Potential Causes of Increased Vocalisation in Elderly Cats with Cognitive Dysfunction Syndrome as Assessed by Their Owners
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- (i)
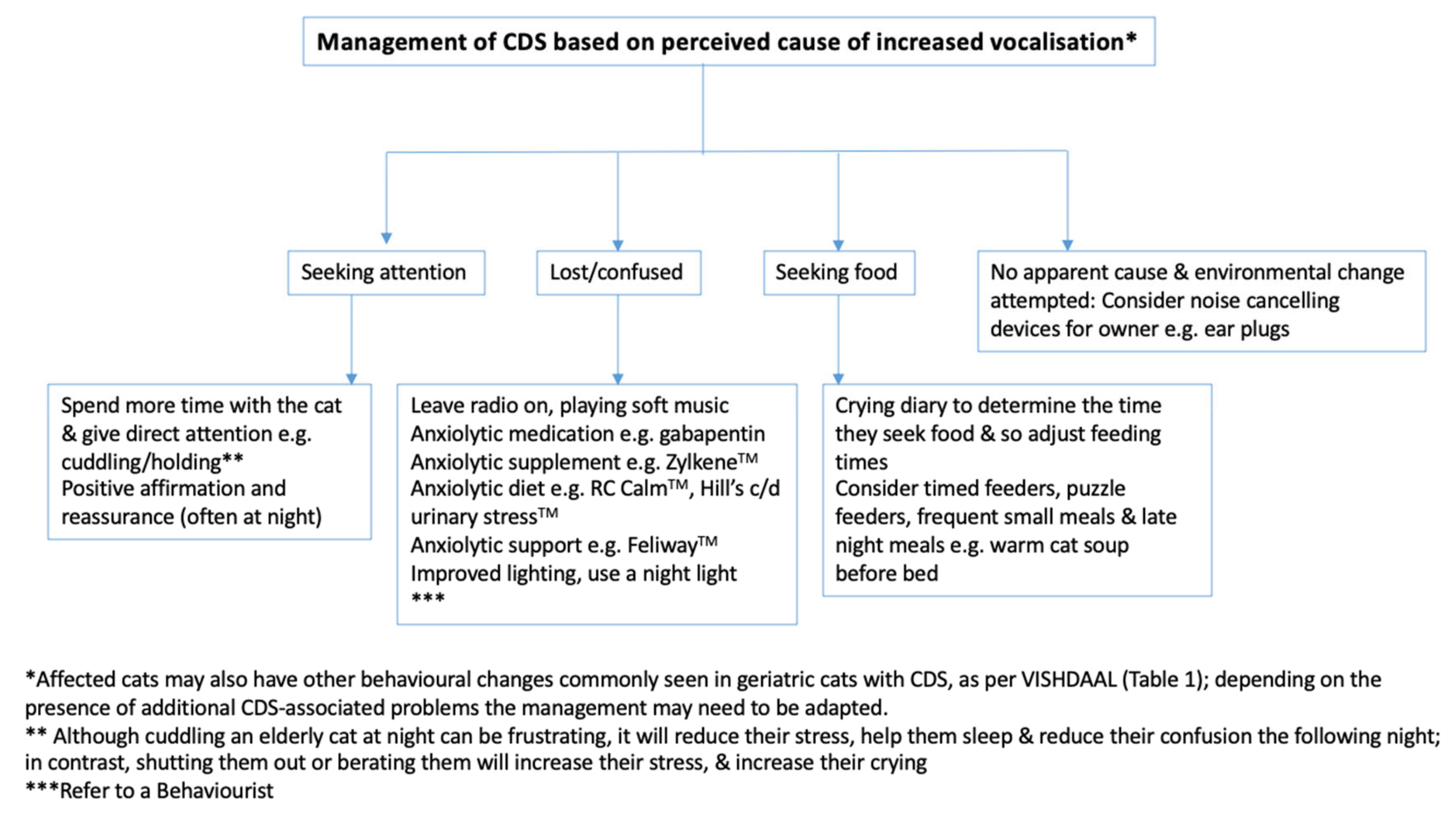
- if it looked directly at them and stopped vocalising when they interacted with it, stroked it, picked it up, etc.—this suggested attention seeking
- (ii)
- if it looked at them, then ran to the food bowl—this suggested the cat was seeking a particular resource, in this case food
- (iii)
- if it was on its own, not looking at them or a particular resource, and appeared confused—this suggested disorientation
- (iv)
- if it appeared to be sore when it moved or was touched—this suggested pain.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Informed Consent Statements
References
- Sordo, L.; Gunn-Moore, D. Cognitive dysfunction in cats: Update on neuropathological and behavioural changes plus clinical management. Vet. Rec. 2020. Submitted. [Google Scholar]
- Chapman, B.; Voith, V. Behavioral problems in old dogs: 26 cases (1984–1987). J. Am. Vet. Med. Assoc. 1990, 196, 944–946. [Google Scholar] [PubMed]
- Ruehl, W.; Bruyette, D.; DePaoli, A.; Cotman, C.; Head, E.; Milgram, N.; Cummings, B. Canine cognitive dysfunction as a model for human age-related cognitive decline, dementia and Alzheimer’s disease: Clinical presentation, cognitive testing, pathology and response to 1-deprenyl therapy. Prog. Brain Res. 1995, 106, 217–225. [Google Scholar] [PubMed]
- Landsberg, G.; Araujo, J.A. Behavior problems in geriatric pets. Vet. Clin. N. Am.-Small. 2005, 35, 675–698. [Google Scholar] [CrossRef]
- Miele, A.; Sordo, L.; Gunn-Moore, D. Feline Aging: Promoting Physiologic and Emotional Well-being. Vet. Clin. North. Am. Small. Anim. Pract. 2020, 50, 719–749. [Google Scholar] [CrossRef] [PubMed]
- Gunn-Moore, D.; Moffat, K.; Christie, L.A.; Head, E. Cognitive dysfunction and the neurobiology of ageing in cats. J. Small Anim. Pract. 2007, 48, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, G.M.; Nichol, J.; Araujo, J.A. Cognitive dysfunction syndrome: A disease of canine and feline brain aging. Vet. Clin. North Am. Small. Anim. Pract. 2012, 42, 749-vii. [Google Scholar] [CrossRef]
