Fiber Metabolism, Procollagen and Collagen Type III Immunoreactivity in Broiler Pectoralis Major Affected by Muscle Abnormalities
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
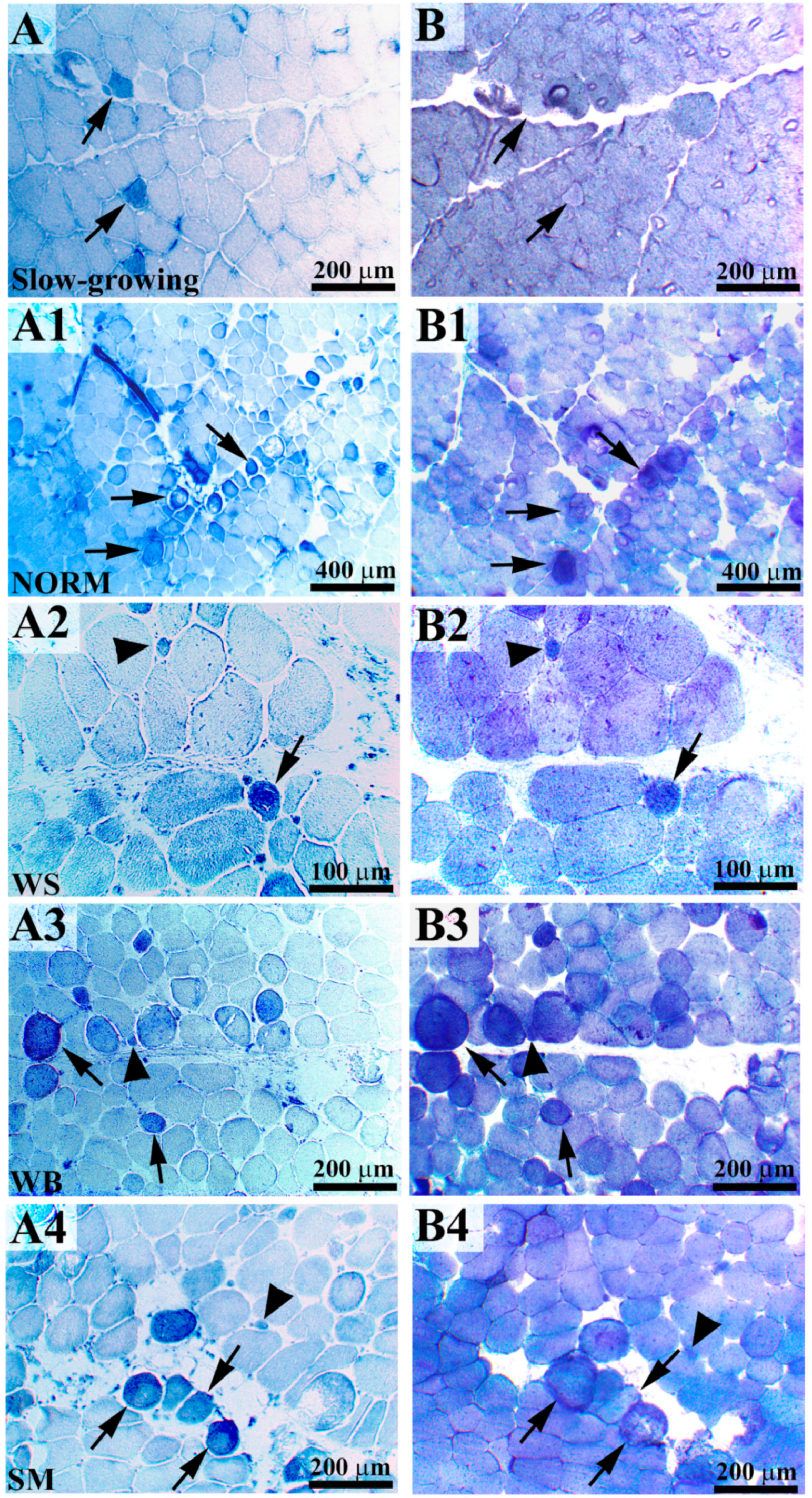
2.1. Histochemical Analyses (NADH-TR and α-GPD) and Morphometric Measurements
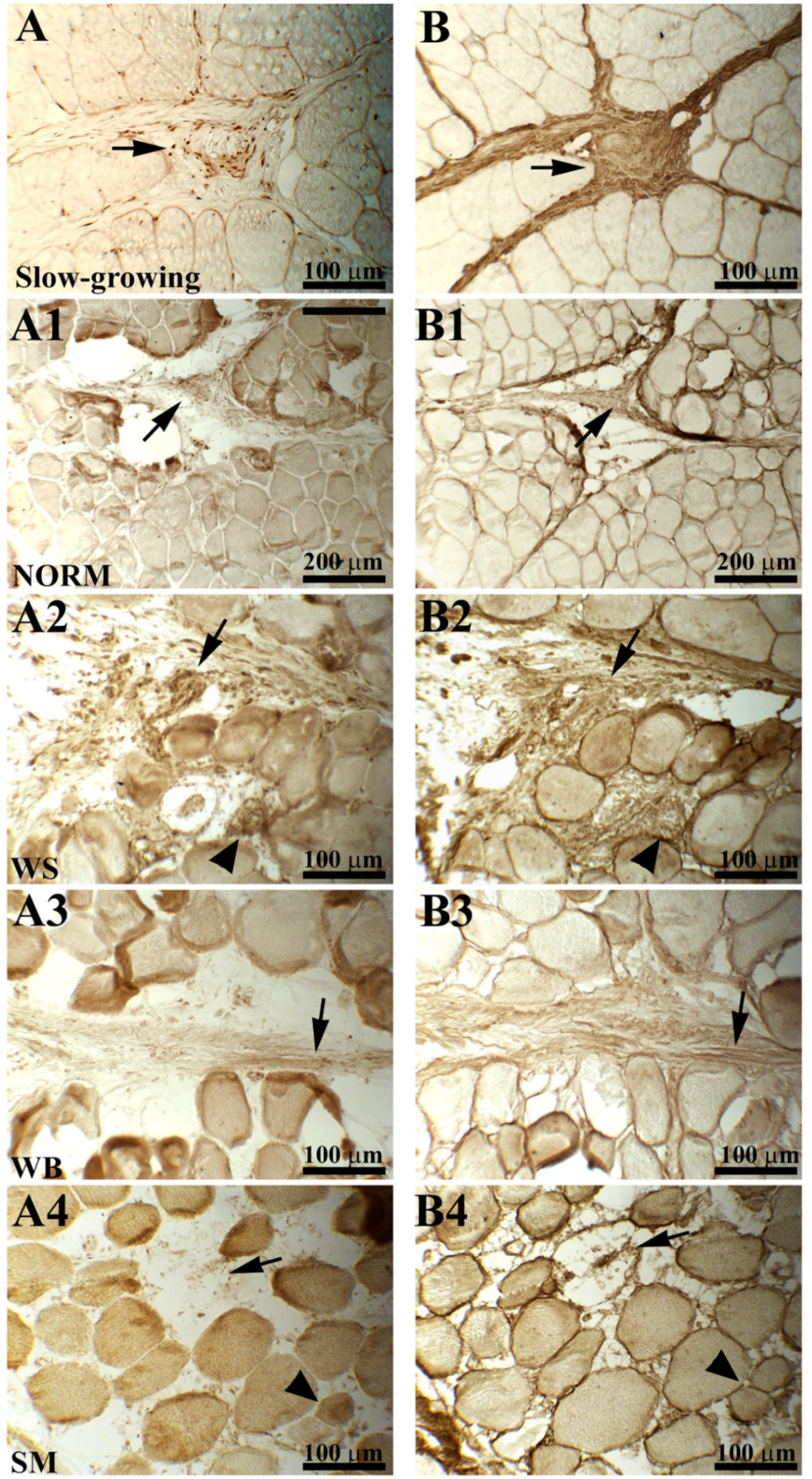
2.2. Procollagen and Collagen Type III
2.3. Antibody Specificity
2.4. Statistical Analysis
3. Results
3.1. Histochemical Analyses (NADH-TR and α-GPD) and Morphometric Measurements
3.2. Procollagen and Collagen Type III
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheuermann, G.N.; Bilgili, S.F.; Tuzun, S.; Mulvaney, D.R. Comparison of chicken genotypes: Myofiber number in pectoralis muscle and myostatin ontogeny. Poult. Sci. 2004, 83, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Artificial selection for improved energy efficiency is reaching its limits in broiler chickens. Sci. Rep. 2018, 8, 1168. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S.G. Recent developments in breast muscle myopathies associated with growth in poultry. Annu. Rev. Anim. Biosci. 2019, 7, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estévez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, Consequences and Consumer Perception of Emerging Broiler Meat Abnormalities. Compr. Rev. Food Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Tompkins, G.; Doerr, L. Postnatal development of muscle fibers in domestic animals. J. Anim. Sci. 1972, 34, 37–41. [Google Scholar] [CrossRef]
- Barnard, E.A.; Lyles, J.M.; Pizzey, J.A. Fiber types in chicken skeletal muscles and their changes in muscular dystrophy. J. Physiol. 1982, 331, 333–354. [Google Scholar] [CrossRef]
- Horak, V.; Sevcíková, K.; Knízetová, H. Histochemical fiber types in the thigh muscles of 4 chicken inbred lines. Ann. Anat. 1989, 169, 313–320. [Google Scholar]
- Ono, Y.; Iwamoto, H.; Takahara, H. The relationship between muscle growth and the growth of different fiber types in the chicken. Poult. Sci. 1993, 72, 568–576. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef]
