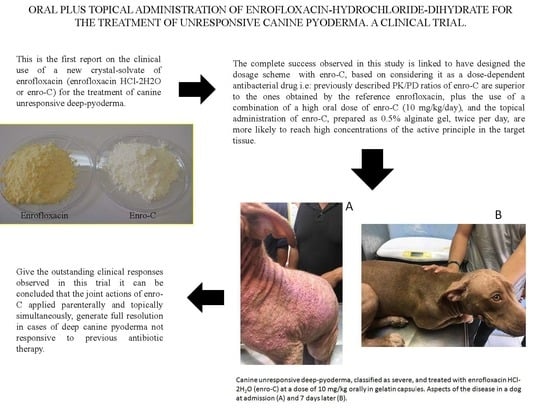
Oral Plus Topical Administration of Enrofloxacin-Hydrochloride-Dihydrate for the Treatment of Unresponsive Canine Pyoderma. A Clinical Trial
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Drug Preparation and Administration
2.3. Experimental Design
2.4. Antimicrobial Susceptibility
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Beco, L.; Guaguère, E.; Lorente Méndez, C.; Noli, C.; Nuttall, T.; Vroom, M. Suggested guidelines for using systemic antimicrobials in bacterial skin infections diagnosis based on clinical presentation, cytology and culture. Vet. Rec. 2013, 172, 72–78. [Google Scholar] [PubMed]
- Gortel, K. Recognizing pyoderma: More difficult than it may seem. Vet. Clin. Small Anim. Pract. 2013, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.F.; Hendricks, A.; Brodbelt, D.C. Prescribing practices of primary-care veterinary practitioners in dogs diagnosed with bacterial pyoderma. BMC Vet. Res. 2014, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Yoon, J.W.; Lee, S.Y.; Park, H.M. High prevalence of fluoroquinolone and methicillin-resistant Staphylococcus pseudintermedius isolates from canine pyoderma and otitis externa in veterinary teaching hospital. J. Microbiol. Biotechnol. 2010, 20, 798–802. [Google Scholar] [PubMed]
- Faires, M.C.; Gard, S.; Aucoin, D.; Weese, J.S. Inducible clindamycin-resistance in methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius isolates from dogs and cats. Vet. Microbiol. 2009, 139, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.E.; Ball, K.R.; Chirino-Trejo, M. Antimicrobial susceptibility of Staphylococcus aureus and Staphylococcus pseudintermedius isolated from various animals. Can. Vet. J. 2011, 52, 153–157. [Google Scholar]
- Kang, M.H.; Chae, M.J.; Yoon, J.W.; Lee, S.Y.; Yoo, J.H.; Park, H.M. Resistance to fluoroquinolones and methicillin in ophthalmic isolates of Staphylococcus pseudintermedius from companion animals. Can. Vet. J. 2014, 55, 678–682. [Google Scholar]
- Van Duijkeren, E.; Van Laar, P.; Houwers, D.J. Methicillin-resistant staphylococci isolated from animals. Vet. Microbiol. 2004, 103, 91–97. [Google Scholar] [CrossRef]
- Weese, J.S. Methicillin-resistant Staphylococcus aureus: An emerging pathogen in small animals. J. Am. Anim. Hosp. Assoc. 2005, 41, 150–157. [Google Scholar] [CrossRef]
- Baptiste, K.E.; Williams, K.; Willams, N.J.; Wattret, A.; Clegg, P.D.; Dawson, S.; Corkill, J.E.; O’Neill, T.; Hart, C.A. Methicillin-resistant Staphylococci in Companion Animals. Emerg. Infect. Dis. 2005, 11, 1942–1944. [Google Scholar]
- Gortel, K.; Campbell, K.L.; Kakoma, I.; Whittem, T.; Schaeffer, D.J.; Weisiger, R.M. Methicillin resistance among staphylococci isolated from dogs. Am. J. Vet. Res. 1999, 60, 1526–1530. [Google Scholar] [PubMed]
- Frank, L.A.; Kania, S.A.; Hnilica, K.A.; Wilkes, R.P.; Bemis, D.A. Isolation of Staphylococcus schleiferi from dogs with pyoderma. J. Am. Vet. Med. Assoc. 2003, 222, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.S.; Stephan, B. Pradofloxacin in the treatment of canine deep pyoderma: A multicentred, blinded, randomized parallel trial. Vet. Dermatol. 2007, 18, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Horspool, L.J.; Van Laar, P.; Van Den Bos, R.; Mawhinney, I. Treatment of canine pyoderma with ibafloxacin and marbofloxacin-fluoroquinolones with different pharmacokinetic profiles. J. Vet. Pharmacol. Ther. 2004, 27, 147–153. [Google Scholar] [CrossRef]
