Long-Term Phenotypic and Proteomic Changes Following Vitrified Embryo Transfer in the Rabbit Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Vitrified Embryo Transfer Procedure
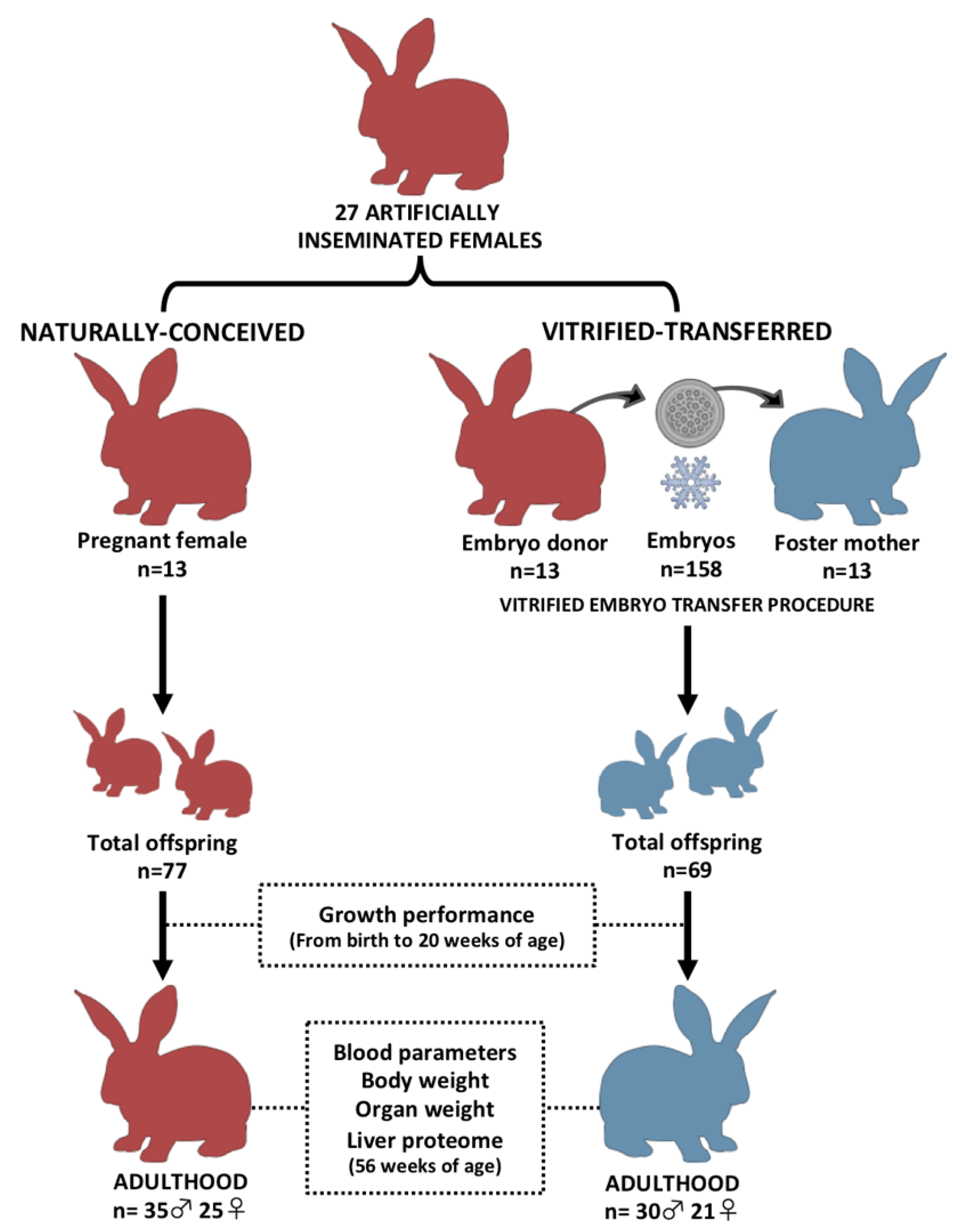
2.2. Experimental Design
2.3. Growth Performance during Postnatal Development
2.4. Adult Body Weight and Organ Phenotypic Comparison
2.5. Determination of Hematological and Biochemical Parameters of Peripheral Blood in Adulthood
2.6. Statistical Analysis of Phenotypic Data
2.7. Comparative Proteomic Analysis: Sampling, Protein Extraction and Quantification
2.8. Complete Proteome: Spectral Library Building by In-Gel Digestion and LC-MS/MS—Data-Dependent Acquisition Analysis
2.9. LC-SWATH-MS Acquisition: Analysis of Individual Samples
2.10. Protein Identification, Validation and Quantification
2.11. Statistical Analysis of the Proteome and Functional Annotation of the Differentially Expressed Proteins
3. Results
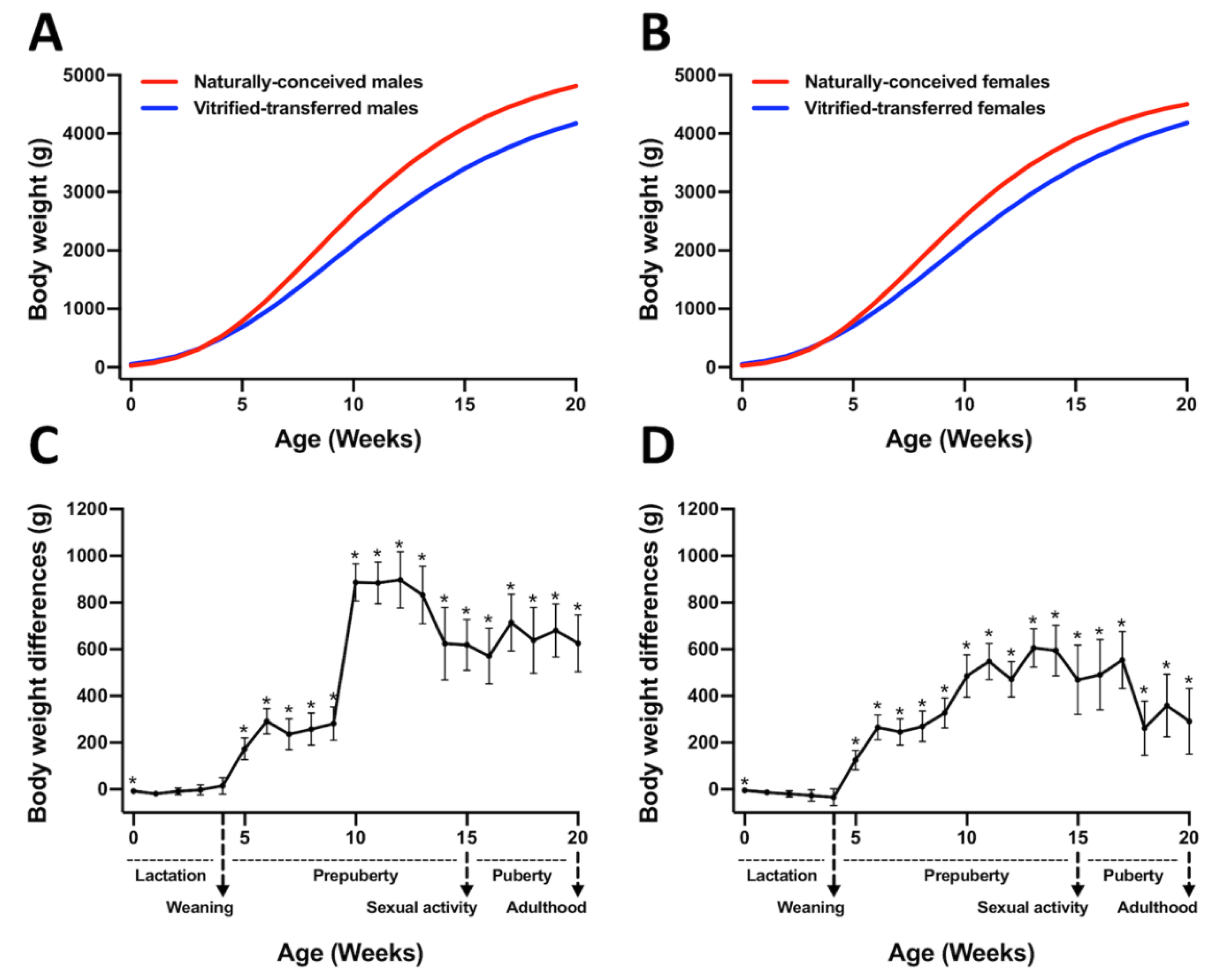
3.1. Animals Derived after Vitrified-Thawed Embryo Transfer Procedure Exhibit Higher Birth Weight, but Lower Growth Performance, until Adulthood
3.2. At Adulthood, Animals Derived from Vitrified-Transferred Embryos Showed Lower Body Weight and Reduced Weight in Some Vital Organs
3.3. The Peripheral Blood Parameters (Healthy Status) of the Vitrified-Transferred Progeny Were Similar to Those of the NC Group
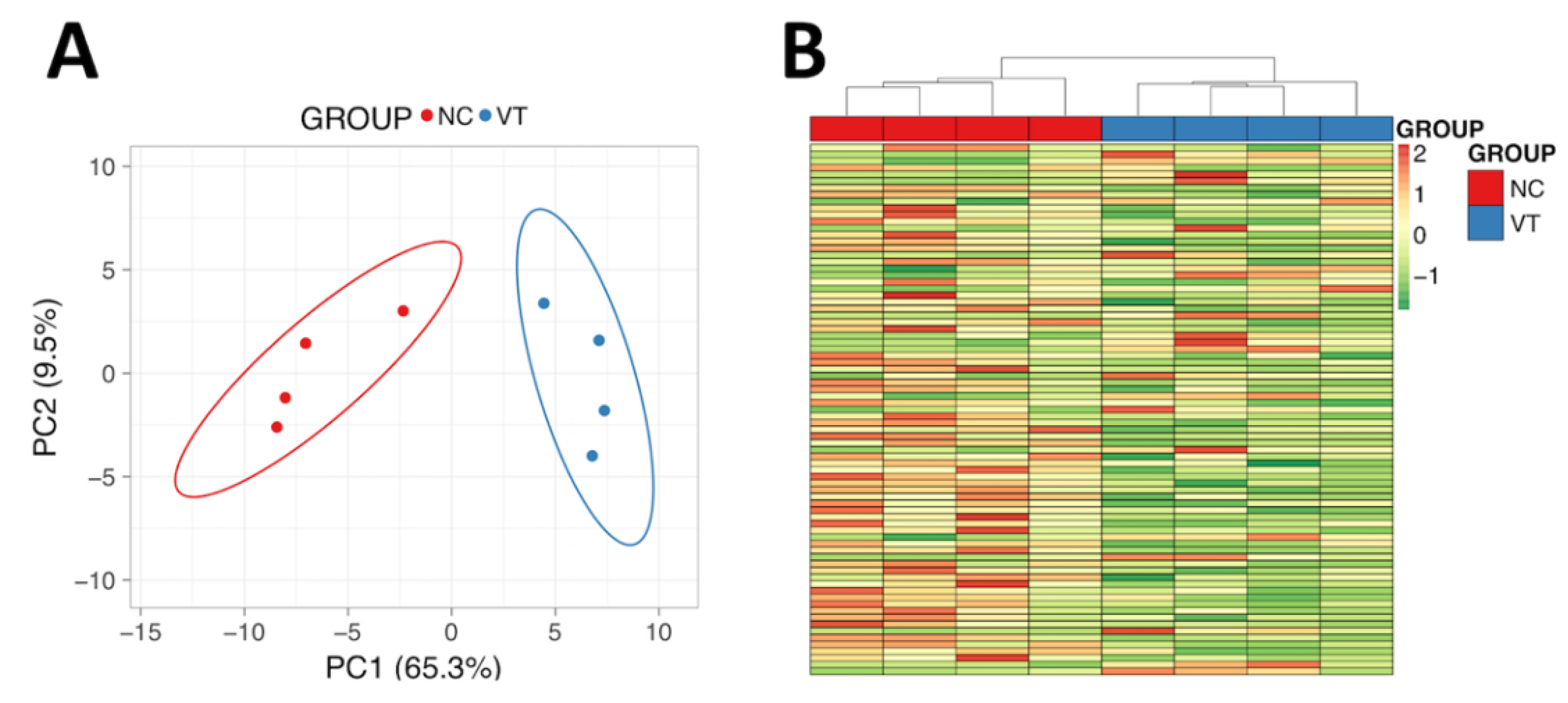
3.4. The Liver Protein Profile Was Influenced by Vitrified-Thawed Embryo Transfer Procedure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| ARTs | Assisted reproductive technologies |
| DDA | Data-dependent acquisition |
| DEPs | Differentially expressed proteins |
| DMSO | Dimethyl sulfoxide |
| DOHaD | Developmental Origins of Health and Disease |
| EG | Ethylene glycol |
| ESHRE | European Society of Human Reproduction and Embryology |
| FDR | False discovery rate |
| FA | Formic acid |
| GLM | General linear model |
| GO | Gene Ontology |
| HM | Heat-Map |
| IVF | In vitro fertilization |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NC | Naturally conceived |
| OXPHO | Oxidative phosphorylation |
| PCA | Principal component analysis |
| SN | Supernatant |
| TFA | Trifluoroacetic acid |
| VET | Vitrified embryo transfer |
| VT | Vitrified-transferred |
| Zn | Zinc |
References
