The Dynamic Changes of African Elephant Milk Composition over Lactation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Determination of Density, Ash and Minerals
2.3. Vitamin Analysis
2.4. Protein Analysis
2.5. Lipid Analysis
2.6. Carbohydrate Analysis
2.7. Determination of Energy
2.8. Statistical Analysis
3. Results and Discussion
3.1. Density, Ash and Minerals
3.2. Vitamins
3.3. Proteins
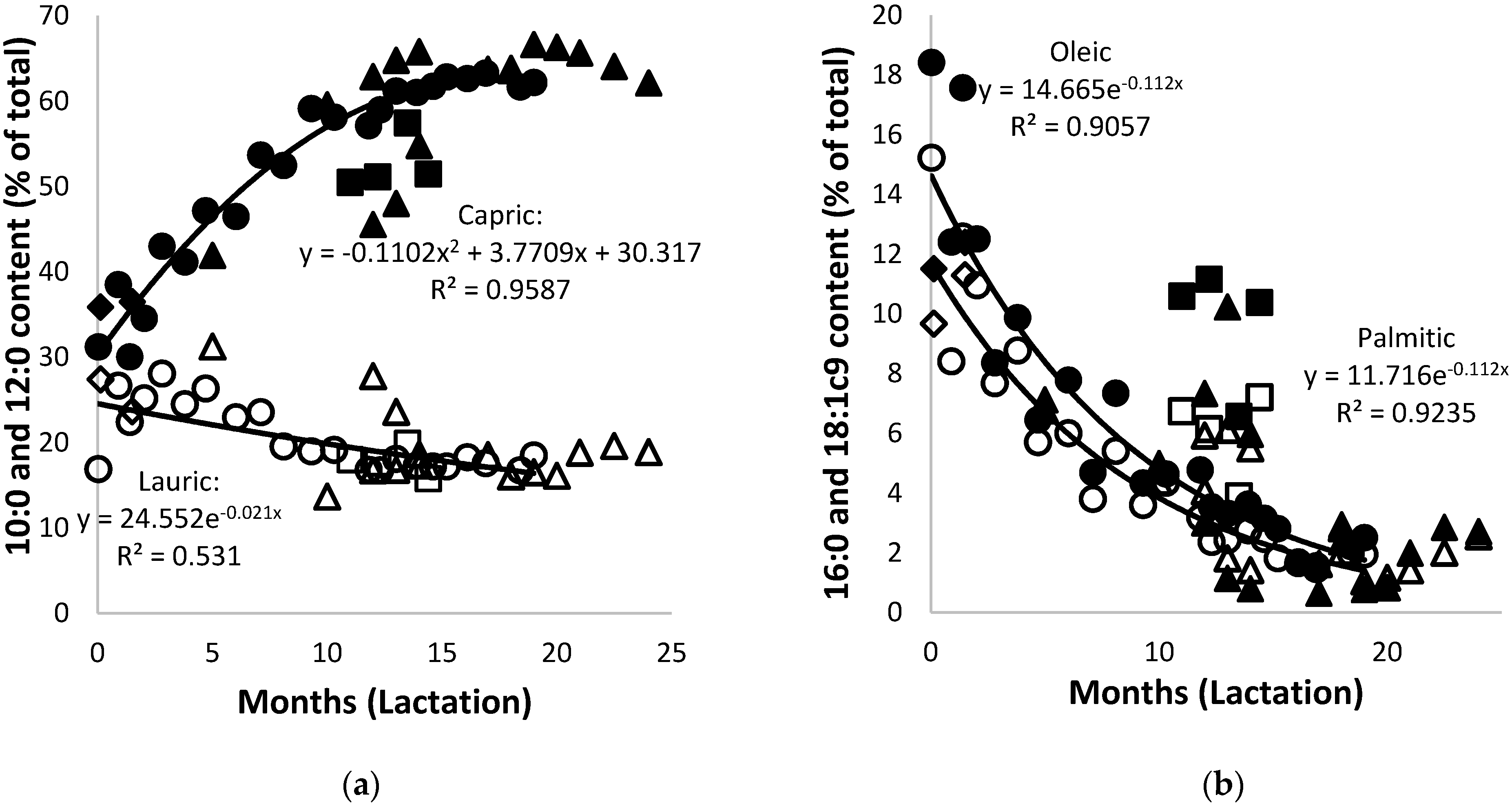
3.4. Lipids
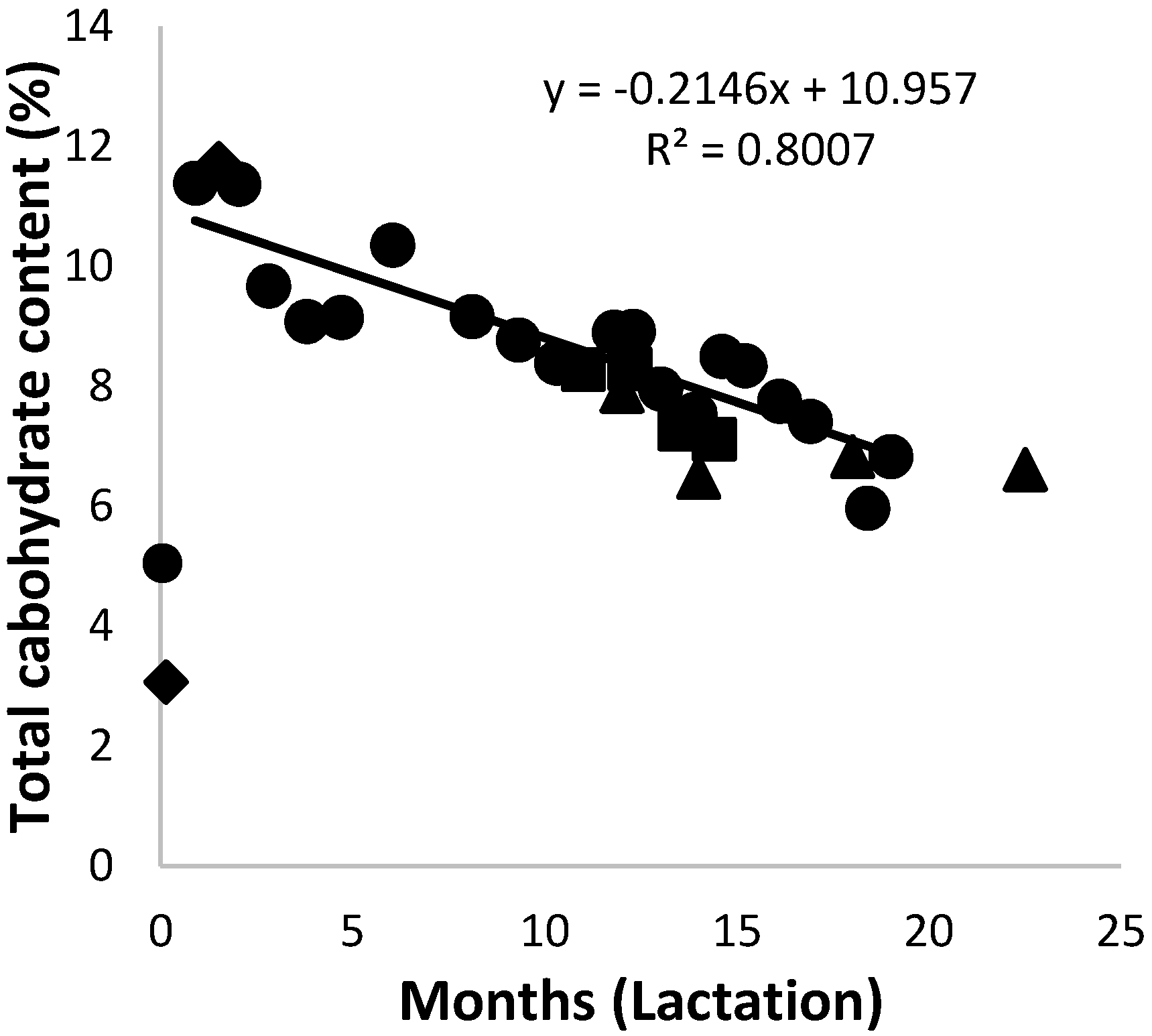
3.5. Carbohydrates
3.6. Energy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mainka, S.A.; Cooper, R.M.; Black, S.R.; Dierenfeld, E.S. Asian elephant (Elephas maximus) milk composition during the first 280 days of lactation. Zoo Biol. 1994, 13, 389–393. [Google Scholar] [CrossRef]
- Abbondanza, F.N.; Power, M.L.; Dickson, M.A.; Brown, J.; Oftedal, O.T. Variation in the composition of milk of Asian elephants (Elephas maximus) throughout lactation. Zoo Biol. 2013, 32, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, G. Elephant milk. In Elephants: Ecology, Behaviour and Conservationcology, Behaviour and Conservation; Aranovich, M., Dufresne, O., Eds.; Nova Publishing: New York, NY, USA, 2012; pp. 97–117. [Google Scholar]
- McCullagh, K.G.; Widdowson, E.M. The milk of the African elephant. Br. J. Nutr. 1970, 24, 109–117. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; Joubert, C.C.; Swarts, J.C. DSC of milk fats from various animals with high levels of medium-chain, unsaturated and polyunsaturated fatty acids. S. Afr. J. Chem. 2011, 64, 241–250. [Google Scholar]
- Uemura, Y.; Asakuma, S.; Yon, L.; Saito, T.; Fukuda, K.; Arai, I.; Urashima, T. Structural determination of the oligosaccharides in the milk of an Asian elephant (Elephas maximus). Comp. Biochem. Physiol. A 2006, 145, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, G.; Dickens, L.; Urashima, T.; Bonnet, S.L.; Uemura, Y.; Van der Westhuizen, J.H. Structural characterization of oligosaccharides in the milk of an African elephant (Loxodonta africana Africana). Comp. Biochem. Physiol. B 2008, 150, 74–84. [Google Scholar] [CrossRef]
- Madende, M.; Kemp, G.; Stoychev, S.; Osthoff, G. Characterisation of African elephant beta casein and its relevance to the chemistry of caseins and casein micelles. Int. Dairy J. 2018, 85, 112–120. [Google Scholar] [CrossRef]