- Paradis, M.; Lemay, S.; Scott, D.W.; Miller, W.H.; Wellington, J.; Papich, R. Efficacy of enrofloxacin in the treatment of canine bacterial pyoderma. Vet. Dermatol. 1990, 1, 123–127. [Google Scholar] [CrossRef]
- McKellar, Q.A.; Sanchez Bruni, S.F.; Jones, D.G. Pharmacokinetic/pharmacodynamics relationships of antimicrobial drugs used in veterinary medicine. J. Vet. Pharmacol. Ther. 2004, 27, 503–514. [Google Scholar] [CrossRef]
- Papich, M.G. Saunders Handbook of Veterinary Drugs, 2nd ed.; Saunders Elsevier: St. Louis, MO, USA, 2007; pp. 236–238. [Google Scholar]
- Meinen, J.B.; McClure, J.T.; Rosin, E. Pharmacokinetics of enrofloxacin in clinically normal dogs and mice and drug pharmacodynamics in neutropenic mice with Escherichia coli and staphylococcal infections. Am. J. Vet. Res. 1995, 56, 1219–1224. [Google Scholar]
- Chakravarthy, V.A.; Sailaja, B.B.; Kumar, A.P. Stability-indicating RP-HPLC method for simultaneous estimation of enrofloxacin and its degradation products in tablet dosage forms. J. Anal. Methods Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Bidgood, T.L.; Papich, M.G. Plasma and interstitial fluid pharmacokinetics of enrofloxacin, its metabolite ciprofloxacin, and marbofloxacin after oral administration and a constant rate intravenous infusion in dogs. J. Vet. Pharmacol. Ther. 2005, 28, 329–341. [Google Scholar] [CrossRef]
- Simon, Z.; Katja, B.; Darko, U.; Marjan, V.; Albin, K. Metal cation-fluoroquinolone complexes do not permeate through the intestinal absorption barrier. J. Pharm. Biomed. Anal. 2010, 53, 655–659. [Google Scholar] [CrossRef]
- Sumano, L.H.; Gutiérrez, O.L.; Zamora, M.A. Bioequivalence of four preparations of enrofloxacin in poultry. J. Vet. Pharmacol. Ther. 2001, 24, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Sumano, L.H.; Ocampo, C.L.; Gutiérrez, O.L. Bioequivalence of six generic preparations of enrofloxacin in pigs. Pig J. 2001, 51, 64–73. [Google Scholar]
- Sumano, L.H.; Ocampo, C.L.; Gutiérrez, O.L. Non-bioequivalence of various trademarks of enrofloxacin and Baytril in cows. Dtsch. Tierarztl. Wochenschr. 2001, 108, 311–314. [Google Scholar]
- Miranda-Calderón, J.E.; Gutiérrez, L.; Flores-Alamo, M.; García-Gutiérrez, P.; Sumano, H. Enrofloxacin hydrochloride dihydrate. Acta Crystallogr. Sect. E Struct. Rep. 2014, 70, 468–469. [Google Scholar] [CrossRef]
- Gutierrez, L.; Miranda-Calderon, J.E.; Garcia-Gutierrez, P.; Sumano, H. Physicochemical characterization and pharmacokinetics in broiler chickens of a new recrystallized enrofloxacin hydrochloride dihydrate. J. Vet. Pharmacol. Ther. 2015, 38, 183–189. [Google Scholar] [CrossRef]
- Sumano, H.; Ocampo, L.; Tapia, G.; Mendoza, C.J.; Gutierrez, L. Pharmacokinetics of enrofloxacin HCl-2H2O (Enro-C) in dogs and pharmacokinetic/pharmacodynamic Monte Carlo simulations against Leptospira spp. J. Vet. Sci. 2018, 19, 600–607. [Google Scholar] [CrossRef]
- Mexican Official Standard NOM-062-ZOO-1999. Technical Specifications for the Production, Care and Use of Laboratory Animals. United Mexican States Department of Agriculture, Livestock, Rural Development, Fisheries and Food. 1999. Available online: www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO (accessed on 13 January 2020).