- Crawford, G.E.; Ledger, W.L. In vitro fertilisation/intracytoplasmic sperm injection beyond 2020. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Findlay, J.K.; Holland, M.K.; Wong, B.B.M. Reproductive science and the future of the planet. Reproduction 2019, 158, R91–R96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrooman, L.A.; Bartolomei, M.S. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 2017, 68, 72–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roseboom, T.J. Developmental plasticity and its relevance to assisted human reproduction. Hum. Reprod. 2018, 33, 546–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Feuer, S.; Rinaudo, P. From Embryos to Adults: A DOHaD Perspective on In Vitro Fertilization and Other Assisted Reproductive Technologies. Healthcare 2016, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Feuer, S.K.; Rinaudo, P.F. Physiological, metabolic and transcriptional postnatal phenotypes of in vitro fertilization (IVF) in the mouse. J. Dev. Orig. Health Dis. 2017, 8, 403–410. [Google Scholar] [CrossRef]
- Duranthon, V.; Chavatte-Palmer, P. Long term effects of ART: What do animals tell us? Mol. Reprod. Dev. 2018, 85, 348–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Ibeas, P.; Heras, S.; Gómez-Redondo, I.; Planells, B.; Fernández-González, R.; Pericuesta, E.; Laguna-Barraza, R.; Pérez-Cerezales, S.; Gutiérrez-Adán, A. Embryo responses to stress induced by assisted reproductive technologies. Mol. Reprod. Dev. 2019, 86, 1292–1306. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Heilbronn, L.K. The health outcomes of human offspring conceived by assisted reproductive technologies (ART). J. Dev. Orig. Health Dis. 2017, 8, 388–402. [Google Scholar] [CrossRef]
- Novakovic, B.; Lewis, S.; Halliday, J.; Kennedy, J.; Burgner, D.P.; Czajko, A.; Kim, B.; Sexton-Oates, A.; Juonala, M.; Hammarberg, K.; et al. Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Belva, F.; Bonduelle, M.; Roelants, M.; Michielsen, D.; Van Steirteghem, A.; Verheyen, G.; Tournaye, H. Semen quality of young adult ICSI offspring: The first results. Hum. Reprod. 