- Madende, M.; Osthoff, G.; Patterton, H.; Martin, P.; Opperman, D.J. Characterization of casein and alpha lactalbumin of African elephant (Loxodonta africana) milk. J. Dairy Sci. 2015, 98, 8308–8318. [Google Scholar] [CrossRef]
- Trott, J.F.; Simpson, K.L.; Moyle, R.L.; Hearn, C.M.; Shaw, G.; Nicholas, K.R.; Renfree, M.B. Maternal regulation of milk composition, milk production, and pouch young development in the Tammar wallaby (Macropus eugenii). Biol. Reprod. 2003, 68, 929–936. [Google Scholar] [CrossRef]
- Sikes, R.S.; The Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; De Waal, H.O.; Botes, P. The composition of African elephant (Loxodonta Africana) milk collected a few days postpartum. Comp. Biochem. Physiol. A 2005, 141, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T.; Iverson, S.J. Comparative analysis of non-human milks: Phylogenetic variation in the gross composition of milks In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: New York, NY, USA, 1995; pp. 749–788. [Google Scholar]
- Oguntunde, A.O.; Akintoye, O.A. Measurement and comparison of density, specific heat and viscosity of cow’s milk and soymilk. J. Food Eng. 1991, 13, 221–230. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- American Public Health Association. Method 3120 B–Inductively coupled plasma method. In American Water Works Association/American Public Works Association/Water Environment Federation, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Comstock, G.W.; Alberg, A.J.; Helzlsouer, K.J. Reported effects of long-term freezer storage on concentrations of retinol, ß-carotene, and α-tocopherol in serum or plasma summarized. Clin. Chim. Acta 1993, 39, 1075–1078. [Google Scholar]
- Sushil, K.J.; Mc Coy, B.; Wise, R. Vitamin E and the hypercoagulability of neonatal blood. Clin. Chim. Acta 1994, 225, 97–103. [Google Scholar]
- Hodges, S.J.; Akesson, K.; Vergnaud, P.; Obrant, K.; Delmas, P.D. Circulating levels of vitamin K1 and K2 decreased in elderly women with hip fracture. J. Bone Miner. Res. 1993, 8, 1241–1245. [Google Scholar] [CrossRef]
- Szulc, P.; Arlot, M.; Chapuy, M.C.; Duboef, F.; Meunier, P.J.; Delmas, P.D. Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly woman. J. Bone Miner. Res. 1994, 9, 1591–1595. [Google Scholar] [CrossRef]
- Friedrich, W. Vitamins; De Gruyter: Berlin, Germany, 1988; p. 1058. [Google Scholar]