- Adityan, B.; Kumari, R.; Thappa, D.M. Scoring systems in acne vulgaris. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 323–326. [Google Scholar]
- Gandhi, S.; Ojha, A.K.; Ranjan, K.P.; Neelima, K. Clinical and Bacteriological Aspects of Pyoderma. N. Am. J. Med. Sci. 2012, 4, 492–495. [Google Scholar]
- Hnilica, K.; Patterson, A. Small Animal Dermatology, 4th ed.; Saunders/Elsevier: St. Louis, MO, USA, 2016. [Google Scholar]
- Quinn, P.J.; Carter, M.E.; Markey, B.; Carter, G.R. Clinical Veterinary Microbiology, 7th ed.; Wolfe: London, UK, 1994. [Google Scholar]
- Kloos, W.E.; Schleifer, K.H. Simplified scheme for routine identification of human Staphylococcus species. J. Clin. Microbiol. 1975, 1, 82–88. [Google Scholar] [CrossRef]
- Bannerman, T.L. Staphylococcus, Micrococcus, and other catalase-positive cocci that grow aerobically. In Manual of Clinical Microbiology; Murray, P.R., Baron, E.J., Jorgensen, J.H., Pfaller, M.A., Yolken, R.H., Eds.; American Society Microbiology: Washington, DC, USA, 2003; pp. 384–404. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 2nd ed.; Approved Standard M31-A2; NCCLS: Wayne, PA, USA, 2002. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Informational Supplement M31-S1; NCCLS: Wayne, PA, USA, 2004. [Google Scholar]
- Paulus, J.K.; Dahabreh, I.; Balk, E.M.; Avendano, E.E.; Lauc, J.; Ipa, S. Opportunities and challenges in using studies without a control group in comparative effectiveness reviews. Res. Synth. Methods 2014, 5, 152–161. [Google Scholar] [CrossRef]
- Kramer, M.; Font, E. Reducing sample size in experiments with animals: Historical controls and related strategies. Biol. Rev. Camb. Philos. Soc. 2017, 92, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.F.; Brodbelt, D.C.; Forsythe, P.J.; Loeffler, A.; Hendricks, A. The effectiveness of systemic antimicrobial treatment in canine superficial and deep pyoderma, a systematic review. Vet. Dermatol. 2012, 23, 305–329. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Pedersen, K.; Jensen, H.; Finster, K.; Jensen, V.F.; Heuer, O.E. Occurrence of antimicrobial resistance in bacteria from diagnostic samples from dogs. J. Antimicrob. Chemother. 2007, 60, 775–781. [Google Scholar]
- Shah, B.; Mathakiya, R.; Rao, N.; Nauriyal, D.S. Organisms recovered from cases of canine pyoderma and their antibiogram pattern. J. Anim. Res. 2017, 7, 1067–1073. [Google Scholar] [CrossRef]
- Oliveira, A.; Devesa, J.S.P.; Hill, P.B.; Silva, V.; Poeta, P. Treatment of selected canine dermatological conditions in Portugal—A research survey. J. Vet. Res. 2018, 62, 563–570. [Google Scholar] [CrossRef]
- Koch, H.J.; Peters, S. Antimicrobial therapy in German Shepherd dog pyoderma (GSP). An open clinical study. Vet. Dermatol. 2008, 7, 177–181. [Google Scholar] [CrossRef]
- Wetzstein, H.G. Comparative mutant preventive concentrations of pradofloxacin and other veterinary fluoroquinolones indicate different potentials in preventing selection of resistance. Antimicrob. Agents Chemother. 2005, 49, 4166–4173. [Google Scholar]
