1. Introduction
The
Lidia cattle constitutes the most numerous autochthonous bovine breed of Spain, with 199,662 heads distributed among 917 farms, and second, in censuses, after the holstein-friesian breed [
1]. The current economic and social transcendence of this livestock production is corroborated by the large number of bullfighting shows, both in our country, 19,219 shows in 2018 [
2], and in the South of France, Portugal and in many regions of Latin America (Ecuador, Mexico, Colombia, Peru and Venezuela).
The origin of this breed goes back to the Middle Ages. Feudal lords raised these animals for hunting and war training purposes. The first reported evidence of artificial selection for
Lidia bulls point to the fifteenth and sixteenth centuries. The developing farms in different areas of the Iberian Peninsula were clearly orientated to their use in taurine celebrations [
3].
The modern Lidia breed can be considered the result of continuous selective pressure for behavioral phenotypes. Since the beginning of the 18th century, farmers used behavioral tests to choose certain ethological characteristics. Desired behaviors included prolonged durations of contest-behaviors.
These sequences of behaviors included charging against objects, people or animals, considering the morphology of the animal as secondary. Breeders deemed these patterns of behavior “
bravura”, indicating bravery because instead of fleeing, the animal displays strength and fierceness while facing danger. Therefore, from the zootechnical point of view, it is a unique breed in the world that has a valuable morphology and genetic resource, the latter, widely studied [
4,
5,
6,
7]. However, the desired behaviors also make the modern
Lidia animals challenging to study. Handling and restraining these cattle are very dangerous due to their surly disposition, which may have prevented the realization of traditional manual morphological studies. Nonetheless, morphological characterization is important for the conservation and biodiversity of cattle breeds [
8]. By studying the phenotypes within a breed, it is possible to improve diversity and selection for adaptation to the environment and functionality [
9,
10].
Visual methods provide a non-invasive mechanism to evaluate cattle but are currently limited because the work requires skilled observers, is very laborious, and precision requires a high sampling rate [
11,
12,
13,
14,
15,
16]. Researchers previously described morphology and phaneroptics of this breed; however, few studies focused on zoometrical measures in
Lidia cattle [
17,
18,
19]. In addition, previous research was focused on the biometry of the horns rather than all of the features [
20,
21,
22].
Previous zoometric-tool sets included standard measuring sticks, non-elastic measuring tape, a compass, goniometers, and calipers, which [
23] required handling and restraint. In addition, handling and restraint may cause distress. Very temperamental animals may need to be immobilized using cattle crushes or containment boxes and possibly tranquilized. These procedures increase the risk of injury for both the animal and humans involved. Over the last two decades, technology advanced to improve various image-analysis techniques. One of them, known as near object photogrammetry, allows for morphological measurements on dangerous and elusive animals, such as the
Lidia cattle, without risk to the operator or stress to the animal [
24,
25]. The technique was previously used to study wild animals such as elephants [
26], orcas [
27], and Wedded seals [
28] and was validated for pigs, horses, and dairy cows [
29,
30,
31]. The objective of this work was to adapt the photogrammetric technology to the
Lidia breed and carry out the first zoometric and morphological characterization of the breed.
2. Materials and Methods
The morphometry and phaneroptics data of 264 adult individuals (4–6 years) of
Lidia cattle (184 males and 80 females), belonging to 21 herds, selected as representative herds in the purity of the 15 genetic lines (called “
encastes”) that are currently preserved of the breed:
Miura, Pablo Romero, Veragua, Murube, Santa Coloma-Buendía, Santa Coloma -Graciliano, Gamero Cívico, Conde de la Corte, Atanasio-Lisardo, Domecq, Torrestrella, Núñez, Albaserrada, Vega-Villar and Navarra [
6] were enrolled. Birth records provided by the farmer were used to determine the age of the animals, which correlated with their ear tag.
All farms were located throughout the entire national territory of Spain (from Navarra in the north to Andalusia in the south), all of them in a common ecosystem called “Dehesa” or Mediterranean forest, characterized by a wooded grassland (30–100 trees per ha), mainly dedicated to extensive livestock with a Mediterranean climate with significant summer drought [
1,
11].
The equipment used (
Figure 1) is an adaptation of the one described by Gaudioso et al. [
32] that consists of a rigid and tubular structure supported in its central part by a vertical peg (
Figure 1). Three cameras were attached to the structure using articulated supports. These supports allowed for adjusting the orientation of the cameras according to the size of the object to be measured and the average distance to which it is located. The lateral cameras were synchronized and remotely controlled by cable.