2016, 31, 2811–2820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calle, A.; Fernandez-Gonzalez, R.; Ramos-Ibeas, P.; Laguna-Barraza, R.; Perez-Cerezales, S.; Bermejo-Alvarez, P.; Ramirez, M.A.; Gutierrez-Adan, A. Long-term and transgenerational effects of in vitro culture on mouse embryos. Theriogenology 2012, 77, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Feuer, S.K.; Liu, X.; Donjacour, A.; Lin, W.; Simbulan, R.K.; Giritharan, G.; Piane, L.D.; Kolahi, K.; Ameri, K.; Maltepe, E.; et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology 2014, 155, 1956–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Dominguez, X.; Vicente, J.S.; Marco-Jiménez, F. Developmental Plasticity in Response to Embryo Cryopreservation: The Importance of the Vitrification Device in Rabbits. Animals 2020, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Dulioust, E.; Toyama, K.; Busnel, M.C.; Moutier, R.; Carlier, M.; Marchaland, C.; Ducot, B.; Roubertoux, P.; Auroux, M. Long-term effects of embryo freezing in mice. Proc. Natl. Acad. Sci. USA 1995, 92, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Santos, A.N.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 2012, 144, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Servick, K. Unsettled questions trail IVF’s success. Science (80-) 2014, 345, 744–746. [Google Scholar] [CrossRef]
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V.; et al. ART in Europe, 2015: Results generated from European registries by ESHRE. Hum. Reprod. Open 2020, 2020, hoz038. [Google Scholar] [CrossRef] [Green Version]
- Sparks, A.E.T. Human embryo cryopreservation-methods, timing, and other considerations for optimizing an embryo cryopreservation program. Semin. Reprod. Med. 2015, 33, 128–144. [Google Scholar] [CrossRef]
- Feuer, S.K.; Liu, X.; Donjacour, A.; Simbulan, R.; Maltepe, E.; Rinaudo, P.F. Transcriptional signatures throughout development: The effects of mouse embryo manipulation in vitro. Reproduction 2017, 153, 107–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estany, J.; Camacho, J.; Baselga, M.; Blasco, A. Selection response of growth rate in rabbits for meat production. Genet. Sel. Evol. 1992, 24, 527–537. [Google Scholar] [CrossRef]
- Vicente, J.S.; Viudes-De-Castro, M.P.; García, M.L. In vivo survival rate of rabbit morulae after vitrification in a medium without serum protein. Reprod. Nutr. Dev. 1999, 39, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dominguez, X.; Marco-Jimenez, F.; Viudes-de-Castro, M.P.; Vicente, J.S. Minimally invasive embryo transfer and embryo vitrification at the optimal embryo stage in rabbit model. J. Vis. Exp. 2019, 147, e58055. [Google Scholar] [CrossRef] [Green Version]