- LECO Corporation. FP-528 Protein/Nitrogen Determinator. FP-528 Instruction Manual, Version 1.2.; Leco® Corporation: Benton Harbor, MI, USA, 2001. [Google Scholar]
- Igarashi, Y. An improved procedure for the preliminary fractionation of milk proteins. Int. Dairy J. 1995, 5, 305–310. [Google Scholar] [CrossRef]
- International Dairy Federation Standard. A Milk-Fat Content (Rose Gottlieb). IDF Standard 22B; International Dairy Federation Standard: Brussels, Belgium, 1987. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Park, P.W.; Goins, R.E. In Situ Preparation of Fatty Acid Methyl Esters for Analysis of Fatty Acid Composition in Foods. J. Food Sci. 1994, 59, 1262–1266. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid Fatty Acid Profiles and Substrate. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Perrin, D.R. The calorific value of milk of different species. J. Dairy Res. 1958, 25, 215–220. [Google Scholar] [CrossRef]
- Addinsoft, 2020. XLSTAT Statistical and Data Analysis Solution. NCCS 11 Statistical Software; NCSS, LLC: Kaysville, UT, USA, 2016. [Google Scholar]
- Sherbon, J.W. Physical Properties of Milk. In Fundamentals of Dairy Chemistry, 3rd ed.; Wong, N.P., Jenness, N.P., Keeney, M., Marth, E.H., Eds.; Springer: Boston, MA, USA, 1988; pp. 409–460. [Google Scholar]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rum. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Vaughan, L.A.; Kemberling, S.R. Longitudinal of human in the mineral content. Am. J. Clin. Nutr. 1979, 32, 2301–2306. [Google Scholar] [CrossRef]
- Schryver, H.; Oftedal, O.T.; Williams, J.; Soderholm, L.; Hintz, H. Lactation in the horse: The mineral composition of a mare’s milk. J. Nutr. 1986, 116, 2142–2147. [Google Scholar] [CrossRef]
- Summer, A.; Sabbioni, A.; Formaggioni, P.; Mariani, P. Trend in ash and mineral element content of milk from Haflinger nursing mares throughout six lactation months. Livest. Prod. Sci. 2004, 88, 55–62. [Google Scholar] [CrossRef]
- Auldist, M.J.; Walsh, B.J.; Thomson, N.A. Seasonal and lactational influences on bovine milk composition in New Zealand. J. Dairy Res. 1998, 65, 401–411. [Google Scholar] [CrossRef]
- Shennan, D.B.; Peaker, M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000, 80, 925–951. [Google Scholar] [CrossRef]
