Habitat Suitability Based Models for Ungulate Roadkill Prognosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
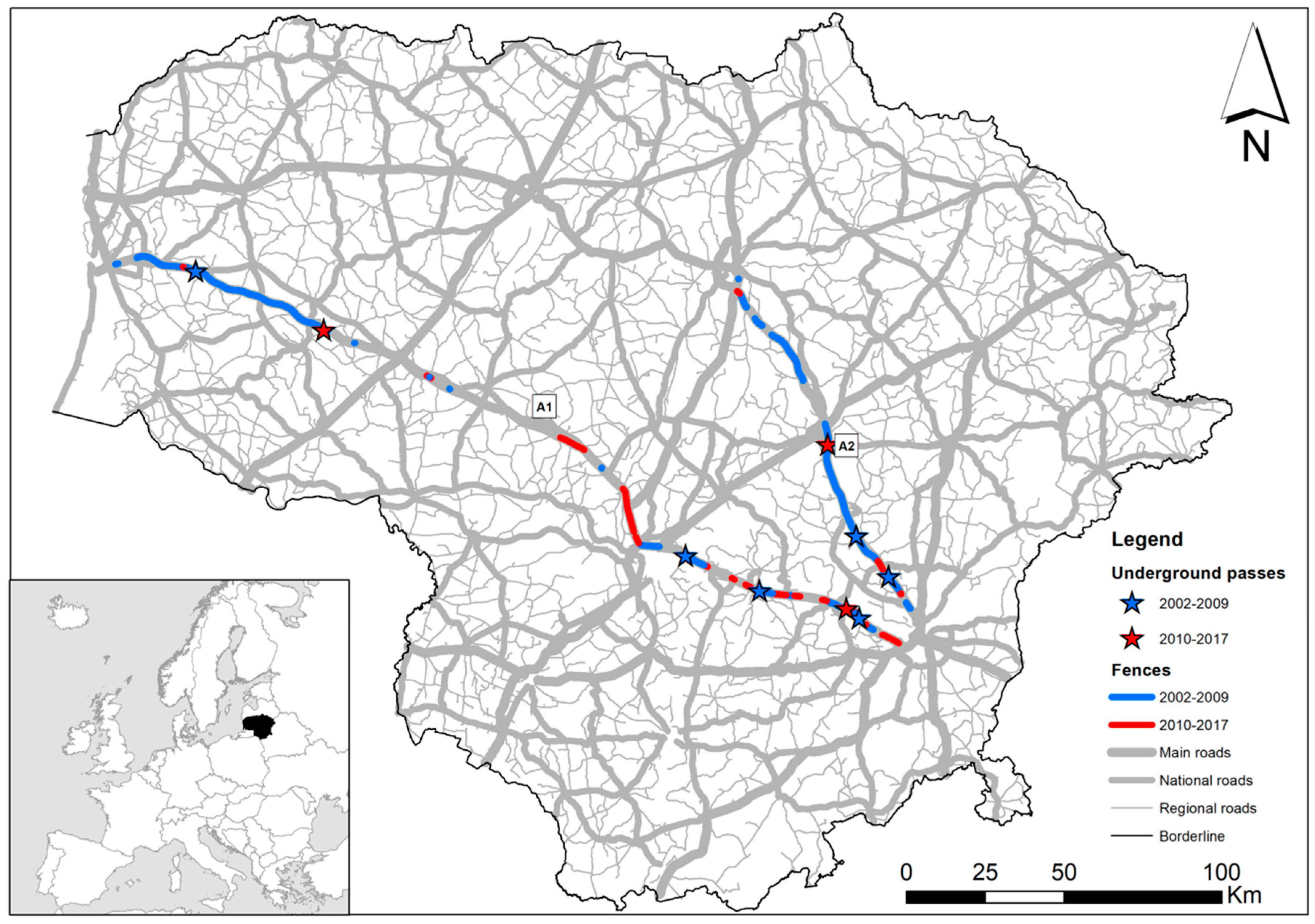
2.1. Study Area
2.2. Assessment of the Habitat Suitability
2.3. Assessment of the Human Impacts
2.4. Usage of the NDVI Index
2.5. Effective Habitats for the Three Ungulate Species Concerned
- ROEh = roe deer habitat ranking layer
- WBh = wild boar habitat ranking layer
- Rdf = road density factor (equation shown in Figure 2a)
- Bdf = built-up areas density factor (equation shown in Figure 2b)
- NDVI = Normalized Difference Vegetation Index rescaled from 0 to 1 at 0.01 increments
- Ba = Built-up areas factor (50% reduction of habitat rank within 210 m around built-up areas).