- Besenfelder, U.; Brem, G. Laparoscopic embryo transfer in rabbits. J. Reprod. Fertil. 1993, 99, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Vicente, J.S.; Llobat, L.; Viudes-de-Castro, M.P.; Lavara, R.; Baselga, M.; Marco-Jiménez, F. Gestational losses in a rabbit line selected for growth rate. Theriogenology 2012, 77, 81–88. [Google Scholar] [CrossRef]
- Zucker, I.; Beery, A.K. Males still dominate animal studies. Nature 2010, 465, 690. [Google Scholar] [CrossRef]
- Kineman, R.D.; del Rio-Moreno, M.; Sarmento-Cabral, A. 40 years of IGF1: Understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J. Mol. Endocrinol. 2018, 61, T187–T198. [Google Scholar] [CrossRef] [Green Version]
- Adamek, A.; Kasprzak, A. Insulin-like growth factor (IGF) system in liver diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef] [Green Version]
- Lavara, R.; Baselga, M.; Marco-Jiménez, F.; Vicente, J.S. Embryo vitrification in rabbits: Consequences for progeny growth. Theriogenology 2015, 84, 674–680. [Google Scholar] [CrossRef]
- Ding, C.; Li, Y.; Guo, F.; Jiang, Y.; Ying, W.; Li, D.; Yang, D.; Xia, X.; Liu, W.; Zhao, Y.; et al. A cell-type-resolved liver proteome. Mol. Cell. Proteom. 2016, 15, 3190–3202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Shilov, I.V.; Seymourt, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The paragon algorithm, a next generation search engine that uses sequence temperature values sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell. Proteom. 2007, 6, 1638–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.M.; Zimmerman, K.; Smith, S.A. Hematological Assessment in Pet Rabbits: Blood Sample Collection and Blood Cell Identification. Clin. Lab. Med. 2015, 35, 617–627. [Google Scholar] [CrossRef]
- Fiorello, C.V.; Divers, S.J. Rabbits. In Exotic Animal Formulary; Carpenter, J.W., James, W., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 546–595. [Google Scholar]
- Kamel, R.M. Assisted reproductive technology after the birth of Louise Brown. J. Reprod. Infertil. 2013, 14, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Auroux, M.; Cerutti, I.; Ducot, B.; Loeuillet, A. Is embryo-cryopreservation really neutral? A new long-term effect of embryo freezing in mice: Protection of adults from induced cancer according to strain and sex. Reprod. Toxicol. 2004, 18, 813–818. [Google Scholar] [CrossRef]
