Endometrial Cytology During the Different Phases of the Estrous Cycle in Jennies: New Evidences
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
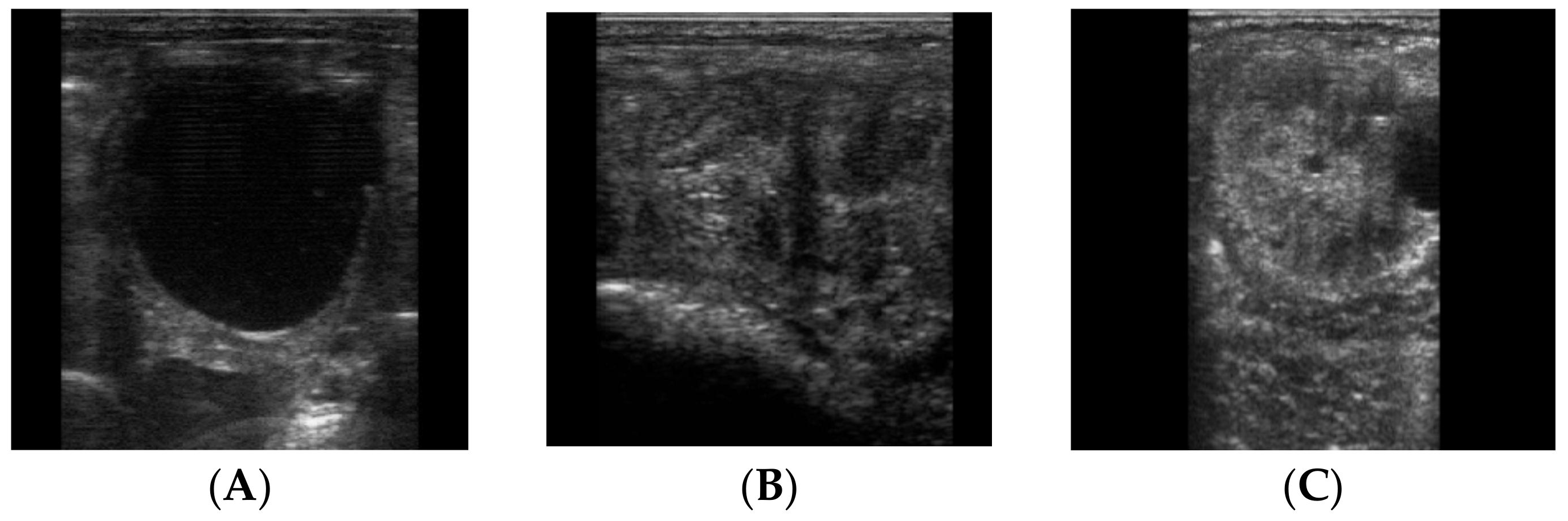
2.2. Samples Collection
2.3. Cytobrush
2.4. Citology
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ley, W.B.; Digrassie, W.A.; Holyoak, G.R.; Slusher, S.H. Endometrium. In Diagnostic Cytology and Hematology in the Horse Cowell; Cowel, R.D., Tyler, R.L., Eds.; Mosby: St. Louis, MO, USA, 2002; pp. 180–186. [Google Scholar]
- Riddle, W.T.; LeBlanc, M.M.; Stromberg, A.J. Relationships between uterine culture, cytology and pregnancy rates in a Thoroughbred practice. Theriogenology 2007, 68, 395–402. [Google Scholar] [CrossRef]
- Defontis, M.; Vaillancourt, D.; Grand, F.X. Comparison of three methods of sampling for endometrial cytology in the mare. Preliminary study. Tierarztl Prax Ausg G Grosstiere 2011, 39, 171–175. [Google Scholar]
- Kozdrowski, R.; Sikora, M.; Buczkowska, J.; Nowak, M.; Raś, A.; Dzięcioł, M. Effects of cycle stage and sampling procedure on interpretation of endometrial cytology in mares. Anim Reprod Sci. 2015, 154, 56–62. [Google Scholar] [CrossRef]
- LeBlanc, M.M.; Magsig, J.; Stromberg, A.J. Use of a low volume uterine flush for diagnosing endometritis in chronically infertile mares. Theriogenology 2007, 68, 403–412. [Google Scholar] [CrossRef]
- Bourke, M.; Mills, J.; Barnes, A. Collection of endometrial cells in the mare. Aust. Vet. J. 1997, 75, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Wehrend, A. Exfoliative endometrium cytology in the breeding mare - specimen sampling and interpretation of findings. Tierarztl Prax Ausg G Grosstiere Nutztiere 2009, 37, 409–416. [Google Scholar]
- Overbeck, W.; Witte, T.S.; Heuwieser, W. Comparison of three diagnostic methods to identify subclinical endometritis in mares. Theriogenology 2011, 75, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Cocchia, N.; Paciello, O.; Auletta, L.; Uccello, V.; Silvestro, L.; Mallardo, K.; Paraggio, G.; Pasolini, M.P. Comparison of the cytobrush, cottonswab, and low-volume uterine flush techniques to evaluate endometrial cytology for diagnosing endometritis in chronically infertile mares. Theriogenology 2012, 77, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Neuberg, K.P.; Failing, K.; Wehrend, A. Cytological diagnosis of endometritis in the mare: Investigations of sampling techniques and relation to bacteriological results. Anim Reprod Sci. 2012, 132, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Miró, J.; Papas, M. Post–Arti!cial Insemination Endometrial In ammation and Its Control in Donkeys. J. Equine Vet. Sci. 2018, 65, 38–43. [Google Scholar]
- Katila, T. Evaluation of diagnostic methods in equine endometritis. Reprod. Biol. 2016, 16, 189–196. [Google Scholar] [CrossRef]
- Bohn, A.A.; Ferris, R.A.; McCue, P.M. Comparison of equine endometrial cytology samples collected with uterine swab, uterine brush, and low-volume lavage from healthy mares. Vet. Clin. Pathol. 2014, 43, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Buczkowska, J.; Kozdrowski, R.; Nowak, M.; Raś, A.; Staroniewicz, Z.; Siemieniuch, M.J. Comparison of the biopsy and cytobrush techniques for diagnosis of subclinical endometritis in mares. Reprod. Biol. Endocrinol. 2014, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Dascanio, J.J. Endometrial cytology. In Current Therapy in Euine Medicine; Robinson, N.E., Ed.; Saunders: St. Louis, MO, USA, 2003; pp. 226–228. [Google Scholar]