2.6. Animal Movement Component of the Model
2.7. Model Testing
3. Results
3.1. Habitat Suitability for the Three Ungulate Species in the Buffer Zone
3.2. Ungulate Animal Movements across Highways A1 and A1 in 2002–2007, as Found in the Habitat Models
3.3. Roadkills and Roadkill Clusters in 2002–2009 and 2010–2017: Was the Model Predictable?
4. Discussion
5. Error Evaluation
6. Conclusions and Recommendations
- Model-predicted pathways of roe deer significantly better described animal migration and roadkill locations than random choice in both periods, and a much higher percentage of exact matches of roadkills to the model was observed.
- Exact predictions for roadkill locations of the wild boar were good in both periods.
- The biggest differences between roadkills of red deer, roe deer and wild boar were observed in the built-up parts of the highways (vicinity of cities), and were excluded in the modeling.
- Roadkill clusters of roe deer and multi-species clusters including all three species were properly predicted by the models, especially in terms of locations with exact fit to the predicted pathways
- To check if registered roadkills in the area occur in the built-up areas before modeling ungulate movements, and, if so, lower the barrier sensitivity for the human settlements and outlying industrial areas (see Table 2).
- Before fencing considerable lengths of the highways, check the multi-species pathways and use these locations as a basis when selecting locations for the artificial wildlife structures.
- Re-run wildlife movement models after considerable changes in populations or habitat structure in the buffer zones.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Fahrig, L.; Rytwinski, T. Effects of roads on animal abundance: An empirical review and synthesis. Ecol. Soc. 2009, 14, 21. Available online: http://www.ecologyandsociety.org/vol14/iss1/art21/ (accessed on 15 April 2016). [CrossRef]
- Hill, J.E.; DeVault, T.L.; Belant, J.L. Research note: A 50-year increase in vehicle mortality of North American mammals. Landsc. Urban Plan. 2020, 197, 103746. [Google Scholar] [CrossRef]
- Bennett, A.F. Roads, roadsides and wildlife conservation: A review. In Nature Conservation 2: The Role of Corridors; Sannders, D.A., Hobbs, R.J., Eds.; Beatty and Sons Pty Ltd.: Surrey Beatty, UK, 1991; pp. 99–117. [Google Scholar]
- Hourdequin, M. Special section: Ecological effects of roads. Conserv. Biol. 2000, 14, 16–17. [Google Scholar] [CrossRef]
- Barbosa, P.; Schumaker, N.H.; Brandon, K.R.; Bager, A.; Grilo, C. Simulating the consequences of roads for wildlife population dynamics. Landsc. Urban Plan. 2020, 193, 103672. [Google Scholar] [CrossRef]
- Ascensão, F.; Desbiez, A.L.; Medici, E.P.; Bager, A. Spatial patterns of road mortality of medium–large mammals in Mato Grosso do Sul, Brazil. Wildl. Res. 2017, 44, 135–146. [Google Scholar] [CrossRef]
- Plante, J.; Bélanger-Smith, K.; Spanowicz, A.G.; Clevenger, A.P.; Jaeger, J.A. Road mortality locations of small and medium-sized mammals along a partly-fenced highway in Quebec, Canada, 2012–2015. Data brief 2018, 21, 1209–1215. [Google Scholar] [CrossRef]