- Cifre, J.; Baselga, M.; Gómez, E.A.; De La Luz, G.M. Effect of embryo cryopreservation techniques on reproductive and growth traits in rabbits. Anim. Res. 1999, 48, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Saenz-De-Juano, M.D.; Marco-Jimenez, F.; Schmaltz-Panneau, B.; Jimenez-Trigos, E.; Viudes-De-Castro, M.P.; Penaranda, D.S.; Jouneau, L.; Lecardonnel, J.; Lavara, R.; Naturil-Alfonso, C.; et al. Vitrification alters rabbit foetal placenta at transcriptomic and proteomic level. Reproduction 2014, 147, 789–801. [Google Scholar] [CrossRef] [Green Version]
- Spijkers, S.; Lens, J.W.; Schats, R.; Lambalk, C.B. Fresh and Frozen-Thawed Embryo Transfer Compared to Natural Conception: Differences in Perinatal Outcome. Gynecol. Obstet. Investig. 2017, 82, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Hann, M.; Roberts, S.A.; D’Souza, S.W.; Clayton, P.; Macklon, N.; Brison, D.R. The growth of assisted reproductive treatment-conceived children from birth to 5 years: A national cohort study. BMC Med. 2018, 16, 224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Robbins, K.M.; Wells, K.D.; Rivera, R.M. Large offspring syndrome: A bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith-Wiedemann. Epigenetics 2013, 8, 591–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidenne, T.; Combes, S.; Feugier, A.; Jehl, N.; Arveux, P.; Boisot, P.; Briens, C.; Corrent, E.; Fortune, H.; Montessuy, S.; et al. Feed restriction strategy in the growing rabbit. 2. Impact on digestive health, growth and carcass characteristics. Animal 2009, 3, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Velazquez, M.A.; Sheth, B.; Smith, S.J.; Eckert, J.J.; Osmond, C.; Fleming, T.P. Insulin and branched-chain amino acid depletion during mouse preimplantation embryo culture programmes body weight gain and raised blood pressure during early postnatal life. Biochim. Biophys. Acta—Mol. Basis Dis. 2018, 1864, 590–600. [Google Scholar] [CrossRef]
- Donjacour, A.; Liu, X.; Lin, W.; Simbulan, R.; Rinaudo, P.F. In Vitro Fertilization Affects Growth and Glucose Metabolism in a Sex-Specific Manner in an Outbred Mouse Model1. Biol. Reprod. 2014, 90, 80. [Google Scholar] [CrossRef] [PubMed]