- Yadav, M.C.; Singh, V.B. Studies of the calcium and phosphorus contents of buffalo milk. Milchwissenschaft 1970, 25, 529–531. [Google Scholar]
- Peters, J.M.; Maier, R.; Hawthorne, B.E.; Storvick, A.C. Composition and Nutrient Content of Elephant (Elephas maximus) Milk. J. Mammal. 1972, 53, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, Z.; Tsuda, M.; Yamada, A.; Matsumoto, H.; Takai, A.; Takeda, Y.; Takase, M. Elephant’s breast milk contains large amounts of glucosamine. J. Vet. Med. Sci. 2017, 79, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, G.; De Wit, M.; Hugo, A.; Kamara, B.I. Milk composition of three free-ranging African elephant (Loxodonta africana Africana) cows during mid lactation. Comp. Biochem. Physiol. B 2007, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Adkins, Y.; Zicker, S.C.; Lepine, A.; Lönnerdal, B. Changes in nutrient and protein composition of cat milk during lactation. Am. J. Vet. Res. 1997, 58, 370–375. [Google Scholar]
- Park, Y.W. Sow milk. In Handbook of Milk of Non-Bovine Mammals; Park, Y.W., Haenlein, G.F.W., Eds.; Blackwell: Oxford, UK, 2006; pp. 371–382. [Google Scholar]
- Csapó, J.; Martin, T.G.; Csapó-Kiss, Z.S.; Hazas, Z. Protein, Fats, Vitamin and mineral concentrations in porcine colostrums and milk from partition to 60 days. Int. Dairy J. 1996, 6, 881–902. [Google Scholar]
- De Waal, H.O.; Osthoff, G.; Hugo, A.; Myburgh, J.; Botes, P. The composition of African lion (Panthera leo) milk collected a few days postpartum. Mammal. Biol. 2004, 69, 375–383. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; De Wit, M. The composition of cheetah (Acinonyx jubatus) milk. Comp. Biochem. Physiol. B 2006, 145, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Zhang, H.; Zhang, B.; Zhang, L. Mare milk. In Handbook of Milk of Non-Bovine Mammals; Park, Y.W., Haenlein, G.F.W., Eds.; Blackwell: Oxford, UK, 2006; pp. 275–296. [Google Scholar]
- Salimei, E.; Fantuz, F.; Coppola, R.; Chiofalo, B.; Polidori, P.; Varisco, G. Composition and characterization of ass’s milk. Anim. Res. 2004, 53, 67–78. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Milk Lipids. In Dairy Chemistry and Biochemistry, 2nd ed.; Springer: Cork, Ireland, 2015; pp. 69–144. [Google Scholar]
- Skinner, J.D.; Smithers, R.H.N. The Mammals of the Southern African Subregion, 2nd ed.; University of Pretoria: Pretoria, South Africa, 1990; pp. 534–540. [Google Scholar]