- Awji, E.G.; Tassew, D.D.; Lee, J.S.; Lee, S.J.; Choi, M.J.; Reza, M.A.; Rhee, M.-H.; Kim, T.H.; Park, S.C. Comparative mutant prevention concentration and mechanism of resistance to veterinary fluoroquinolones in Staphylococcus pseudintermedius. Vet. Dermatol. 2012, 23, 376–380. [Google Scholar] [CrossRef]
- Blondeau, J.M.; Hansen, G.; Metzler, K. The role of PK/PD parameters to avoid selection and increase of resistance: Mutant prevention concentration. J. Chemother. 2004, 16, 1–19. [Google Scholar]
- Ihrke, P.J.; Papich, M.G.; DeManuelle, T.C. The Use of fluoroquinolones in veterinary dermatology. Vet. Dermatol. 1999, 10, 193–204. [Google Scholar] [CrossRef]
- Pallo-Zimerman, L.M.; Byron, J.K.; Graves, T.K. Fluoroquinolones: Then and now. Compend. Contin. Educ. Vet. 2010, 32, E1–E9. [Google Scholar]




| Sign | Classification of Pyoderma | |
|---|---|---|
| Severe | Very Severe | |
| Pruritus cessation or marked reduction | ≤3 days | ≤5 (days) |
| Initial resolution of skin lesions | ≤7 days | ≤10 days |
| Initial fur growth | ≤15 days | ≤15 days |
| Absence of peculiar odor | ≤3 days | ≤5 days |
| Absence of other signs (fever, hyporexia, postural discomfort, etc.) | ≤3 days | ≤5 days |
| Absence of recurrence | 2 months | 2 months |
| Classification | Signs | |
|---|---|---|
| Severe | Very Severe | |
| Pruritus | Moderate to severe (2) | Severe-constant (3) |
| Fever | No (zero) | Slightly increased (3) |
| Appetite | Not affected—slightly reduced (0–2) | Greatly reduced (3) |
| Papules | Few—some # (1,2) | Many (3) |
| Pustules | Few—some # (1,2) | Many (3) |
| Erythema | Localized in few areas (2) | In almost the whole body surface (3) |
| Crusts | Few—some # (1,2) | Many (3) |
| Comedo | Few—Some # (1,2) | Many (3) |
| Fistulae | Few—Some # (1,2) | Many (3) |
| Cellulitis | Slight–moderate (1,2) | Marked (3) |
| Estimated affected body surface | ≥50% (2) | ≥75% (3) |
| Total score | From 10 to19 * | 20–33 |
| Feature | Deep-Pyoderma | |
|---|---|---|
| Severe | Very Severe | |
| No of cases treated | 32 | 23 |
| No of days on treatment * | 8.03 ± 2.1 | 12.0 ± 2.4 |
| Time to control of pruritus | 2.2 ± 0.6 | 3.8 ± 0.8 |
| Adverse drug reactions º | none | Hyporexia cases 5 cases; loose feces 7 cases; muscle pain 1 case |
| Treatment success | 100% | 100% |
| possibly facilitated by: | ||
| Dog-flea collar reaction | 4 | 8 |
| Flea allergy | 3 | 6 |
| Post-grooming furunculosis | 8 | 5 |
| After a dog bite or fight | 2 | 1 |
| Lesions in pressure points | 2 | 2 |
| Previous history of demodicosis | 2 | none |
| Unidentified | 11 | 1 |
| Previously treated with #, ∞: | ||
| Amoxicillin/K-clavulanate: (5:1) 20 mg/kg PO bid | 12 | 8 |
| Cefovecin: 8 mg/kg SC once every 7 days | 9 | 8 |
| Marbofloxacin: mg/kg PO, every 24 h | 6 | 6 |
| Enrofloxacin: 10 mg/kg PO, every 24 h | 5 | 6 |
| Cephalexin: 20 mg/kg PO tid | 4 | 4 |
| Clindamycin: 20 mg/kg PO bid | 0 | 2 |
