Acute Heat Stress Induces the Differential Expression of Heat Shock Proteins in Different Sections of the Small Intestine of Chickens Based on Exposure Duration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Experimental Design
2.2. Sample Collection
2.3. Serum Analyses
2.4. Western Blotting
2.5. RNA Extraction and qRT-PCR
2.6. Statistical Analyses
3. Results
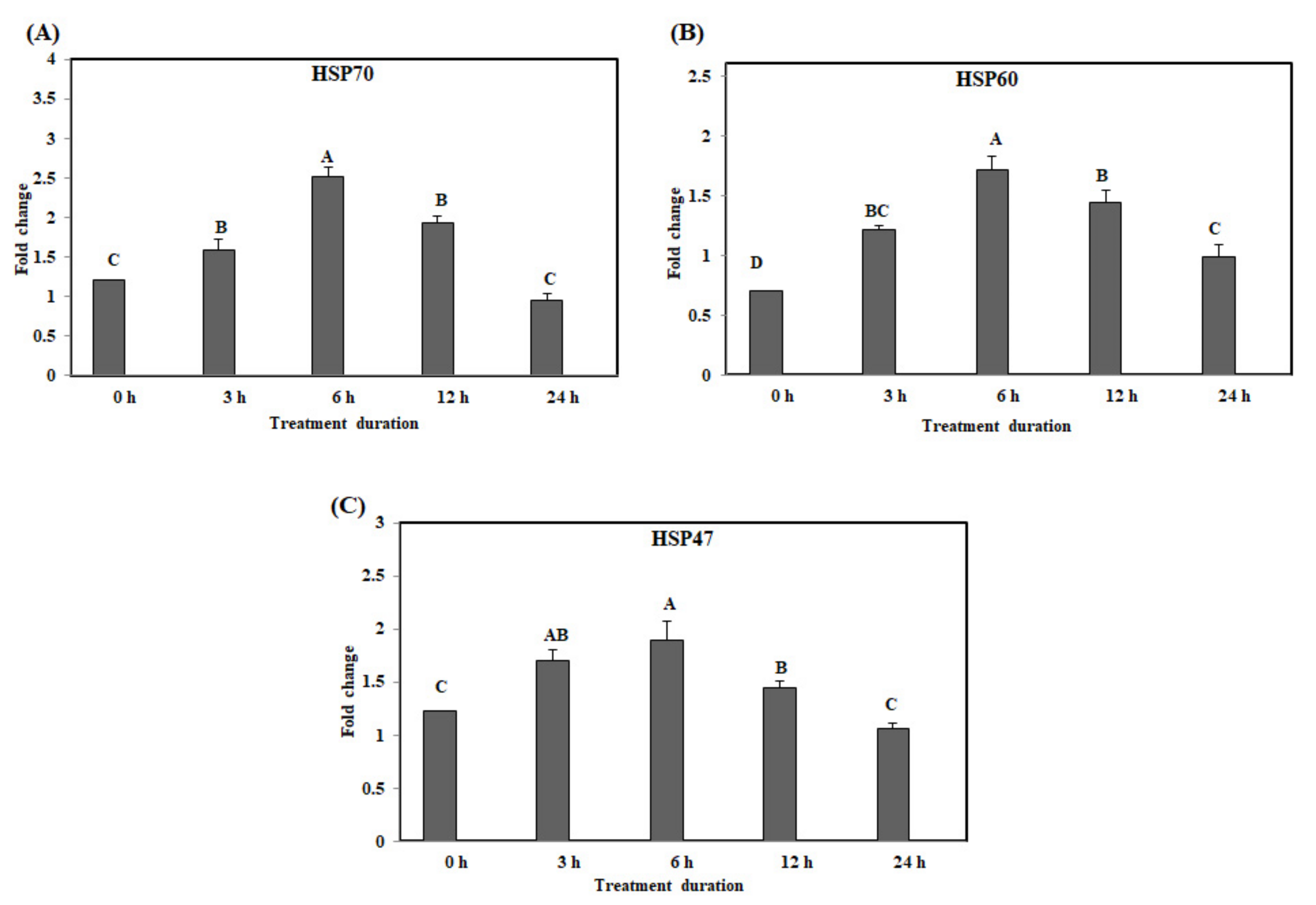
3.1. Effect of Acute Heat Stress on the mRNA Expression Levels of the HSPs
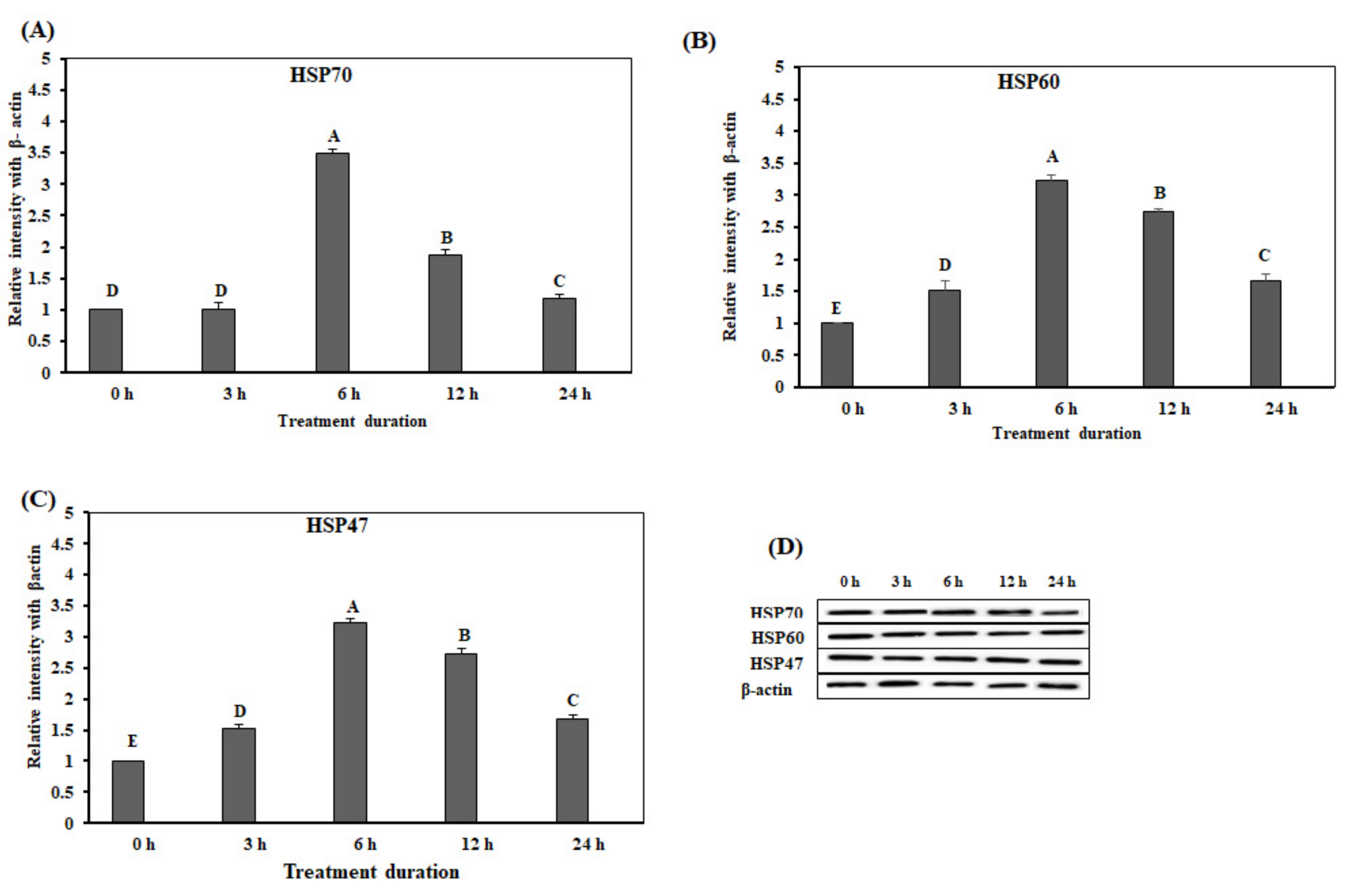
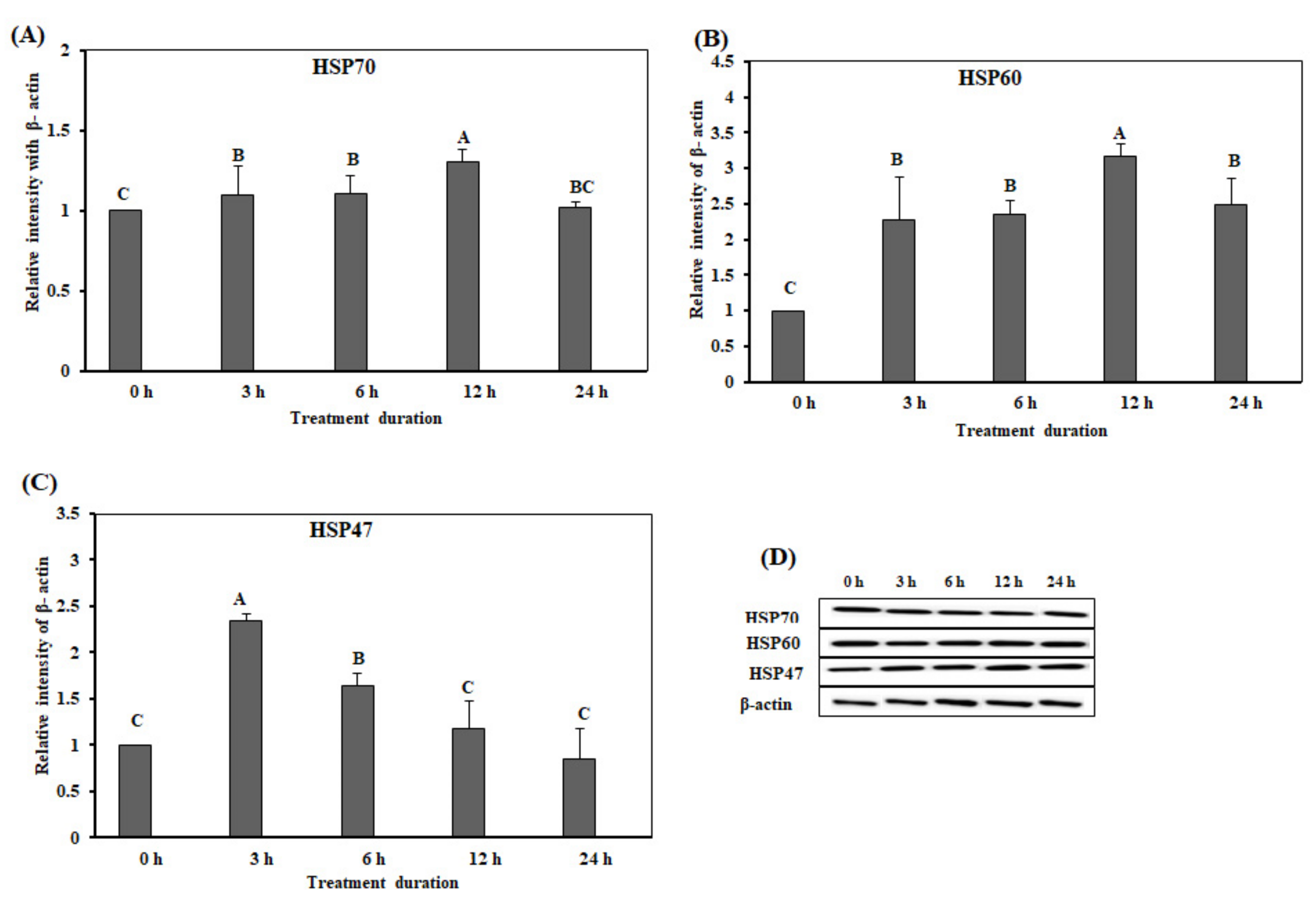
3.2. Effect of Acute Heat Stress on the Protein Expression Levels of the HSPs
3.3. Effect of Acute Heat Stress on the Levels of SGPT and SGOT
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Mignon-Grasteau, S.; Moreri, U.; Narcy, A.; Rousseau, X.; Rodenburg, T.B.; Tixier-Boichard, M.; Zerjal, T. Robustness to chronic heat stress in laying hens: A meta-analysis. Poult. Sci. 2015, 94, 586–600. [Google Scholar] [CrossRef]
- Deyhim, F.; Teeter, R.G. Research note: Sodium and potassium chloride drinking water supplementation effects on acid-base balance and plasma corticosterone in broilers reared in thermoneutral and heat-distressed environments. Poult. Sci. 1991, 70, 2551–2553. [Google Scholar] [CrossRef] [PubMed]
- Amrutkar, S.A.; Saxena, V.K.; Tomar, S. Influence of different tropical stress conditions on biochemical parameters in various broiler strains. Indian J. Anim. Res. 2016, 50, 945–955. [Google Scholar] [CrossRef][Green Version]
- Hosseinivashan, S.J.; Golian, A.; Yaghobfar, A. Growth immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int. J. Biometeorol. 2016, 60, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Jiang, L.; Zhong, H.; Lu, Y.; Zhang, L.; Wang, T. Effects of enzymatically treated Artemisia annua L. on growth performance and some blood parameters of broilers exposed to heat stress. Anim. Sci. J. 2017, 88, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Ferket, P.R.; Gernat, A.G. Factors that affect feed intake of meat birds: A review. Int. J. Poult. Sci. 2006, 5, 905–911. [Google Scholar]
