throughout development, from weaning to 180 days old.
There were no main differences between LBW and NBW pigs in the BIRTH-WEIGHT trial in the FA profile of brain, liver, and subcutaneous fat—results which are very different from previous studies in Iberian pigs [
19,
20]. However, we should bear in mind that, in contrast to these previous studies, the pigs currently studied were sampled at an earlier age, prior to the fattening period. In the muscle, the FA composition of the polar fraction was also similar in both groups (a foreseeable result, since membrane lipids of the polar fraction are more stable than storage lipids of the neutral fraction [
53]). On the other hand, there were significant differences in the neutral fraction, where MUFA content and desaturation index increased in LBW pigs (confirming results found at weaning and therefore predisposition to metabolic disorders in these pigs [
34,
49]). Conversely, SFA values and the ratio of Σn−6/Σn FA decreased, which is protective against metabolic disorders by diminishing pro-inflammatory status and favoring the action of insulin [
54].
On the other hand, there was a plethora of differences in the FA composition of pigs from large and small litters in the LITTER-SIZE experiment. Viscerae (brain and liver) of pigs from large litters showed a decrease in Σn−6/Σn−3 FA ratio in both neutral and polar lipid fractions, which was related to a decrease in Σn−6 FA concentrations in the brain and an increase in Σn−3 levels in the liver. These changes were all protective for the individual. Higher availability of Σn−3 has been found to improve pro-/anti-inflammatory status, insulin function, and physical and mental development during the first years of life [
55,
56,
57]; higher availability of Σn−3 FA is related to higher availability of anti-inflammatory lipid mediators, which reduce pathological risks [
58,
59]. Higher availability of Σn−6 FA would be related to higher availability of pro-inflammatory lipid mediators [
60]. Therefore, an increase of Σn−6/Σn−3 FA ratio in the liver would indicate a pro-inflammatory state related to increased peripheral lipolysis and increased flux of FAs [
54].
Assessment of the FA content of triglycerides in the subcutaneous fat showed increases of PUFA, Σn−6, and Σn−3 FA values in both outer and inner layers of pigs from large litters. The outer layer also showed a decrease in the SFA content and an increase in the desaturation index. The same changes (increases of concentrations of PUFA, Σn−6 FA, and Σn−3 FA and desaturation index, and decrease in SFA values) were found in the neutral fraction (triglycerides) of the GM muscle. This finding is logical from the physiological point of view, since FAs are mostly distributed in the neutral fraction (around 70% of total FAs [
61]) and, besides that, the polar fraction is more stable than the neutral fraction, as previously discussed [
53]. On the other hand, only the increase in the desaturation index and the decrease in SFA values were found in the LD muscle (which were the same changes found in LBW). These differences in the FA profiles between GM and LD muscle were likely related to the content of oxidative muscle fibers [
62], because fibers of the GM have a predominantly oxidative metabolism while fibers of the LD have a mainly glycolytic metabolism.The LD muscle also showed a decrease in the ratio of Σn−6/Σn−3 FA, but in the polar fraction (coincidentally with changes in the polar fraction of brain and liver). In the skeletal muscle, the Σn−3 FA content of cellular membranes plays a main role favoring the action of insulin; a high Σn−6/Σn−3 FA ratio would be deleterious to insulin sensitivity [
63] and ultimately would result in insulin resistance [
52].
Hence, the FA profile found in NBW piglets from large litters was mostly protective for their metabolic health (higher levels of PUFA and Σn−6 FA levels with lower ratios Σn−6/Σn−3 FA and lower content of SFA); only the increase in desaturation indexes was a warning. On the other hand, from a productive point of view, increases in the PUFA content and decreases in the SFA content and Σn−6/Σn−3 FA ratio found in the muscle and subcutaneous fat this imply health and sensorial benefits and therefore enhance consumer acceptance [
64,
65,
66].