- Jensen, R.G.; Ferris, A.M.; Lammi-Keefe, C.J.; Henderson, R.A. Lipids of Bovine and Human Milks: A Comparison. J. Dairy Sci. 1990, 73, 223–240. [Google Scholar] [CrossRef]
- Hall, A.J. Fatty acid composition of rabbit (Oryctolagus cuniculus) milk fat throughout lactation. Int. J. Biochem. 1971, 2, 414–418. [Google Scholar] [CrossRef]
- Klös, H.G.; Jarofke, D.; Langner, H.J.; Siems, H.; Malek, E. The chemical and microbiological compositive of rhinoceros milk [continued]. Reprod. Dom. Anim. 1974, 9, 150–153. [Google Scholar] [CrossRef]
- Demarne, Y.; Lhuillery, C.; Pihet, J.; Martinet, L.; Flanzy, J. Comparative study of triacylglycerol fatty acids in milk fat from two Leporidae species: Rabbit (Oryctolagus cuniculus) and hare (Lepus europaeus). Comp. Biochem. Physiol. B 1978, 61, 223–226. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Lipids and Membranes. In Biochemistry, 4th ed.; Hoboken, N., Ed.; John Wiley & Sons, Inc: New York, NY, USA, 2011; p. 386. [Google Scholar]
- Lauber, E.; Reinhardt, M. Studies on the quality of breast milk during 23 months of lactation in a rural community of the Ivory Coast. Am. J. Clin. Nutr. 1979, 32, 1159–1173. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, D.L.; Mehta, N.R.; Hamosh, P.; Hamosh, M. Comparison of the phospholipid composition of breast milk from mothers of term and preterm infants during lactation. Am. J. Clin. Nutr. 1984, 40, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Gallier, S. Nature’s complex emulsion: The fat globules of milk. Food Hydrocol. 2017, 68, 81–89. [Google Scholar] [CrossRef]
- Mueller, C. Changes in the nutrient composition of milk of black-tailed deer during lactation. J. Mammal. 1977, 58, 421–423. [Google Scholar] [CrossRef] [PubMed]
- White, R.G.; Luick, J.R. Plasticity and constraints in the lactational strategy of reindeer and caribou. Symp. Zool. Soc. Lond. 1984, 51, 215–232. [Google Scholar]
- Gjøstein, H.; Holand, Ø.; Weladji, R.B. Milk production and composition in reindeer (Rangifer tarandus): Effect of lactational stage. Comp. Biochem. Physiol. A 2004, 137, 649–656. [Google Scholar] [CrossRef]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; Kimura, K. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br. J. Nutr. 2003, 89, 61–69. [Google Scholar] [CrossRef]