- Gunn-Moore, D.A.; McVee, J.; Bradshaw, J.M.; Pearson, G.R.; Head, E.; Gunn-Moore, F.J. Ageing changes in cat brains demonstrated by β-amyloid and AT8-immunoreactive phosphorylated tau deposits. J. Feline Med. Surg. 2006, 8, 234–242. [Google Scholar] [CrossRef]
- Gunn-Moore, D.A. Cognitive dysfunction in cats: Clinical assessment and management. Top. Companion Anim. Med. 2011, 26, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Volicer, L.; Crino, P.B. Involvement of free radicals in dementia of the Alzheimer type: A hypothesis. Neurobiol. Aging 1990, 11, 567–571. [Google Scholar] [CrossRef]
- Dimakopoulos, A.C.; Mayer, R.J. Aspects of neurodegeneration in the canine brain. J. Nutr. 2002, 132, 1579S–1582S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellows, J.; Center, S.; Daristotle, L.; Estrada, A.H.; Flickinger, E.A.; Horwitz, D.F.; Lascelles, B.D.X.; Lepine, A.; Perea, S.; Scherk, M. Evaluating aging in cats: How to determine what is healthy and what is disease. J. Feline Med. Surg. 2016, 18, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, F.; Noble, P.-J.M.; Jones, P.H.; Menacere, T.; Buchan, I.; Reynolds, S.; Dawson, S.; Gaskell, R.M.; Everitt, S.; Radford, A.D. Demographics of dogs, cats, and rabbits attending veterinary practices in Great Britain as recorded in their electronic health records. BMC Vet. Res. 2017, 13, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landsberg, G. Behavior problems of older cats. In Proceedings of the 135th annual meeting of the American Veterinary Medical Association, Baltimore, MD, USA, 25–29 July 1998; pp. 317–320. [Google Scholar]
- Moffatt, K.; Landsberg, G. An investigation of the prevalence of clinical signs of cognitive dysfunction syndrome (CDS) in cats. J. Am. Anim. Hosp. Assoc. 2003, 39, 512. [Google Scholar]
- Gunn-Moore, D.A.; Pearson, G.R.; Harbour, D.A.; Whiting, C.V. Encephalitis associated with giant cells in a cat with naturally occurring feline immunodeficiency virus infection demonstrated by in situ hybridization. Vet. Pathol. 1996, 33, 699–703. [Google Scholar] [CrossRef]
- Meeker, R.B.; Hudson, L. Feline Immunodeficiency Virus Neuropathogenesis: A Model for HIV-Induced CNS Inflammation and Neurodegeneration. J. Vet. Sci. 2017, 4, E14. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J. Optimizing animal models for HIV-associated CNS dysfunction and CNS reservoir research. J. Neurovirol. 2018, 24, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Power, C. Neurologic disease in feline immunodeficiency virus infection: Disease mechanisms and therapeutic interventions for NeuroAIDS. J. Neurovirol. 2018, 24, 220–228. [Google Scholar] [CrossRef]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 2018, 32, 1803–1822. [Google Scholar] [CrossRef]