- Mahsoudi, B.; Li, A.; O’Neill, C. Assessment of the Long-Term and Transgenerational Consequences of Perturbing Preimplantation Embryo Development in Mice1. Biol. Reprod. 2007, 77, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermejo-Alvarez, P.; Rizos, D.; Lonergan, P.; Gutierrez-Adan, A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction 2011, 141, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Feuer, S.K.; Donjacour, A.; Simbulan, R.K.; Lin, W.; Liu, X.; Maltepe, E.; Rinaudo, P.F. Sexually dimorphic effect of In Vitro Fertilization (IVF) on adult mouse fat and liver metabolomes. Endocrinology 2014, 155, 4554–4567. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Gonzalez, R.; Moreira, P.; Bilbao, A.; Jiménez, A.; Pérez-Crespo, M.; Ramírez, M.A.; De Fonseca, F.R.; Pintado, B.; Gutiérrez-Adán, A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 5880–5885. [Google Scholar] [CrossRef] [Green Version]
- Calle, A.; Miranda, A.; Fernandez-Gonzalez, R.; Pericuesta, E.; Laguna, R.; Gutierrez-Adan, A. Male Mice Produced by In Vitro Culture Have Reduced Fertility and Transmit Organomegaly and Glucose Intolerance to Their Male Offspring1. Biol. Reprod. 2012, 87, 1–9. [Google Scholar] [CrossRef]
- Riesche, L.; Bartolomei, M.S. Assisted Reproductive Technologies and the Placenta: Clinical, Morphological, and Molecular Outcomes. Semin. Reprod. Med. 2018, 36, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, M.A.; Budge, H.; Symonds, M.E. Early developmental influences on hepatic organogenesis. Organogenesis 2008, 4, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Peterside, I.E.; Selak, M.A.; Simmons, R.A. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 1258–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Kleist-Retzow, J.C.; Cormier-Daire, V.; Viot, G.; Goldenberg, A.; Mardach, B.; Amiel, J.; Saada, P.; Dumez, Y.; Brunelle, F.; Saudubray, J.M.; et al. Antenatal manifestations of mitochondrial respiratory chain deficiency. J. Pediatr. 2003, 143, 208–212. [Google Scholar] [CrossRef]
- Hüttemann, M.; Lee, I.; Samavati, L.; Yu, H.; Doan, J.W. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1701–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, K.; Halliday, J.L.; Kirby, D.M.; Yaplito-Lee, J.; Thorburn, D.R.; Boneh, A. Mitochondrial oxidative phosphorylation disorders presenting in neonates: Clinical manifestations and enzymatic and molecular diagnoses. Pediatrics 2008, 122, 1003–1008. [Google Scholar] [CrossRef]