- Amano, J.; Messer, M.; Kobata, A. Structures of the oligosaccharides isolated from milk of the platypus. Glycoconj. J. 1985, 2, 121–135. [Google Scholar] [CrossRef]
- Green, B.; Merchant, J.C. The composition of marsupial milk. In The Developing Marsupial; Tyndale-Biscoe, C.H., Janssens, P.A., Eds.; Springer: Berlin, Germany, 1988; pp. 41–54. [Google Scholar]
- Kunz, C.; Rudloff, S.; Schad, W.; Braun, D. Lactose-derived oligosaccharides in the milk of elephants: Comparison with human milk. Br. J. Nutr. 1999, 82, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Yamamoto, M.; Nakamura, T.; Arai, I.; Saito, T.; Namiki, M.; Kawahara, K. Chemical characterisation of the oligosaccharides in a sample of milk of a white-nosed coati, Nasua narica (Procyonidae: Carnivora). Comp. Biochem. Physiol. A 1999, 123, 187–193. [Google Scholar] [CrossRef]
- Nakamura, T.; Urashima, T.; Mizukami, T.; Arai, I.; Senshu, T.; Imazu, K.; Wu, K. Composition and oligosaccharides of a milk sample of the giant panda, Ailuropoda melanoleuca. Comp. Biochem. Physiol. B 2003, 135, 439–448. [Google Scholar] [CrossRef]
- Coppa, V.; Pierani, P.; Giorgi, P.L. Changes in Carbohydrate Composition in Human Milk Over 4 Months of Lactation. Pediatrics 1993, 91, 637–641. [Google Scholar]
- Urashima, T.; Kitaoka, M.; Asakuma, S.; Messer, M. Milk Oligosaccharides. In Advanced Dairy Chemistry; McSweeney, P.L.H., Fox, P.F., Eds.; Springer: New York, NY, USA, 2009; pp. 295–349. [Google Scholar]
- Petzinger, C.; Oftedal, O.T.; Jacobsen, K.; Murtough, K.L.; Irlbeck, N.A.; Power, M.L. Proximate composition of milk of the bongo (Tragelaphus eurycerus) in comparison to other African bovids and to hand-rearing formulas. Zoo Biol. 2014, 33, 305–313. [Google Scholar] [CrossRef]
- Simon, K.J. Preliminary studies on composition of milk of Indian elephants. Ind. Vet. J. 1959, 36, 500–503. [Google Scholar]






| Lactation Time (Months) | Vit A (mg/kg) | Vit D3 (µg/kg) | Vit E (mg/kg) | Vit K (µg/kg) |
|---|---|---|---|---|
| 0.03 | 0.1 | ND | 0.36 | ND |
| 0.1 | ND | ND | 0 | ND |
| 0.9 | ND | ND | 0 | ND |
| 1.4 | ND | ND | <0.3 | <1.0 |
| 2.03 | ND | <1.0 | <0.3 | <1.0 |
| 2.8 | ND | ND | 0.33 | ND |
| 3.8 | ND | ND | 0.31 | ND |
| 4.7 | ND | <1.0 | <0.3 | <1.0 |
| 6.03 | <0.1 | ND | 0.3 | <1.0 |
| 7.1 | <0.1 | ND | <0.3 | <1.0 |
| 8.1 | <0.1 | ND | <0.3 | <1.0 |
| 9.3 | <0.1 | ND | <0.3 | <1.0 |