- McLean, J.L.; Lobetti, R.G.; Schoeman, J.P. Worldwide prevalence and risk factors for feline hyperthyroidism: A review. J. S. Afr. Vet. Assoc. 2014, 85, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, K.; Launer, L.J.; Dewey, M.E.; Letenneur, L.; Ott, A.; Copeland, J.; Dartigues, J.-F.; Kragh-Sorensen, P.; Baldereschi, M.; Brayne, C. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology 1999, 53, 1992. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.L.; Resnick, E.M.; Mallampalli, M.; Kalbarczyk, A. Sex and gender differences in Alzheimer’s disease: Recommendations for future research. J. Womens Health 2012, 21, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin. Epidemiol. 2014, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.; Knapp, M.; Guerchet, M.; McCrone, P.; Prina, M.; Comas-Herrera, A.; Wittenberg, R.; Adelaja, B.; Hu, B.; King, D. Dementia UK Update; Alzheimer’s Society: London, UK, 2014. [Google Scholar]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Longevity and mortality of cats attending primary care veterinary practices in England. J. Feline Med. Surg. 2015, 17, 125–133. [Google Scholar] [CrossRef]
- Ryan, D.P.; Tainsh, S.M.; Kolodny, V.; Lendrum, B.L.; Fisher, R.H. Noise-making amongst the elderly in long term care. Gerontologist 1988, 28, 369–371. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Werner, P.; Marx, M.S. Screaming in nursing home residents. J. Am. Geriatr. Soc. 1990, 38, 785–792. [Google Scholar] [CrossRef]
- Cariaga, J.; Burgio, L.; Flynn, W.; Martin, D. A controlled study of disruptive vocalizations among geriatric residents in nursing homes. J. Am. Geriatr. Soc. 1991, 39, 501–507. [Google Scholar] [CrossRef]
- Matteau, E.; Landreville, P.; Laplante, L.; Laplante, C. Disruptive vocalizations: A means to communicate in dementia? Am. J. Alzheimers Dis. Other Demen. 2003, 18, 147–153. [Google Scholar] [CrossRef]
- Schake, C. Sundowner Syndrome in Dogs. Available online: https://positively.com/contributors/sundowner-syndrome-in-dogs/ (accessed on 28 December 2019).