- Howlider, M.A.R.; Rose, S.P. Temperature and growth of broilers. World’s Poult. Sci. J. 1987, 43, 228–237. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Reed, J.C.; Homma, S. Heat-shock proteins as regulators of apoptosis. Oncogene 2003, 22, 9041–9047. [Google Scholar] [CrossRef]
- Al-Aqil, A.; Zulkifli, I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult. Sci. 2009, 88, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Gabai, V.L.; Meriin, A.B.; Mosser, D.D.; Caron, A.W.; Rits, S.; Shifrin, V.I.; Sherman, M.Y. HSP70 prevents activation of stress kinase—A novel pathway of cellular thermotolerance. J. Biol. Chem. 1997, 272, 18033–18037. [Google Scholar] [CrossRef] [PubMed]
- Pirkkala, L.; Nykanen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef]
- Jonsson, H.; Schiedek, D.; Goksøyr, A.; Grøsvik, B.E. Expression of cytoskeletal proteins, cross-reacting with antiCYP1A, in Mytilus sp. exposed to organic contaminants. Aquat. Toxicol. 2006, 78, S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Burkart, V.; Flohè, S.; Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef]
- Taguchi, T.; Nazneen, A.; Al-Shihri, A.A.; Turkistani, K.A.; Razzaque, M.S. Heat shock protein 47: A novel biomarker of phenotypically altered collagen-producing cells. Acta Histochem. Cytochem. 2011, 44, 35–41. [Google Scholar] [CrossRef]
- Miyaishi, O.; Ito, Y.; Kozaki, K.; Sato, T.; Takechi, H.; Nagata, K.; Saga, S. Agerelated attenuation of HSP47 heat response in fibroblasts. Mech. Ageing Dev. 1995, 77, 213–226. [Google Scholar] [CrossRef]
- Mott, C.R.; Siegel, P.B.; Webb, K.E., Jr.; Wong, E.A. Gene expression of nutrient transporters in the small intestine of chickens from lines divergently selected for high or low juvenile body weight. Poult. Sci. 2008, 87, 2215–2224. [Google Scholar] [CrossRef]