| Time of Lactation (Months) | Shorty 12.3 | Shorty 13.0 | Shorty Average (n = 16) | Mussina Average (n = 4) | Shan Average (n = 2) | |
|---|---|---|---|---|---|---|
| Fatty Acid Composition (%): | ||||||
| Common Name: | Abbreviation: | |||||
| Butyric | C4:0 | 0.0 | 0.0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| Caproic | C6:0 | 0.0 | 0.0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| Caprylic | C8:0 | 13.3 | 0.0 | 1.96 ± 4.49 | 0.00 ± 3.93 | 0.00 |
| Capric | C10:0 | 54.7 | 6.9 | 20.17 ± 14.55 | 6.30 ± 13.61 | 6.34 |
| Hendecanoic | C11:0 | 1.0 | 4.5 | 1.05 ± 1.13 | 1.56 ± 1.00 | 1.43 |
| Lauric | C12:0 | 7.4 | 1.5 | 7.68 ± 4.60 | 1.93 ± 4.55 | 5.36 |
| Tridecanoic | C13:0 | 0.0 | 0.0 | 0.03 ± 0.07 | 0.00 ± 0.06 | 0.00 |
| Myristic | C14:0 | 0.5 | 1.2 | 1.85 ± 0.98 | 1.24 ± 0.88 | 2.85 |
| Myristoleic | C14:1c9 | 0.0 | 0.0 | 0.04 ± 0.08 | 0.51 ± 0.24 | 0.08 |
| Pentadecylic | C15:0 | 0.1 | 1.7 | 0.24 ± 0.42 | 0.00 ± 0.37 | 0.16 |
| Pentadecenoic | C15:1c10 | 0.0 | 0.0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| Palmitic | C16:0 | 3.9 | 20.3 | 15.91 ± 6.74 | 21.46 ± 6.56 | 23.42 |
| Palmitoleic | C16:1c9 | 0.3 | 1.8 | 0.81 ± 0.70 | 0.90 ± 0.68 | 1.24 |
| Margaric | C17:0 | 0.3 | 0.5 | 0.55 ± 0.35 | 0.50 ± 0.31 | 0.63 |
| Heptadecenoic | C17:1c10 | 0.4 | 0.1 | 0.41 ± 0.36 | 0.98 ± 0.42 | 0.24 |
| Stearic acid | C18:0 | 3.6 | 10.9 | 13.13 ± 7.20 | 18.64 ± 7.00 | 12.74 |
| Elaidic | C18:1t9 | 0.1 | 0.5 | 0.18 ± 0.24 | 0.13 ± 0.22 | 0.08 |
| Oleic | C18:1c9 | 11.1 | 30.0 | 24.12 ± 6.73 | 30.50 ± 7.99 | 30.12 |
| Vaccenic | C18:1c7 | 0.1 | 2.7 | 0.80 ± 0.91 | 1.19 ± 0.91 | 1.06 |
| Linolelaidic | C18:2t9,12 (n−6) | 0.0 | 0.6 | 0.29 ± 0.41 | 1.30 ± 0.63 | 0.00 |
| Linoleic | C18:2c9,12 (n−6) | 1.0 | 9.0 | 2.89 ± 2.19 | 3.19 ± 2.69 | 6.37 |
| Conjugated linoleic acid (CLA) | C18:2c9t11 (n−6) (CLA) | 0.0 | 0.0 | 0.03 ± 0.05 | 0.14 ± 0.07 | 0.00 |
| Conjugated linoleic acid (CLA) | C18:2t10,c12(n−6)(CLA) | 0.0 | 0.3 | 0.04 ± 0.08 | 0.91 ± 0.62 | 0.02 |
| γ-Linolenic | C18:3c6,9,12 (n−3) | 0.0 | 0.4 | 0.18 ± 0.32 | 1.35 ± 0.63 | 0.32 |
| α-Linolenic | C18:3c9,12,15 (n−3) | 0.8 | 0.1 | 0.73 ± 0.58 | 0.76 ± 0.54 | 0.69 |
| Nonadecanoic | C19:0 | 0.0 | 0.0 | 0.50 ± 0.89 | 0.17 ± 0.78 | 1.71 |
| Arachidic | C20:0 | 0.1 | 0.0 | 0.26 ± 0.17 | 0.19 ± 0.91 | 0.19 |
| Eicosenoic | C20:1c11 | 0.0 | 0.2 | 0.23 ± 0.35 | 0.00 ± 0.31 | 0.06 |