- Volicer, L.; Harper, D.G.; Manning, B.C.; Goldstein, R.; Satlin, A. Sundowning and circadian rhythms in Alzheimer’s disease. Am. J. Psychiatry 2001, 158, 704–711. [Google Scholar] [CrossRef]
- Reinberg, A.; Ashkenazi, I. Concepts in human biological rhythms. Dialogues Clin. Neurosci. 2003, 5, 327. [Google Scholar] [PubMed]
- Moore-Ede, M.C.; Sulzman, F.M.; Fuller, C.A. The Clocks that Time Us: Physiology of the Circadian Timing System; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Sordo, L.; Breheny, C.; Halls, V.; Gunn-Moore, D. Behaviour and health of elderly cats in the UK: Changes over 20 years. Vet. Sci. 2020. under review. [Google Scholar]
- Epstein, M.; Rodan, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.; Robertson, S.; Simpson, W. 2015 AAHA/AAFP Pain Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2015, 51, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Brown, D.C.; Conzemius, M.G.; Gill, M.; Oshinsky, M.L.; Sharkey, M. Measurement of chronic pain in companion animals: Discussions from the Pain in Animals Workshop (PAW) 2017. Vet. J. 2019, 250, 71–78. [Google Scholar] [CrossRef]
- Bennett, D.; Zainal Ariffin, S.M.; Johnston, P. Osteoarthritis in the cat: 1. how common is it and how easy to recognise? J. Feline Med. Surg. 2012, 14, 65–75. [Google Scholar] [CrossRef]
- Hardie, E.M.; Roe, S.C.; Martin, F.R. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J. Am. Anim. Hosp. Assoc. 2002, 220, 628–632. [Google Scholar] [CrossRef]
- Landsberg, G. Therapeutic options for cognitive decline in senior pets. J. Am. Anim. Hosp. Assoc. 2006, 42, 407–413. [Google Scholar] [CrossRef]
- Galvan, V.; Bredesen, D.E. Neurogenesis in the adult brain: Implications for Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2007, 6, 303–310. [Google Scholar] [CrossRef]
- Pop, V.; Head, E.; Hill, M.-A.; Gillen, D.; Berchtold, N.C.; Muggenburg, B.A.; Milgram, N.W.; Murphy, M.P.; Cotman, C.W. Synergistic effects of long-term antioxidant diet and behavioral enrichment on β-amyloid load and non-amyloidogenic processing in aged canines. J. Neurosci. 2010, 30, 9831–9839. [Google Scholar] [CrossRef] [Green Version]
- Ellis, S.L.H.; Rodan, I.; Carney, H.C.; Heath, S.; Rochlitz, I.; Shearburn, L.D.; Sundahl, E.; Westropp, J.L. AAFP and ISFM Feline Environmental Needs Guidelines. J. Feline Med. Surg. 2013, 15, 219–230. [Google Scholar] [CrossRef]
- Slingerland, L.; Hazewinkel, H.; Meij, B.; Picavet, P.; Voorhout, G. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet. J. 2011, 187, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X. Feline Degenerative Joint Disease. Vet. Surg. 2010, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Ikeda-Douglas, C.; Zicker, S.; Estrada, J.; Jewell, D.; Milgram, N. Prior experience, antioxidants, and mitochondrial cofactors improve cognitive function in aged beagles. Vet. Ther. 2004, 5, 5–16. [Google Scholar] [PubMed]
- Head, E.; Zicker, S.C. Nutraceuticals, aging, and cognitive dysfunction. Vet. Clin. North Am. Small Anim. Pract. 2004, 34, 217–228. [Google Scholar] [CrossRef]
- Roudebush, P.; Zicker, S.C.; Cotman, C.W.; Milgram, N.W.; Muggenburg, B.A.; Head, E. Nutritional management of brain aging in dogs. J. Am. Vet. Med. Assoc. 2005, 227, 722–728. [Google Scholar] [CrossRef]
- Heath, S.E.; Barabas, S.; Craze, P.G. Nutritional supplementation in cases of canine cognitive dysfunction—A clinical trial. Appl. Anim. Behav. Sci. 2007, 105, 284–296. [Google Scholar] [CrossRef]