- Burkholder, K.M.; Thompson, K.L.; Einstein, M.E.; Applegate, T.J.; Patterson, J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poult. Sci. 2008, 87, 1734–1741. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Musch, M.W.; Kojima, K.; Boone, D.; Ma, A.; Chang, E.B. Short fatty acids induce intestinal epithelial heat shock protein 25 and IEC18 cells. Gastroenterology 2001, 121, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, T.; Nishihira, J.; Takeda, H.; Katsurada, T.; Kato, K.; Yoshiki, T.; Sugiyama, T.; Asaka, M. Protective effect of geranylgeranylacetone on trinitrobenzene sulfonic acid-induced colitis in mice. Int. J. Mol. Med. 2006, 17, 229–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tchernitchko, D.; Bourgeois, M.; Martin, M.E.; Beaumont, C. Expression of the two mRNA isoforms of the iron transporter Nramp2/DMT1 in mice and function of the iron responsive element. Biochem. J. 2002, 363, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gonzalez-Esquerra, R.; Leeson, S. Physiological and metabolic responses of broilers to heat stress: Implications for protein and acid nutrition. World’s Poult. Sci. J. 2006, 62, 282–295. [Google Scholar] [CrossRef]
- Bogin, E.; Avidar, Y.; Pech-Waffenschmidt, V.; Doron, Y.; Israeli, E.; Kevkhayev, E. The relationship between heat stress, survivability and blood composition of the domestic chicken. Eur. J. Clin. Chem. Clin. Biochem. 1996, 34, 463–469. [Google Scholar] [CrossRef][Green Version]
- Bynum, G.D.; Pandolf, K.B.; Schuette, W.H.; Goldman, R.F.; Lees, D.E.; Whang-Peng, J.; Atkinson, E.R.; Bull, J.M. Induced hyperthermia in sedated humans and the concept of critical thermal maximum. Am. J. Physiol. 1978, 235, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Spurr, G.B. Serum enzymes following repetitive hyperthermia. Proc. Soc. Exp. Biol. Med. 1972, 139, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Das, A. Heat stress-induced hepatotoxicity and its prevention by resveratrol in rats. Toxicol. Mech. Methods 2011, 21, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, H.K.; Kim, S.Y.; Cho, J.H. Analysis of the relationship between liver regeneration rate and blood levels. Pak. J. Med. Sci. 2015, 31, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B. Linking intestinal homeostasis and liver disease. Curr. Opin. Gastroenterol. 