| Eicosadienoic | C20:2c11,14 (n−6) | 0.0 | 0.4 | 1.03 ± 3.20 | 0.50 ± 2.76 | 0.47 |
| Eicosatrienoic | C20:3c8,11,14 (n−6) | 0.1 | 0.2 | 0.30 ± 0.25 | 0.70 ± 0.41 | 0.03 |
| Eicosatrienoic | C20:3c11,14,17 (n−3) | 0.1 | 0.0 | 0.05 ± 0.05 | 0.31 ± 0.19 | 0.09 |
| Arachidonic | C20:4c5,8,11,14 (n−6) | 0.3 | 2.8 | 0.45 ± 0.64 | 0.48 ± 0.57 | 1.84 |
| Eicosapentaenoic | C20:5c5,8,11,14,17 (n−3) | 0.0 | 0.6 | 0.15 ± 0.20 | 0.09 ± 0.17 | 0.03 |
| Heneicosanoic | C21:0 | 0.0 | 0.1 | 0.04 ± 0.04 | 0.32 ± 0.20 | 0.00 |
| Behenic | C22:0 | 0.0 | 0.1 | 0.14 ± 0.24 | 0.16 ± 0.22 | 0.15 |
| Erucic | C22:1c13 | 0.1 | 0.1 | 0.43 ± 0.81 | 0.38 ± 0.70 | 0.27 |
| Docosadienoic | C22:2c13,16 (n−6) | 0.0 | 0.1 | 0.06 ± 0.09 | 0.20 ± 0.16 | 0.01 |
| Docosapentaenoic | C22:5c7,10,13,16,19 (n−3) | 0.01 | 0.92 | 0.33 ± 0.35 | 1.39 ± 0.64 | 0.41 |
| Docosahexanoic | C22:6c4,7,10,13,16,19 (n−3) | 0.0 | 0.0 | 0.05 ± 0.07 | 0.05 ± 0.06 | 0.06 |
| Tricosanoic | C23:0 | 0.5 | 1.4 | 1.30 ± 1.80 | 0.77 ± 1.61 | 0.42 |
| Lignoceric | C24:0 | 0.0 | 0.0 | 0.07 ± 0.08 | 0.22 ± 0.09 | 0.09 |
| Nervonic | C24:1c15 | 0.1 | 0.2 | 0.44 ±1.05 | 0.58 ± 0.91 | 1.05 |
| Fatty Acid Ratios: | ||||||
| SFA (%) | 85.4 | 49.0 | 65.97 ± 9.10 | 53.45 ± 15.14 | 55.47 | |
| MUFA (%) | 12.3 | 35.7 | 27.45 ± 7.56 | 35.18 ± 8.86 | 34.20 | |
| PUFA (%) | 2.3 | 15.3 | 6.58 ± 3.85 | 11.37 ± 3.93 | 10.33 | |
| n−6 (%) | 1.4 | 14.2 | 5.36 ± 4.05 | 8.67 ± 3.84 | 9.08 | |
| n−3 (%) | 0.92 | 1.63 | 1.30 ± 0.72 | 2.60 ± 0.97 | 1.28 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobeni, S.; Osthoff, G.; Madende, M.; Hugo, A.; Marabini, L. The Dynamic Changes of African Elephant Milk Composition over Lactation. Animals 2020, 10, 948. https://doi.org/10.3390/ani10060948
Kobeni S, Osthoff G, Madende M, Hugo A, Marabini L. The Dynamic Changes of African Elephant Milk Composition over Lactation. Animals. 2020; 10(6):948. https://doi.org/10.3390/ani10060948
Chicago/Turabian StyleKobeni, Sibusiso, Gernot Osthoff, Moses Madende, Arnold Hugo, and Lisa Marabini. 2020. "The Dynamic Changes of African Elephant Milk Composition over Lactation" Animals 10, no. 6: 948. https://doi.org/10.3390/ani10060948
APA StyleKobeni, S., Osthoff, G., Madende, M., Hugo, A., & Marabini, L. (2020). The Dynamic Changes of African Elephant Milk Composition over Lactation. Animals, 10(6), 948. https://doi.org/10.3390/ani10060948