To minimize the risks for operator and disturbance of the animals, the photographs were taken from different angles and perspectives in the animal’s own habitat, from a distance of 10 to 15 m, without altering the normal behavior or posture. For the subsequent morphometric analysis, photographs were transformed into three dimension files using software (PhotoModeller Scanner) that allows for the possibility of performing 20 body measurements as can be seen in
Figure 2, following reviewed literature standards [
33,
34,
35].
In addition, complementary phaneroptic variables were recorded. The profile and horns conformation, layer, pigmentation of mucous membranes and hooves were measured. Also, the development of the dewlap (very developed when the dewlap reaches the carpal joint and little developed when it ends in the sternum) and tail (very developed when the tail reaches the ground, little development when it reaches the hock) were recorded.
In addition, the following zoometric indexes have been calculated [
34,
36,
37]:
- -
Cephalic index = (head width/head length) × 100.
- -
Proportionality index = (height at the withers/body length) × 100.
- -
Relative depth of thorax index = (back-sternal diameter/height at withers) × 100.
- -
Posterior foot index = (height at hock/height at tail) × 100.
- -
Relative thickness of cannon bone index = (perimeter of the cannon/height at withers) × 100.
- -
Saddling index = (height at withers + height at rump/2) − height at loins.
A descriptive statistical analysis of the variables was carried out, considering gender, for all the animals. Normality was verified using the Kolmogorov–Smirnov test. In turn, the data were subjected to a Student t-test for an independent samples test. Pearson correlation coefficients were estimated to analyze the relationships between the studied parameters. Chi-square tests (Χ
2) were used for the phaneroptic measurements determined. These statistical analyses were done with the SPSS
® version 19.0 package for Windows. Finally, a principal component analysis (PCA) was performed on the morphometrical data of the males selected by the breeders as representatives of the morphology of their genetic line (
encaste). PCA was carried out using The Unscrambler (Camo Analytics AS. Oslo, Norway) version 11.0 software under the UEX Department of Animal Production and Food Science License. This software of chemometric origin in addition to develop models validate how the model will behave in the future in the face of new cases. The following variables were used in the PCA: exterior length of the horn, head length, head width, height at withers, height at loins, height at rump, height at tail, height at shoulder, hock height, back-sternal diameter, back length, trunk length, rump length and body length. The explained variance for principal components (PC) is expressed in % and is calculated from the residual variance as:
where
VRes is any of the residual variance matrices listed in individual residual variance calculations and
VExp is the corresponding explained variance matrix.
Accumulative explained variances for principal components (PC), also expressed as a percentage, are computed according to the following equation where VExp,cum is accumulative explained variances and VRes is any of the residual variance matrices listed in Individual Residual Variance Calculations:
3. Results
The twenty morphological parameters were first evaluated (
Table 1), which included means, standard deviations, distributions, and Sexual dimorphism quotient SD (male/female). The exterior length of the horn was less variable in males than females (
Table 1). In addition, compared to females, males had greater head width and length, horizontal and vertical diameter of the horn, back-sternal diameter, trunk, back, rump and body length, height at withers, loins, rump and height at tail. The dimorphism differences were corroborated numerically (
Table 1); male body measurement/female body measurement (m/f) presented a global mean value of 1.1, indicating bulls’ morphology predominance over
Lidia cows.
Table 2 showed the mean, standard deviation, and maximum/minimum values for the six morphometric indexes. Significant sexual dimorphism was observed in all the indexes.
Lidia males were mesocephalic (50.6), while females were slightly dolicocephalic (44.6). The relative depth of the thorax index was significantly higher in the males as well as the relative thickness of cannon bone index. Posterior foot index and proportionality index were higher in the females. Finally, the males studied presented a marked degree of deviation of the thoracolumbar line compared with the
Lidia cows, registering a saddling index of 4.8 cm.
Table 3 shows the linear correlations between the 20 variables analyzed in the males and females sampled. Horn morphometric variables were significant and positively correlated both in males and in females with higher values in the bulls. All the height variables were correlated, and the highest correlation value was obtained between height at rump and height at tail, 0.96 in males and 0.82 in females. Both genders presented a negative correlation between sternum height and back-sternal diameter (females −0.77 and males −0.73). Males had 59% of their parameters positively correlate, showing a harmonic morphostructural model. Females had slightly fewer harmonic features (40% parameters correlated).
Table 4 shows the percentage of different categories of morphological and phaneroptic variables studied and the results of the chi-square test depending on the gender. Studied males presented significantly less straight and more saddleback thoracolumbar lines than
Lidia cows. Dewlap was little and developed in females and developed or very developed in males. Development of the tail was more evident in the males than in the females. No differences were observed between genders in coat and mucous membranes coloration (predominantly black), profile (mainly subconcave), and type of horns that were procerus (e.g., growth above the nape forward and curved in its medial and distal part, giving rise to crown forms and hook).