2013, 29, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Otaka, M.; Mikami, K.; Yoneyama, K.; Goto, T.; Miura, K.; Ohshima, S.; Lin, J.G.; Shibuya, T.; Segawa, D. Expression of a 72-kDa heat shock protein, and its cytoprotective function, in gastric mucosa in cirrhotic rats. J. Gastroenterol. 2004, 39, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Oyake, J.; Otaka, M.; Matsuhashi, T.; Jin, M.; Odashima, M.; Komatsu, K.; Wada, I.; Horikawa, Y.; Ohba, R.; Hatakeyama, N.; et al. Over-expression of 70-kDa heat shock protein confers protection against monochloramine-induced gastric mucosal cell injury. Life Sci. 2006, 79, 300–305. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, T.; Zhang, X.; Li, W. Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones 2010, 15, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Naito, H.; Tsurukawa, T.; Ichinoseki-Sekine, N.; Saga, N.; Sugiura, T.; Katamoto, S. Microwave hyperthermia treatment increases heat shock proteins in human skeletal muscle. Br. J. Sports Med. 2007, 41, 453–455. [Google Scholar] [CrossRef]
- Loc, N.H.; Macrae, T.H.; Musa, N.; Bin Abdullah, M.D.; Abdul Wahid, M.E.; Sung, Y.Y. Non-lethal heat shock increased Hsp70 and immune protein transcripts but not Vibrio tolerance in the white-leg shrimp. PLoS ONE 2013, 8, e73199. [Google Scholar]
- Achary, B.G.; Campbell, K.M.; Co, I.S.; Gilmour, D.S. RNAi screen in Drosophila larvae identifies histone deacetylase 3 as a positive regulator of the hsp70 heat shock gene expression during heat shock. Biochim. Biophys. Acta 2014, 1839, 355–363. [Google Scholar] [CrossRef]
- Zulkifli, I.; Che Norma, M.T.; Israf, D.A.; Omar, A.R. The effect of early-age food restriction on heat shock protein 70 response in heat-stressed female broiler chickens. Br. Poult. Sci. 2002, 43, 141–145. [Google Scholar] [CrossRef]
- Zhen, F.S.; Du, H.L.; Xu, H.P.; Luo, Q.B.; Zhang, X.Q. Tissue and allelic-specific expression of hsp70 gene in chickens: Basal and heat-stress-induced mRNA level quantified with realtime reverse transcriptase polymerase chain reaction. Br. Poult. Sci. 2006, 47, 449–455. [Google Scholar] [CrossRef]