- Sokkar, S.M.; Hamouda, M.A.; El-Rahman, S.M. Endometritis in she donkeys in Egypt. J. Vet. Med. Binfect. Dis. Vet. Public Health 2001, 48, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Taberner, E.; Medrano, A.; Pefia, A.; Rigau, T.; Miró, J. Oestrus cycle characteristics and prediction of ovulation in Catalonian jennies. Theriogenology 2008, 70, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Renner-Martin, T.F.; Forstenpointner, G.; Weissengruber, G.E.; Eberhardt, L. Gross anatomy of the female genital organs of the domestic donkey (Equus asinus Linné, 1758). Anat. Histol. Embryol. 2009, 38, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Climent, F.; Vilés, K.; Miró, J. Vaginoplastia in two young infertile Catalan jennies. A clinical case. Reprod Domest Anim. 2012, 47 (Suppl. 3), 113. [Google Scholar]
- Vilés, K.; Rabanal, R.; Rodríguez-Prado, M.; Miró, J. Effect of ketoprofen treatment on the uterine inflammatory response after AI of jennies with frozen semen. Theriogenology 2013, 79, 1019–1026. [Google Scholar] [CrossRef]
- Couto, M.; Hughes, J. Technique and interpretation of cervical and endometrial cytology in the mare. J. Equine Vet. Sci. 1986, 4, 265–273. [Google Scholar] [CrossRef]
- Reiswig, J.D.; Threlfall, W.R.; Rosol, T.J. A comparison of endometrial biopsy, culture and cytology during oestrus and dioestrus in the horse. Equine Vet. J. 1993, 25, 240–241. [Google Scholar] [CrossRef]
- Knudsen, O. Endometrial cytology as a diagnostic aid in mares. Cornell Vet. 1964, 54, 415–422. [Google Scholar] [PubMed]
- Vidament, M.; Vincent, P.; Martin, F.; Magistrini, M.; Blesbois, E. Differences in ability of jennies and mares to conceive with cooled and frozen semen containing glycerol or not. Anim. Reprod. Sci. 2009, 112, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.V.; Papa, F.O.; Melo Oña, C.M.; Monteiro, G.A.; Puoli-Filho, J.N.P.; Alvarenga, M.A. New procedures to freeze donkey semen and its influence on mares and jennies fertility. J. Equine Vet Sci. 2012, 32, 503–504. [Google Scholar] [CrossRef]
- Pugh, D.G. Donkey reproduction. Proc. Am. Assoc. Equine Pract. 2002, 48, 113–114. [Google Scholar]
- Miró, J.; Marín, H.; Catalán, J.; Papas, M.; Gacem, S.; Yeste, M. Seminal Plasma, Sperm Concentration, and Sperm-PMN Interaction in the Donkey: An in vitro model to study endometrial inflammation at post-insemination. Int. J. Mol. Sci. 2020, 21, 3478. [Google Scholar] [CrossRef]
- Miró, J.; Vilés, K.; García, W.; Jordana, J.; Yeste, M. Effect of donkey seminal plasma on sperm movement and sperm-polimorphonuclear neutrophils attachment in vitro. Anim. Reprod. Sci. 2013, 140, 164–172. [Google Scholar] [CrossRef]
- Vendramini, O.; Guintard, C.; Moreau, J.; Tainturier, D. Cervix conformation: A first anatomical approach in baudet du poitou jenny asses. Anim. Sci. 1998, 66, 741–744. [Google Scholar] [CrossRef]
- Miró, J.; Vilés, K.; Fernández, M.; Fábregas, N.; Soares, J.; García, W. Induced acute endometritis by frozen semen insemination in donkey. Anim. Reprod Sci. 2011, 46, 130. [Google Scholar]
- Rota, A.; Panzani, D.; Sabatini, C.; Camillo, F. Donkey Jack (Equus Asinus) semen cryopreservation: Studies of seminal parameters, Post Breeding inflammatory response, and fertility in Donkey jennies. Theriogenology 2012, 78, 1846–1854. [Google Scholar] [CrossRef]
- Gerstenberg, C.; Allen, W.R.; Stewart, F. Factors controlling epidermal growth factor (EGF) gene expression in the endometrium of the mare. Mol. Reprod. Dev. 1999, 53, 255–265. [Google Scholar] [CrossRef]