- Pan, Y.; Larson, B.; Araujo, J.A.; Lau, W.; De Rivera, C.; Santana, R.; Gore, A.; Milgram, N.W. Dietary supplementation with medium-chain TAG has long-lasting cognition-enhancing effects in aged dogs. Br. J. Nutr. 2010, 103, 1746–1754. [Google Scholar] [CrossRef] [Green Version]
- Fragua, V.; Lepoudère, A.; Leray, V.; Baron, C.; Araujo, J.; Nguyen, P.; Milgram, N. Effects of dietary supplementation with a mixed blueberry and grape extract on working memory in aged beagle dogs. J. Nutr. Sci. 2017, 6, 6:e35. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Kennedy, A.D.; Jönsson, T.J.; Milgram, N.W. Cognitive enhancement in old dogs from dietary supplementation with a nutrient blend containing arginine, antioxidants, B vitamins and fish oil. Br. J. Nutr. 2018, 119, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Araujo, J.A.; Landsberg, G.M.; Milgram, N.W.; Miolo, A. Improvement of short-term memory performance in aged beagles by a nutraceutical supplement containing phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine. Can. Vet. J. 2008, 49, 379. [Google Scholar]
- Pan, Y.; Landsberg, G.; Mougeot, I.; Kelly, S.; Xu, H.; Bhatnagar, S.; Gardner, C.L.; Milgram, N.W. Efficacy of a Therapeutic Diet on Dogs With Signs of Cognitive Dysfunction Syndrome (CDS): A Prospective Double Blinded Placebo Controlled Clinical Study. Front. Nutr. 2018, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, G.M.; DePorter, T.; Araujo, J.A. Clinical signs and management of anxiety, sleeplessness, and cognitive dysfunction in the senior pet. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 565–590. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Faubert, M.L.; Brooks, M.L.; Landsberg, G.M.; Lobprise, H. NOVIFIT®(NoviSAMe®) Tablets Improve Executive Function in Aged Dogs and Cats: Implications for Treatment of Cognitive Dysfunction Syndrome. Int. J. Appl. Res. Vet. M. 2012, 10, 90. [Google Scholar]
- Bottiglieri, T. S-Adenosyl-L-methionine (SAMe): From the bench to the bedside—molecular basis of a pleiotrophic molecule. Am. J. Clin. Nutr. 2002, 76, 1151S–1157S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rème, C.-A.; Dramard, V.; Kern, L.; Hofmans, J.; Halsberghe, C.; Mombiela, D.V. Effect of S-adenosylmethionine tablets on the reduction of age-related mental decline in dogs: A double-blinded, placebo-controlled trial. Vet. Ther. 2008, 9, 69–82. [Google Scholar] [PubMed]
- Pan, Y.; Araujo, J.A.; Burrows, J.; de Rivera, C.; Gore, A.; Bhatnagar, S.; Milgram, N.W. Cognitive enhancement in middle-aged and old cats with dietary supplementation with a nutrient blend containing fish oil, B vitamins, antioxidants and arginine. Br. J. Nutr. 2013, 110, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houpt, K.; Levine, E.; Landsberg, G.; Moffat, K.S.; Zicker, S.C. Antioxidant fortified food improves owner perceived behaviour in the aging cat. In Proceedings of the ESFM Conference, Prague, Czech Republic, 21–23 September 2007; Moffat, K.S., Ed.; 2007. [Google Scholar]
- Hill, A.; Werner, J.; Rogers, Q.; O’Neill, S.; Christopher, M.M. Lipoic acid is 10 times more toxic in cats than reported in humans, dogs or rats. J. Anim. Physiol. Anim. Nutr. 2004, 88, 150–156. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Hunthausen, W.L.; Ackerman, L.J. Handbook of Behavior Problems of the Dog and Cat; Landsberg, G., Hunthausen, W., Ackerman, L., Eds.; Saunders: Philadelphia, PA, USA, 2003. [Google Scholar]