- Bailey, A.J.; Shellswell, G.B.; Duance, V.C. Identification and change of collagen types in differentiating myoblasts and developing chick muscle. Nature 1979, 278, 67–69. [Google Scholar] [CrossRef]
- DeMichele, S.J.; Brown, R.G.; Krasin, B.W.; Sweeny, P. R Connective tissue metabolism in muscular dystrophy. Amino acid composition of native types I, III, IV and V collagen isolated from the gastrocnemius muscle of embryonic chickens with genetic muscular dystrophy. Comp. Biochem. Phys. A 1985, 81, 149–157. [Google Scholar] [CrossRef]
- Goldspink, G.; Fernandes, K.; Williams, P.E.; Wells, D.J. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul. Disord. 1994, 4, 183–191. [Google Scholar] [CrossRef]
- Soglia, F.; Mazzoni, M.; Zappaterra, M.; Di Nunzio, M.; Babini, E.; Bordini, M.; Sirri, F.; Clavenzani, P.; Davoli, R.; Petracci, M. Distribution and expression of vimentin and desmin in broiler Pectoralis major affected by the growth-related muscular abnormalities. Front. Physiol. 2020, 10, 1581. [Google Scholar] [CrossRef] [PubMed]
- Novikoff, A.B.; Shin, W.Y.; Drucker, J. Mitochondrial localization of oxidative enzymes: Staining results with two tetrazolius salts. J. Cell Biol. 1961, 9, 47–61. [Google Scholar] [CrossRef]
- Ishimoto, S.; Goto, I.; Kuroiwa, Y. Early morphological changes in the striated muscles in normal and dystrophic chickens. J. Comp. Pathol. 1988, 98, 69–79. [Google Scholar] [CrossRef]
- Brooke, M.H.; Kaiser, K.K. Muscle fiber types: How many and what kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar] [CrossRef]
- Suzuki, A.; Hayama, S. Histochemical classification of myofiber types in the triceps surae and flexor digitorum superficialis muscle of Japanese macaques. Acta Histochem. Cytochem. 1991, 24, 323–328. [Google Scholar] [CrossRef]