Finally, a principal component analysis has been made on 15 morphometric variables analyzed in males classified according to their genetic line. Variables that have no influence or weight were not included in the model. Males (
n = 184) are represented spatially in
Figure 3. Combining information presented in
Table 5 and
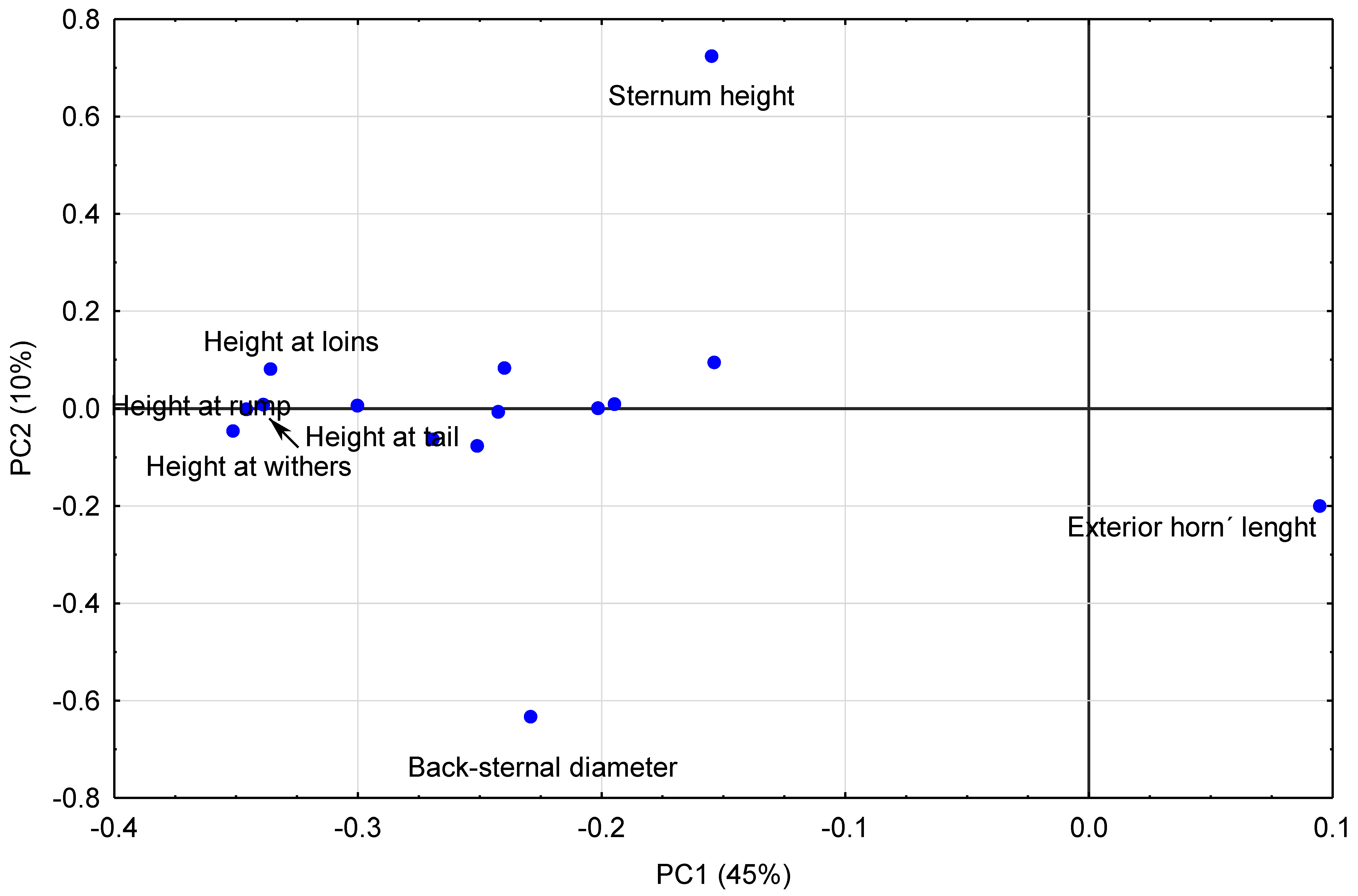
Figure 4, it could be observed that the PC1 (45% of explained variance,
Table 6) is determined mainly by the measurements of heights in the negative quadrant and the exterior length of the horn in the positive but with less influence. Two variables clearly conditioned PC2 (10% of explained variance,
Table 6)): sternum height and back-sternal diameter (
Table 5 and
Figure 4).