- Studzinski, C.M.; Araujo, J.A.; Milgram, N.W. The canine model of human cognitive aging and dementia: Pharmacological validity of the model for assessment of human cognitive-enhancing drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 489–498. [Google Scholar] [CrossRef]
- Kume, K.; Hanyu, H.; Sakurai, H.; Takada, Y.; Onuma, T.; Iwamoto, T. Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer’s disease. Geriatr. Gerontol. Int. 2012, 12, 207–214. [Google Scholar] [CrossRef]
- Wang, J.; Pang, T.; Hafko, R.; Benicky, J.; Sanchez-Lemus, E.; Saavedra, J.M. Telmisartan ameliorates glutamate-induced neurotoxicity: Roles of AT1 receptor blockade and PPARγ activation. Neuropharmacology 2014, 79, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Wincewicz, D.; Braszko, J.J. Telmisartan attenuates cognitive impairment caused by chronic stress in rats. Pharmacol. Rep. 2014, 66, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.; Lu, F.; Ma, M.; Wang, J.; Suo, A.; Bai, Y.; Liu, H. Effects of telmisartan on the level of Aβ1-42, interleukin-1β, tumor necrosis factor α and cognition in hypertensive patients with Alzheimer’s disease. Zhonghua yi xue za zhi 2012, 92, 2743–2746. [Google Scholar] [PubMed]
- Pan, G.; Zhou, X.; Zhao, J. Effect of Telmisartan on Atrial Fibrillation Recurrences in Patients with Hypertension: A Systematic Review and Meta-Analysis. Cardiovasc. Ther. 2014, 32, 184–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Behavioural Changes Related to CDS (VISHDAAL): |
|---|
| Inappropriate Vocalisation, especially at night |
| Altered social Interaction with the family and/or other pets |
| Changes in Sleep/wake patterns |
| House-soiling |
| Spatial and temporal Disorientation e.g., forgetting the location of their litterbox or that they have been fed |
| Changes in Activity e.g., aimless wandering Anxiety Learning and memory deficits |
| Trigger | Number of Cats |
|---|---|
| No initial trigger | 62.1%; n = 23 |
| Moving house | 10.8%; n = 4 |
| Loss of a sibling | 8.1%; n = 3 |
| Loss of a family member | 8.1%; n = 3 |
| New cat/dog in the family | 5.4%; n = 2 |
| Going to a cattery | 2.7%; n = 1 |
| Significant veterinary treatment * | 2.7%; n = 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černá, P.; Gardiner, H.; Sordo, L.; Tørnqvist-Johnsen, C.; Gunn-Moore, D.A. Potential Causes of Increased Vocalisation in Elderly Cats with Cognitive Dysfunction Syndrome as Assessed by Their Owners. Animals 2020, 10, 1092. https://doi.org/10.3390/ani10061092
Černá P, Gardiner H, Sordo L, Tørnqvist-Johnsen C, Gunn-Moore DA. Potential Causes of Increased Vocalisation in Elderly Cats with Cognitive Dysfunction Syndrome as Assessed by Their Owners. Animals. 2020; 10(6):1092. https://doi.org/10.3390/ani10061092
Chicago/Turabian StyleČerná, Petra, Hannah Gardiner, Lorena Sordo, Camilla Tørnqvist-Johnsen, and Danièlle A. Gunn-Moore. 2020. "Potential Causes of Increased Vocalisation in Elderly Cats with Cognitive Dysfunction Syndrome as Assessed by Their Owners" Animals 10, no. 6: 1092. https://doi.org/10.3390/ani10061092
APA StyleČerná, P., Gardiner, H., Sordo, L., Tørnqvist-Johnsen, C., & Gunn-Moore, D. A. (2020). Potential Causes of Increased Vocalisation in Elderly Cats with Cognitive Dysfunction Syndrome as Assessed by Their Owners. Animals, 10(6), 1092. https://doi.org/10.3390/ani10061092