- Wank, V.; Bauer, R.; Punkt, K.; Ziegan, J. Enzyme activity patterns of myosin ATPase, alpha-glycerophosphate dehydrogenase and succinate dehydrogenase within different muscle fiber types. Acta Histochem. 1994, 96, 213–218. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol. Rev. 1996, 76, 371–423. [Google Scholar] [CrossRef]
- Cavalcanti, G.M.; Oliveira, A.B.; Assis, T.O.; Chimelli, L.M.C.; de Madeiros, P.L.; da Mota, D.L. Histochemistry and Morphometric Analysis of Muscle Fibers from Patients with Duchenne Muscular Dystrophy (DMD). Int. J. Morphol. 2011, 29, 934–938. [Google Scholar] [CrossRef]
- Moens, P.; Baatsen, P.H.; Marechal, G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J. Muscle Res. Cell. Motil. 1993, 14, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.; Silberstein, L.; Hays, A.P.; Blau, H.M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 1988, 52, 503–513. [Google Scholar] [CrossRef]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Masatoshi, S. Skeletal muscle fiber type in neuromuscular disease (chapter 5th). In Muscle Cells and Tissue: Current Status of Research Field; Sakuma, K., Ed.; IntechOpen: London, UK, 2018; pp. 65–79. [Google Scholar]
- Mutryn, M.F.; Brannick, E.M.; Fu, W.; Lee, W.R.; Abasht, B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genom. 2015, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Zambonelli, P.; Zappaterra, M.; Soglia, F.; Petracci, M.; Sirri, F.; Cavani, C.; Davoli, R. Detection of differentially expressed genes in broiler Pectoralis major muscle affected by White Striping-Wooden Breast myopathies. Poult. Sci. 2016, 95, 2771–2785. [Google Scholar] [CrossRef]
- Remignon, H.; Lefaucheur, L.; Blum, J.C.; Ricard, F.H. Effect of divergent selection for body weight on three skeletal muscles characteristics in the chicken. Br. Poult. Sci. 1994, 35, 65–76. [Google Scholar] [CrossRef]
- Hendricks, H.B.; Aberle, E.D.; Jones, D.J.; Martin, T.G. Fiber type, rigor development, and bone strength in doubled muscle cattle. J. Anim. Sci. 1973, 37, 1305–1311. [Google Scholar] [CrossRef]