| Severity | Mean | Median | ||||||
|---|---|---|---|---|---|---|---|---|
| Value | Typical Error | 95% Confidence Interval | Value | Typical Error | 95% Confidence Interval | |||
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||||
| Severe | 8.000 a | 0.577 | 6.868 | 9.132 | 8.000 | 0.707 | 6.614 | 9.386 |
| Very severe | 12.000 b | 0.495 | 11.030 | 12.970 | 12.000 | 0.951 | 10.136 | 13.864 |
| From severe to very severe | 8.043 a | 0.472 | 7.117 | 8.970 | 7.000 | 0.799 | 5.435 | 8.565 |
| Global | 9.691 | 0.399 | 8.910 | 10.472 | 9.000 | 0.494 | 8.031 | 9.969 |
| Isolate | No. of Isolates | Percentage of Isolates Sensitive to the Antibiotic Used | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am | AmC | Mb | Me | Ox | Amp | Cl | Cf | TmS | Dox | En | ||
| Staphylococcus intermedius | 19 | 15.8 | 26.3 | 15.8 | 10.5 | 5.3 | 5.3 | 10.5 | 21.1 | 15.8 | 15.8 | 42.1 |
| Staphylococcus pyogenes | 14 | 14.3 | 42.9 | 7.1 | 7.1 | 7.1 | 7.1 | 0 | 7.1 | 14.3 | 21.4 | 42.9 |
| Staphylococcus aureus | 6 | 16.7 | 33.3 | 50.0 | 16.7 | 0 | 16.7 | 0 | 16.7 | 16.7 | 16.7 | 33.3 |
| Staphylococcus pseudintermedius | 16 | 12.5 | 31.3 | 12.5 | 6.3 | 12.5 | 12.5 | 6.3 | 12.5 | 12.5 | 18.8 | 31.3 |
| Staphylococcus epidermidis | 15 | 13.3 | 40.0 | 6.7 | 6.7 | 6.7 | 13.3 | 6.7 | 13.3 | 6.7 | 20.0 | 40.0 |
| Staphylococcus saprophyticus | 8 | 12.5 | 25.0 | 12.5 | 12.5 | 0 | 12.5 | 12.5 | 12.5 | 12.5 | 25.0 | 37.5 |
| Pseudomonas aeruginosa | 6 | 16.7 | 16.7 | 16.7 | 16.7 | 0.0 | 16.7 | 16.7 | 0.0 | 16.7 | 16.7 | 16.7 |
| Klebsiella sp. | 4 | 0 | 0 | 50.0 | 0 | 0 | 0 | 0 | 0 | 25.0 | 25.0 | 25.0 |
| Streptococcus sp. | 7 | 0 | 0 | 28.6 | 0 | 0 | 0 | 0 | 0 | 14.3 | 14.3 | 0.0 |
| Escherichia coli | 4 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 25.0 | 25.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, L.; Tapia, G.; Ocampo, L.; Monroy-Barreto, M.; Sumano, H. Oral Plus Topical Administration of Enrofloxacin-Hydrochloride-Dihydrate for the Treatment of Unresponsive Canine Pyoderma. A Clinical Trial. Animals 2020, 10, 943. https://doi.org/10.3390/ani10060943
Gutierrez L, Tapia G, Ocampo L, Monroy-Barreto M, Sumano H. Oral Plus Topical Administration of Enrofloxacin-Hydrochloride-Dihydrate for the Treatment of Unresponsive Canine Pyoderma. A Clinical Trial. Animals. 2020; 10(6):943. https://doi.org/10.3390/ani10060943
Chicago/Turabian StyleGutierrez, Lilia, Graciela Tapia, Luis Ocampo, Minerva Monroy-Barreto, and Hector Sumano. 2020. "Oral Plus Topical Administration of Enrofloxacin-Hydrochloride-Dihydrate for the Treatment of Unresponsive Canine Pyoderma. A Clinical Trial" Animals 10, no. 6: 943. https://doi.org/10.3390/ani10060943
APA StyleGutierrez, L., Tapia, G., Ocampo, L., Monroy-Barreto, M., & Sumano, H. (2020). Oral Plus Topical Administration of Enrofloxacin-Hydrochloride-Dihydrate for the Treatment of Unresponsive Canine Pyoderma. A Clinical Trial. Animals, 10(6), 943. https://doi.org/10.3390/ani10060943