4. Discussion
In general, the results obtained from body dimensions place the
Lidia breed as mid-sized compared across the bovine species [
34]. For the present study, significant parameter differences (
Table 1 and
Table 2) corroborates the marked sexual dimorphism that exists in this breed [
13]. There was clear evidence in the dimensions of the heights and body length, which indicate that the male is significantly larger than the female. In turn, significant differences among genders were observed in all indexes: cephalic, relative depth of the thorax and saddle higher in males and posterior foot and proportionality index higher in females, indicated different body proportions. The numerical estimation of dimorphism with an SD global mean value of 1.1 (
Table 1) documented that the genetic selection pressure has been directed to the male morphology [
38,
39].
The mean height at withers observed in this study (
Table 1) is less than previous means reported for
Lidia bulls (136–143 cm) [
17]. Compared with Spanish autochthonous breeds, the
Lidia is framed in the group of mature, small-sized individuals, which includes the
cachena (122 cm male and 117 cm female) and the
albera (126 cm male and 121 cm female) [
1,
40]. Mean
Lidia height at withers values was smaller than the rest of Spanish autochthonous breeds [
1,
34]. Individuals from the
Miura line presented the highest values and the ones from
Vega-Villar and
Navarra lines the lowest results (
Figure 3) in accordance with reviewed literature [
13,
41]. These results may reflect the influence of farmers´ preferences and the differences in the origin of the lines [
5,
6]. The sexual dimorphism of height-measurements was similar to that reported in other European cattle´s breeds, which is approximately 10 cm differences [
33].
The heights of the withers, loins, rump and tail were correlated within both males and females (
Table 3). This work indicates that the
Lidia bull’s rump is slightly less than its withers (3 cm difference) giving a slightly “downhill” figure. On the contrary, female withers and rumps were level (0.8 cm difference). Males also had a marked saddling back line, registering an index of 4.8 cm. The saddling reported in the current study is similar to the previously published descriptions of this breed [
13,
37] and to the morphological prototype described for the rustic European bovine breeds [
34]. The observed thoracolumbar line tended to be more saddled in the male (83.9%) than in the female (48.6%;
Table 4).
In addition, a thorax significantly more developed was recorded in the male, endorsed in the measures of the back-sternal diameter and the length of the back, which generate significant differences between genders, (74.9 cm in the male and 64.5 cm in the female). These results state that males were selected for heavy-muscled shoulders to have a more athletic, masculine appearance than females in accordance with some authors [
41]. The idea that thoracic muscle and bone development is a selected character in the male was corroborated by the index of relative thorax depth (59.5 ± 8.1 cm). All this, the results of genetic improvement, favors a great thoracic capacity that allows for greater oxygenation, leading to better performance of the bull during the taurine celebrations [
5,
13]. However, farmers selected males for variables of anterior third because the “
badanudo” (developed dewlap) was a desired characteristic because it contributes to increase the visual predominance of this third. For the current study, a developed dewlap was presented in 56.2% of males, whereas in the females, 48.1% of the cases had less developed dewlaps (
Table 4).
For the present study, the body length was also shorter than that reported for the main mature Spanish autochthonous breeds [
1,
42] but similar to that reported for the
avileña, also Spanish native beef cattle breed [
43]. Based on the variable trunk length, the
Lidia breed could be framed in the group of medium cattle, although presenting great variations depending on the
encastes (results of principal component analysis are shown in
Figure 3) according to literature reviewed [
12,
13,
14,
15,
16].
On the other hand, the index of proportionality gives us a value lower than 100 for males (85.4) and females (90.6), proving that females presented a poor beef conformation improved in the bulls specially in some genetic lines such as
Pablo Romero that has been described closer to meat production prototype breeds, with a rectangular trunk [
14]. Similar values have been reported for Uruguay breeds (88.2) and
pirenaica breed (86.3) [
44,
45]. Moreover, the relative depth of the thorax index provided an idea of the sarcopoietic aptitude of each breed, considering a better beef conformation for a breed when it exceeds the more from 50 points. Average values of this index in
Lidia individuals are close to 50 (
Table 2) because farmers selected muscular development in this breed focusing on the athletic appearance and performance of charging behavior during the fight [
46]. These characteristics are not desired among cattle raised solely for beef production.
The measures of height at shoulder and sternum provided information about the length of the animals’ extremities, which, in this breed, are of a medium-low size in comparison with the rest of the bovine breeds, as a selection result because breeders have been selecting bulls with less height during the last century [
47,
48].