- Aupperle, H.; SSchoon, H.; Schoon, D.; Hoppen, H.; Sieme, H.; Tannapfel, A. Cyclical endometrial steroid hormone receptor expression and proliferation intensity in the mare. Equine Vet. J. 2000, 32, 228–232. [Google Scholar] [CrossRef] [PubMed]
- McCue, P. Endometrial Biopsy. In Equine Reproductive Procedures; Dascanio, J., McCue, P., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hafez, E.S.E.; Hafez, B. Reproductive Cycles. In Reproduction and Artifical Insemination in Animals; 7ª Intermericana McGraw-Hill: Ciudad de México, México, 2002; pp. 56–69. [Google Scholar]
- Groppetti, D.; Pecile, A.; Arrighi, S.; Di Giancamillo, A.; Cremonesi, F. Endometrial cytology and computerized morphometric analysis of epithelial nuclei: A useful tool for reproductive diagnosis in the bitch. Theriogenology 2010, 73, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Stamatiu, L. Endometrial cytology in the female cat (Felix catus) during diestrous. Rev. Investig. Vet. Perú 2017, 28, 869–875. [Google Scholar]
- Ferris, R.A. Mating-Induced Endometritis. In Current Therapy in Equine Medicine Sprayberry, 7th ed.; Robinson, N.E., Ed.; Saunders: St. Louis, MO, USA, 2015; pp. 692–694. [Google Scholar]
- Nielsen, J.M. Endometritis in the mare: A diagnostic study comparing cultures from swab and biopsy. Theriogenology 2005, 64, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Brook, D. Uterine cytology. In Uterine cytology; McKinnon, A.O., Voss, J.L., Eds.; Lea & Febiger: Philadelphia, PA, USA, 1993; pp. 246–253. [Google Scholar]
- Cadario, M.E. Revisiting the diagnosis and the treatment options for an old problem: Chronic and post-breeding endometritis in the mare. Practitioner 2014, 1, 21–25. [Google Scholar]
- LeBlanc, M.M. Uterine cytology. In Equine Reproduction, 2nd ed.; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Ames, IA, USA, 2011; pp. 1922–1928. [Google Scholar]
- Nielsen, J.M.; Troedsson, M.H.; Pedersen, M.R.; Bojesen, A.M.; Lehn-Jensen, H.; Zent, W.W. Diagnosis of endometritis in the mare based on bacteriological and cytological examinations of the endometrium: Comparison of results obtained by swabs and biopsies. J. Equine Vet. Sci. 2010, 30, 27–30. [Google Scholar] [CrossRef]
- Overbeck, W.; Jäger, K.; Schoon, H.A.; Witte, T.S. Comparison of cytological and histological examinations in different locations of the equine uterus-an in vitro study. Theriogenology 2013, 79, 1262–1268. [Google Scholar] [CrossRef]
- LeBlanc, M.M.; Causey, R.C. Clinical and subclinical endometritis in the mare: Both threats to fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef]
- Causey, R.C. Mucus and the mare: How little we know. Theriogenology 2007, 68, 386–394. [Google Scholar] [CrossRef]
- Ricketts, S.; Troedsson, M. Female reproductive problems: Diagnosis and management. In Current Therapy in Equine Reproduction; Samper, J., Pycock, J., McKinnon, A., Eds.; Saunders: Philadelphia, PA, USA, 2007; pp. 53–69. [Google Scholar]
- Quartuccio, M.; Marino, G.; Mannarino, C.; Cristarella, S. Permanent lateral deviation and stenosis of the cervix in an infertile jennet. Anat. Histol. Embryol. 2016, 45, 145–147. [Google Scholar] [CrossRef]
- LeBlanc, M.M. When to refer an infertile mare to a theriogenologist. Theriogenology 2008, 70, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Brinsko, S.; Blanchard, T.L.; Varner, D.; Schumacher, J.; Love, C.C. Manual of Equine Reproduction; Mosby Elsevier: Maryland Heights, MO, USA; p. 2011.