- Abu-Libdeh, B.; Douiev, L.; Amro, S.; Shahrour, M.; Ta-Shma, A.; Miller, C.; Elpeleg, O.; Saada, A. Mutation in the COX4I1 gene is associated with short stature, poor weight gain and increased chromosomal breaks, simulating Fanconi anemia. Eur. J. Hum. Genet. 2017, 25, 1142–11146. [Google Scholar] [CrossRef]
- Hara, T.; Kin, A.; Aoki, S.; Nakamura, S.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol enhances the clearance of mitochondrial damage by vitrification and improves the development of vitrifiedwarmed bovine embryos. PLoS ONE 2018, 13, e0204571. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Prasad, K.N.; Singh, A.K.; Singh, S.K.; Gupta, K.K.; Paliwal, V.K.; Pandey, C.M.; Gupta, R.K. Human Glutathione S-Transferase Enzyme Gene Polymorphisms and Their Association With Neurocysticercosis. Mol. Neurobiol. 2017, 54, 2843–2851. [Google Scholar] [CrossRef]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.J. Cellular sensing and transport of metal ions: Implications in micronutrient homeostasis. J. Nutr. Biochem. 2015, 26, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.R.; Jiang, S.W.; Zhang, Y.; Hu, Y.F.; Yi, H.G.; Liu, J.; Zhao, N.N.; Chen, J.; Gao, L.; Cui, Y.G.; et al. Serum levels of trace elements in children born after assisted reproductive technology. Clin. Chim. Acta 2019, 495, 664–669. [Google Scholar] [CrossRef]
- Li, B.; Xiao, X.; Chen, S.; Huang, J.; Ma, Y.; Tang, N.; Sun, H.; Wang, X. Changes of Phospholipids in Fetal Liver of Mice Conceived by In Vitro Fertilization1. Biol. Reprod. 2016, 94, 105. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Liu, X.M.; Jin, L.; Wang, T.T.; Ullah, K.; Sheng, J.Z.; Huang, H.F. Cardiovascular and metabolic profiles of offspring conceived by assisted reproductive technologies: A systematic review and meta-analysis. Fertil. Steril. 2017, 107, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, H.L.; Hofman, P.L.; Peek, J.; Harris, M.; Wilson, D.; Robinson, E.M.; Gluckman, P.D.; Cutfield, W.S. In vitro fertilization improves childhood growth and metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 3441–3445. [Google Scholar] [CrossRef] [Green Version]