- Hao, Y.; Gu, X.H.; Wang, X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 1. Intestinal structure and digestive function. Poult. Sci. 2012, 91, 781–789. [Google Scholar] [CrossRef]
- Gu, X.H.; Hao, Y.; Wang, X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult. Sci. 2012, 91, 790–799. [Google Scholar] [PubMed]
- Pierzchalski, P.; Krawiec, A.; Ptak-Belowska, A.; Barańska, A.; Konturek, S.J.; Pawlik, W.W. The mechanism of heat-shock protein 70 gene expression abolition in gastric epithelium caused by Helicobacter pylori infection. Helicobacter 2006, 11, 96–104. [Google Scholar]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in Susceptibility to Heat Stress along the Chicken Intestine and the Protective Effects of Galacto-Oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Feng, Y.; Li, J.; Gu, X. Role of MAPKs in HSP70’s Protection against Heat Stress-Induced Injury in Rat Small Intestine. Biomed. Res. Int. 2018, 2018, 1571406. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Molecular chaperones in cellular protein folding. Nature 1996, 381, 571–579. [Google Scholar] [CrossRef]
- Martin, J.; Horwich, A.L.; Hartl, F.U. Prevention of protein denaturation under heat stress by the chaperonin Hsp60. Science 1992, 258, 995–998. [Google Scholar]
- Sharma, S.; Reddy, P.V.J.; Rohilla, M.S.; Tiwari, P.K. Expression of HSP60 homologue in sheep blowfly Lucilia cuprina during development and heat stress. J. Therm. Biol. 2006, 31, 546–555. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B. Thermal manipulation during broiler chicken embryogenesis increases basal mRNA levels and alters production dynamics of heat shock proteins 70 and 60 and heat shock factors 3 and 4 during thermal stress. Poult. Sci. 2018, 97, 3661–3670. [Google Scholar]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, S.; Yin, B.; Xu, J.; Di, L.; Zhang, J.; Bao, E. Heat stress-induced renal damage in poultry and the