- Slusher, S.H.; Cowell, R.L.; Tyler, R.D. The Endometrium. In Cytology and hematology of the horse; American Veterinary Publications; Cowell, R.L., Tylor, R.D., Eds.; Mosby Elsevier: Maryland Heights, MO, USA, 1992; pp. 173–180. [Google Scholar]
- Snider, T.A.; Sepoy, C.; Holyoak, G.R. Equine endometrial biopsy reviewed: Observation, interpretation, and application of histopathologic data. Theriogenology 2011, 75, 1567–1581. [Google Scholar] [CrossRef] [PubMed]






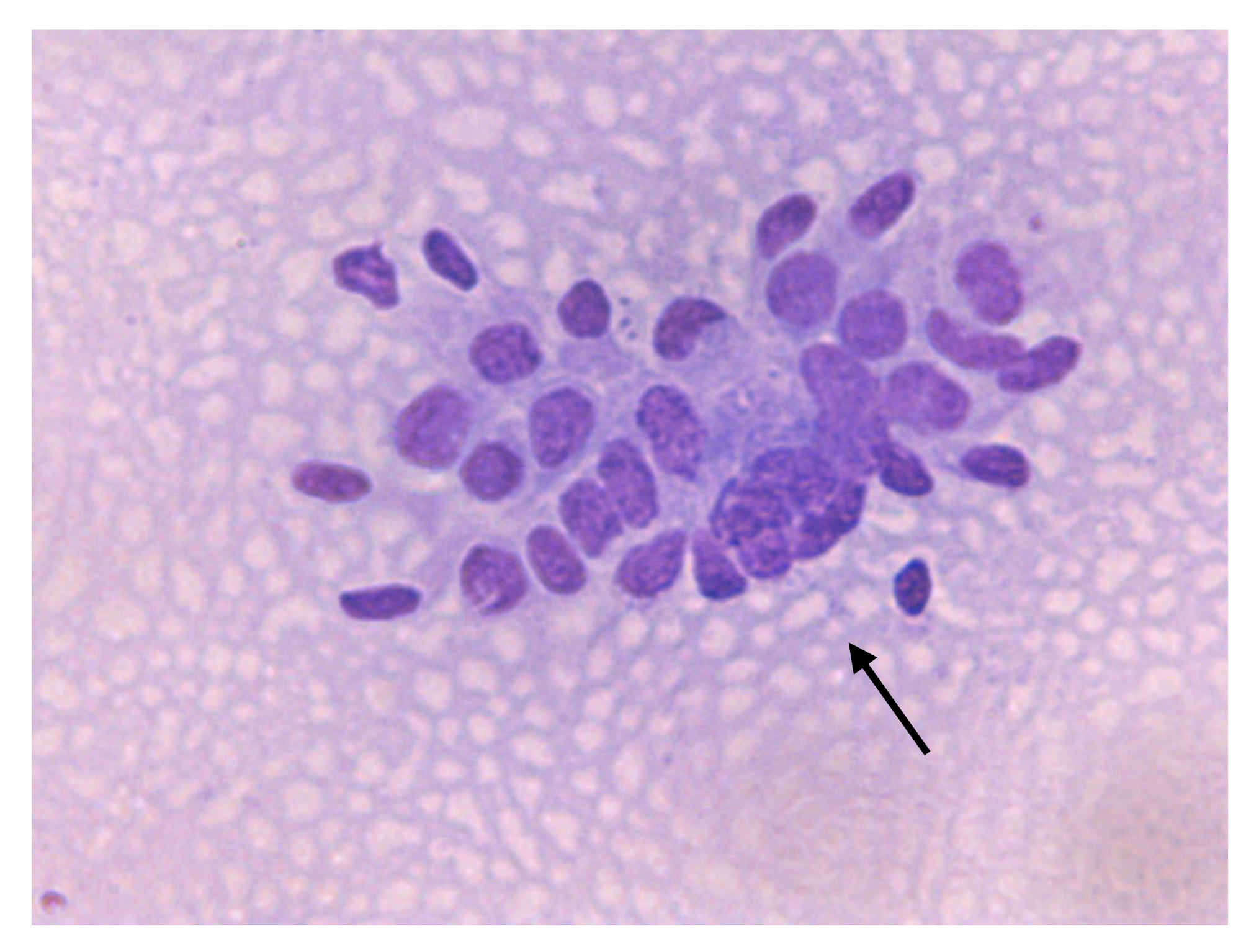




| Cellular Types | Origin | Significance (Indication of) |
|---|---|---|
| Endometrial epithelial cells | Luminal endometrium | Normal |
| Neutrophils (PMNs) | Acute inflammation | |
| Eosinophils | Pneumouterus | |
| Macrophages | Chronic inflammation or resolving acute inflammation | |
| Lymphocytes | Chronic inflammation | |
| Red blood cell (RBC) | Trauma, inflammation, postpartum |
| Groups | N | Endometrial Epithelial Cells | Background | |||
|---|---|---|---|---|---|---|
| Epithelial cells (cells/HPF) | Dense, monolayer and clusters | Intact, distorted or fragmented | Clear, proteinaceous or debris | |||
| Cyclic phase | 8 | Estrous (D-21) | 89.28 ± 2.75 a | Dense +++ and clusters + | Intact | Proteinaceous/Clear |
| 8 | Early diestrus (D-1) | 79.37 ± 1.99 a | Clusters ++ and monolayer | Intact and distorted | Proteinaceous/Clear | |
| 8 | Later diestrus (D-14) | 68.88 ± 4.79 | Clusters + and monolayer | Distorted and fragmented | Clear/debris | |
| Age | 3 | Young: 4–12 years | 79.75 ± 1.94 | Dense and clusters ++ | Intact | Proteinaceous/Clear |
| 5 | Older: >12 years | 83.23 ± 2.41 | Dense and clusters ++ | Distorted and fragmented | Clear/Debris | |