| Traits | Naturally Conceived (n = 35) | Vitrified-Transferred (n = 30) |
|---|---|---|
| Body weight (Kg) | 5.7 ± 0.10 a | 5.3 ± 0.11 b |
| Perirenal fat (g) | 173.7 ± 11.09 | 184.2 ± 12.13 |
| Scapular fat (g) | 58.9 ± 5.32 | 66.1 ± 5.99 |
| Kidneys (g) | 22.7 ± 0.45 | 22.3 ± 0.42 |
| Liver (g) | 102.1 ± 2.51 a | 92.8 ± 2.37 b |
| Spleen (g) | 1.4 ± 0.08 | 1.3 ± 0.07 |
| Lungs (g) | 25.6 ± 1.20 | 26.7 ± 1.13 |
| Heart (g) | 13.1 ± 0.40 a | 11.4 ± 0.42 b |
| Gonads (g) | 6.2 ± 0.36 | 6.9 ± 0.34 |
| Adrenal glands (g) | 0.6 ± 0.04 | 0.7 ± 0.03 |
| Peripheral Blood Parameters | Naturally Conceived (n = 20) | Vitrified-Transferred (n = 20) |
|---|---|---|
| Hematology | ||
| White blood cells (103/mm3) | 9.7 ± 0.77 | 8.7 ± 0.77 |
| Lymphocytes (%) | 42.6 ± 3.14 | 41.8 ± 3.14 |
| Monocytes (%) | 4.2 ± 0.844 | 5.1 ± 0.844 |
| Granulocytes (%) | 42.5 ± 2.71 | 45.9 ± 2.71 |
| Red blood cells (106/mm3) | 6.0 ± 0.14 | 6.1 ± 0.14 |
| Hemoglobin (g/dL) | 12.5 ± 0.32 | 12.7 ± 0.32 |
| Hematocrit (%) | 42.4 ± 1.27 | 42.3 ± 1.27 |
| Serum metabolites + | ||
| Albumin (g/dL) | 4.2 ± 0.05 b | 4.4 ± 0.05 a |
| Bile acids (µmol/L) | 7.2 ± 0.87 | 6.9 ± 0.87 |
| Cholesterol (mg/dL) | 40.1 ± 2.09 a | 31.7 ± 2.09 b |
| Glucose (mg/dL) | 127.7 ± 9.41 b | 141.4 ± 9.41 a |
| Bilirubin total (mg/dL) | 0.1 ± 0.01 | 0.1 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Dominguez, X.; Marco-Jiménez, F.; Peñaranda, D.S.; Vicente, J.S. Long-Term Phenotypic and Proteomic Changes Following Vitrified Embryo Transfer in the Rabbit Model. Animals 2020, 10, 1043. https://doi.org/10.3390/ani10061043
Garcia-Dominguez X, Marco-Jiménez F, Peñaranda DS, Vicente JS. Long-Term Phenotypic and Proteomic Changes Following Vitrified Embryo Transfer in the Rabbit Model. Animals. 2020; 10(6):1043. https://doi.org/10.3390/ani10061043
Chicago/Turabian StyleGarcia-Dominguez, Ximo, Francisco Marco-Jiménez, David S. Peñaranda, and José Salvador Vicente. 2020. "Long-Term Phenotypic and Proteomic Changes Following Vitrified Embryo Transfer in the Rabbit Model" Animals 10, no. 6: 1043. https://doi.org/10.3390/ani10061043
APA StyleGarcia-Dominguez, X., Marco-Jiménez, F., Peñaranda, D. S., & Vicente, J. S. (2020). Long-Term Phenotypic and Proteomic Changes Following Vitrified Embryo Transfer in the Rabbit Model. Animals, 10(6), 1043. https://doi.org/10.3390/ani10061043