protective effects of HSP60 and HSP47. Cell Stress Chaperones 2018, 23, 1033–1040. [Google Scholar] [CrossRef]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Mackei, M.; Molnár, A.; Nagy, S.; Pál, L.; Kővágó, C.; Gálfi, P.; Dublecz, K.; Husvéth, F.; Neogrády, Z.; Mátis, G. Effects of Acute Heat Stress on a Newly Established Chicken Hepatocyte-Nonparenchymal Cell Co-Culture Model. Animals 2020, 10, 409. [Google Scholar] [CrossRef] [PubMed]




| Primer Name | Primer Sequence (5′→ 3′) | Annealing Temperature (°C) |
|---|---|---|
| HSP70 | F-GGTAAGCACAAGCGTGACAATGCT | 55 |
| R-TCAATCTCAATGCTGGCTTGCGTG | ||
| HSP60 | F-AGAAGAAGGACAGAGTTACC | 55 |
| R-GCGTCTAATGCTGGAATG | ||
| HSP47 | F-ACTGGCTCATAAGCTCTCCAGCAT | 57 |
| R-TCATCTTGCTGGCCCA GGTCTTTA | ||
| GAPDH | F-AGAACATCATCCCAGCGTCC | 60 |
| R-CGGCAGGTCAGGTCAACAAC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan Siddiqui, S.; Kang, D.; Park, J.; Choi, H.W.; Shim, K. Acute Heat Stress Induces the Differential Expression of Heat Shock Proteins in Different Sections of the Small Intestine of Chickens Based on Exposure Duration. Animals 2020, 10, 1234. https://doi.org/10.3390/ani10071234
Hasan Siddiqui S, Kang D, Park J, Choi HW, Shim K. Acute Heat Stress Induces the Differential Expression of Heat Shock Proteins in Different Sections of the Small Intestine of Chickens Based on Exposure Duration. Animals. 2020; 10(7):1234. https://doi.org/10.3390/ani10071234
Chicago/Turabian StyleHasan Siddiqui, Sharif, Darae Kang, Jinryong Park, Hyun Woo Choi, and Kwanseob Shim. 2020. "Acute Heat Stress Induces the Differential Expression of Heat Shock Proteins in Different Sections of the Small Intestine of Chickens Based on Exposure Duration" Animals 10, no. 7: 1234. https://doi.org/10.3390/ani10071234
APA StyleHasan Siddiqui, S., Kang, D., Park, J., Choi, H. W., & Shim, K. (2020). Acute Heat Stress Induces the Differential Expression of Heat Shock Proteins in Different Sections of the Small Intestine of Chickens Based on Exposure Duration. Animals, 10(7), 1234. https://doi.org/10.3390/ani10071234