| Number of births | 3 | Primiparous | 89.80 ± 1.73 * | Dense +++ | Intact | Proteinaceous |
| 5 | Multiparous | 83.40 ± 1.73 | Clumps +++ | Intact and distorted | Clear/Debris | |
| N | Groups | PMNs | ||
|---|---|---|---|---|
| % | Cells/Field | |||
| Cyclic phase | 8 | Estrous (D-21) | 0.3 | 1.15 ± 0.22 a |
| 8 | Early diestrus (D-1) | 0.2 | 0.40 ± 0.11 | |
| 8 | Later diestrus (D-14) | 0.1 | 0.17 ± 0.04 | |
| Age | 3 | Young: 4–12 years | 0.2 | 0.34 ± 0.10 |
| 5 | Older: >12 years | 0.3 | 0.55 ± 0.08 | |
| Number of births | 3 | Primiparous | 0.2 | 0.56 ± 0.13 |
| 5 | Multiparous | 0.3 | 1.14 ± 0.13 * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartuccio, M.; Cristarella, S.; Medica, P.; Fazio, E.; Mazzullo, G.; Rifici, C.; Liotta, L.; Satué, K. Endometrial Cytology During the Different Phases of the Estrous Cycle in Jennies: New Evidences. Animals 2020, 10, 1062. https://doi.org/10.3390/ani10061062
Quartuccio M, Cristarella S, Medica P, Fazio E, Mazzullo G, Rifici C, Liotta L, Satué K. Endometrial Cytology During the Different Phases of the Estrous Cycle in Jennies: New Evidences. Animals. 2020; 10(6):1062. https://doi.org/10.3390/ani10061062
Chicago/Turabian StyleQuartuccio, Marco, Santo Cristarella, Pietro Medica, Esterina Fazio, Giuseppe Mazzullo, Claudia Rifici, Luigi Liotta, and Katiuska Satué. 2020. "Endometrial Cytology During the Different Phases of the Estrous Cycle in Jennies: New Evidences" Animals 10, no. 6: 1062. https://doi.org/10.3390/ani10061062
APA StyleQuartuccio, M., Cristarella, S., Medica, P., Fazio, E., Mazzullo, G., Rifici, C., Liotta, L., & Satué, K. (2020). Endometrial Cytology During the Different Phases of the Estrous Cycle in Jennies: New Evidences. Animals, 10(6), 1062. https://doi.org/10.3390/ani10061062