- Pant, M.; Sopariwala, D.H.; Bal, N.C.; Lowe, J.; Delfín, D.A.; Rafael-Fortney, J.; Periasamy, M. Metabolic dysfunction and altered mitochondrial dynamics in the utrophin-dystrophin deficient mouse model of Duchenne muscular dystrophy. PLoS ONE 2015, 10, e0123875. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Doerr, L. Postnatal development of fiber types in normal and dystrophic skeletal muscle of the chick. Exp. Neurol. 1971, 30, 431–446. [Google Scholar] [CrossRef]
- Pfeffer, G.; Chinnery, F. Diagnosis and treatment of mitochondrial myopathies. Ann. Med. 2013, 45, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, J.A.; Graça, C.R.; Ferreira, V.F.M. Quantitation of muscle pathology abnormalities in 18 patients with mitochondrial disorders. J. Bras. Patol. Med. Lab. 2018, 54, 325–332. [Google Scholar] [CrossRef]
- Velleman, S.G.; Clark, D.L. Histopathologic and myogenic gene expression changes associated with Wooden breast in broiler breast muscles. Avian Dis. 2015, 59, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, P.R. Ultrastructure of the developing myotendinous junction of genetic dystrophic chickens. Muscle Nerve 1983, 6, 207–217. [Google Scholar] [CrossRef]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef]
- Fujii, K.; Murota, K. Abnormal collagen synthesis in skeletal muscle of dystrophic chicken. Biochem. Biophys. Res. Commun. 1983, 111, 933–938. [Google Scholar] [CrossRef]
- Listrat, A.; Picard, B.; Geay, Y. Age-related changes and location of type I, III and IV collagens during skeletal muscle development of double-muscled and normal bovine fetuses. J. Muscle Res. Cell Motil. 1998, 19, 1–14. [Google Scholar] [CrossRef]
- Baldi, G.; Soglia, F.; Mazzoni, M.; Sirri, F.; Canonico, L.; Babini, E.; Laghi, L.; Cavani, C.; Petracci, M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal 2018, 12, 164–173. [Google Scholar] [CrossRef]
- Tixier-Boichard, M. From the jungle fowl to highly performing chickens: Are we reaching limits? World Poult. Sci. J. 2020, 76, 1–17. [Google Scholar] [CrossRef]
| +NADH-TR (%) | +α-GPD (%) | +NADH-TR/+ α-GPD (%) | |