The perimeter of the carpal region (31.4 cm in males) was in accordance with data published by other authors in this breed (31.2 cm) [
19] but smaller than that described for other cattle breeds (33 cm for the
retinta and 34 cm for the
berrenda) [
49]. The males’ cannon perimeter (19.7 cm), were slightly higher to values published for this breed [
19,
49] (18.4 cm) and considerably lower than values pointed out by other authors [
17] (21–24 cm). On the other hand, this perimeter is lower than the reported for the
pallaresa [
50]; however, it is similar to that published for the
cachena [
40], both Spanish breeds. This indicates that the
Lidia breed has a greater fineness compared to these Spanish native cattle.
The head conformation is often influenced by the origin area and environment and generally provides important characteristics of each breed [
37,
51]. The males in the current experiment had wider heads than the females, which generated a significantly higher cephalic index (
Table 2). Head length was more variable in the males in the current study (43.3–59.2 cm in the male) than the same breed examined by Fuentes et al. [
19] (44.5–52 cm) and by Barga-Besusán [
17] (45–53 cm). Our results may have differed from the previous reports because we included a larger number of farms and genetic lines, which indicates that this breed may have greater morphological diversity than previously reported. Nevertheless, the means of head length obtained were lower than those described for some Spanish breeds [
50,
52]. The “shortened head” characteristic is often used when several authors described the
Lidia breed [
17,
53]. With regard to males’ width of the head (20.3–31 cm), values presented in this study exceed the range of measurement published (21.5–28 cm) [
19] and were lower than others (26–32 cm) presented in literature reviewed for this breed [
17] and native Spanish breeds [
50,
53]. The average cephalic index in
Lidia bulls is 50.6 and 44.6 in cows, within values considered mesocephalic and slightly dolicocephalic, respectively [
12,
13].
All the cattle enrolled in this study presented proceros-type horns, in the form of a hook and with different proportions and directions in their trajectory [
54,
55]. The results on the variety of horn pigmentation, related to the coat, with the females being significantly thin and longer (
Table 1), are in accordance with the literature consulted [
54,
55]. The mean horizontal diameter (7.8 cm) observed was slightly larger than the vertical one (7.5 cm) describing an elliptical section of the horn as pointed out by Aparicio-Sánchez [
49], which suggests a horizontal flattening of the horn. Fuentes et al. [
19] reported a slightly thicker horn (8 cm) that was practically circular. The horns’ external length mean (58.5 cm) in the current project is considerably higher than that obtained by other authors thirty years ago [
17], which was 46.7 cm. There was likely increased selection for bulls with larger horns because the current results are in accordance with more recent published data [
21,
22,
56].
Considering the phaneroptic variables studied (
Table 4), both genders have similar characteristics, with the black coat being predominant in the breed, not so closely followed by the brown and gray, although the female tends to a greater variability of coatings and mucous membranes and hooves’ pigmentation, as indicated by the bibliographic references consulted [
13,
41,
54,
57,
58,
59,
60,
61].
Generalized positive correlation between most of the parameters studied (
Table 3), previously mentioned in results, supported that males of the fighting breed can be considered as representatives of a highly harmonic morphostructural model and the females of medium harmony according to consulted literature [
62]. The differences between genders were probably due to increased selection pressure in the male as a result of the influence of the “
trapio”, that could be defined as a combination of physical qualities and presence necessary for the taurine celebrations, on the males´ economic value [
55,
57].
Finally, the “
encastes”
Núñez, Domecq, Gamero Cívico, Albaserrada and
Santa Coloma (
Graciliano and
Buendía lines) with a common phylogenetic provenance (called “
Vistahermosa”) [
63] were grouped in the central right part of
Figure 3 in accordance with their similar morphological and genetic characteristics reported in literature reviewed [
6,
13] because the bulls of these
encastes presented mean values of heights and greater exterior length of the horns.
The similarities between
Conde de la Corte and
Atanasio-Lisardo that come from the same livestock line, called “
Tamarón” [
63] and historically sharing their breeding area (Salamanca, Spain [
52]), placed them in the left quadrants of
Figure 3, spread vertically by the greater influence of the sternum height on the
Atanasio line individuals and back-sternal diameter on the
Conde de la Corte ones. On the other hand, differences in the morphology of animals that come from genetically isolated herds [
5,
6] such as the
Miura and
Pablo Romero lines (left above), with larger dimensions and individuals from
Vega-villar (right below) and
Navarra (right above) lines, could be clearly observed with a smaller size in
Figure 3. The males of the “
Veragua” line were dispersed in
Figure 3 due to their lack of morphometric uniformity [
13].