|---|---|---|---|
| NORM | 9.5 c ± 0.3 | 91.1 a ± 0.3 | 7.2 b ± 0.5 |
| WS | 13.4 b ± 0.3 | 87.7 b ± 0.3 | 9.6 b ± 0.3 |
| WB | 10.9 bc ± 0.5 | 89.9 ab ± 0.3 | 8.0 b ± 0.8 |
| SM | 19.7 a ± 3.2 | 82.3 c ± 0.4 | 14.3 a ± 3.6 |
| p-value | <0.001 | <0.001 | <0.001 |
| Fast-growing (NORM) | 9.5 a ± 0.3 | 91.1 b ± 0.3 | 7.2 a ± 0.5 |
| Slow-growing | 2.0 b ± 0.5 | 97.3 a ± 0.2 | 1.4 b ± 0.4 |
| p-value | <0.001 | <0.001 | <0.001 |
| NADH-TR (µm2) | α-GPD (µm2) | |
|---|---|---|
| NORM | 6289 a ± 201 | 6776 b ± 170 |
| WS | 6460 a ± 207 | 7480 a ± 161 |
| WB | 6639 a ± 219 | 6978 ab ± 229 |
| SM | 5544 b ± 158 | 7339 a ± 171 |
| p-value | <0.001 | <0.05 |
| Fast-growing (NORM) | 6289 a ± 201 | 6776 a ± 170 |
| Slow-growing | 3740 b ± 87 | 4499 b ± 90 |
| p-value | <0.001 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzoni, M.; Soglia, F.; Petracci, M.; Sirri, F.; Lattanzio, G.; Clavenzani, P. Fiber Metabolism, Procollagen and Collagen Type III Immunoreactivity in Broiler Pectoralis Major Affected by Muscle Abnormalities. Animals 2020, 10, 1081. https://doi.org/10.3390/ani10061081
Mazzoni M, Soglia F, Petracci M, Sirri F, Lattanzio G, Clavenzani P. Fiber Metabolism, Procollagen and Collagen Type III Immunoreactivity in Broiler Pectoralis Major Affected by Muscle Abnormalities. Animals. 2020; 10(6):1081. https://doi.org/10.3390/ani10061081
Chicago/Turabian StyleMazzoni, Maurizio, Francesca Soglia, Massimiliano Petracci, Federico Sirri, Giulia Lattanzio, and Paolo Clavenzani. 2020. "Fiber Metabolism, Procollagen and Collagen Type III Immunoreactivity in Broiler Pectoralis Major Affected by Muscle Abnormalities" Animals 10, no. 6: 1081. https://doi.org/10.3390/ani10061081
APA StyleMazzoni, M., Soglia, F., Petracci, M., Sirri, F., Lattanzio, G., & Clavenzani, P. (2020). Fiber Metabolism, Procollagen and Collagen Type III Immunoreactivity in Broiler Pectoralis Major Affected by Muscle Abnormalities. Animals, 10(6), 1081. https://doi.org/10.3390/ani10061081