- Rytwinski, T.; Soanes, K.; Jaeger, J.A.G.; Fahrig, L.; Findlay, C.S.; Houlahan, J.; van der Ree, R.; van der Grift, E.A. How Effective Is Road Mitigation at Reducing Road-Kill? A Meta-Analysis. PLoS ONE 2016, 11, e0166941. [Google Scholar] [CrossRef]
- D’Amico, M.; Ascensão, F.; Fabrizio, M.; Barrientos, R.; Gortázar, C. Twenty years of Road Ecology: A Topical Collection looking forward for new perspectives. Eur. J. Wildl. Res. 2018, 64. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Alexander, L.E. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 1998, 29, 207–231. [Google Scholar] [CrossRef]
- Damarad, T.; Bekker, G.J. COST 341—Habitat Fragmentation Due to Transportation Infrastructure: Findings of the COST Action 341; Office for Official Publications of the European Communities: Luxembourg, 2003. [Google Scholar]
- Gunson, K.E.; Mountrakis, G.; Quackenbush, L.J. Spatial wildlife-vehicle collision models: A review of current work and its application to transportation mitigation projects. J. Environ. Manag. 2011, 92, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Van der Grift, E.A.; van der Ree, R.; Fahrig, L.; Findlay, S.; Houlahan, J.; Jaeger, J.A.G.; Klar, N.; Madriñan, L.F.; Olson, L. Evaluating the effectiveness of road mitigation measures. Biodivers. Conserv. 2013, 22, 425–448. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, J.; Wang, Y.; Zhang, H.; Wu, D.; Xu, G.; Lin, Y.; Wu, X. Designing wildlife crossing structures for ungulates in a desert landscape: A case study in China. Transport. Res. D-Tr. E. 2019, 77, 50–62. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, H.; Fu, Y.; He, X.; Wang, W. Associations between road density, urban forest landscapes, and structural-taxonomic attributes in northeastern China: Decoupling and implications. Forests 2019, 10, 58. [Google Scholar] [CrossRef]
- Bekker, G.J.; Hengel, V.D.B.; van der Sluijs, H. Natuur over Wegen; (Nature over motorways, in Dutch and English); Ministry of Transport, Public Works and Water Management: Delft, The Netherlands, 1995.
- Clevenger, A.P.; Wierzchowski, J.; Chruszcz, B.; Gunson, J. GIS-generated expert based models for identifying wildlife habitat linkages and mitigation passage planning. Conserv. Biol. 2002, 16, 503–514. [Google Scholar] [CrossRef]
- Wegmann, M.; Leutner, B.; Dech, S. (Eds.) Remote Sensing and GIS for Ecologists: Using Open Source Software; Pelagic Publishing Ltd.: Exeter, UK, 2016. [Google Scholar]
- Opdam, P.F.M. How to choose the right solution for the right fragmentation problem? In Habitat Fragmentation & Infrastructure; Canters, K., Ed.; Ministry of Transportation, Public Works & Water Management: Delft, The Netherlands, 1997; pp. 55–60. [Google Scholar]
- Marignani, M.; Bruschi, D.; Garcia, D.A.; Frondoni, R.; Carli, E.; Pinna, M.S.; Cumo, F.; Gugliermetti, F.; Saatkamp, A.; Doxa, A.; et al. Identification and prioritization of areas with high environmental risk in Mediterranean coastal areas: A flexible approach. Sci. Total Environ. 2017, 590, 566–578. [Google Scholar] [CrossRef]
- Treweek, J.; Veitch, N. The Potential Application of GIS and Remotely Sensed Data to the Ecological Assessment of Proposed New Road Schemes. Global Ecol. Biogeogr. 1996, 5, 249. [Google Scholar] [CrossRef]
- Schadt, S.; Knauer, F.; Kaczensky, P.; Revilla, E.; Wiegand, T.; Trepl, L. Rule-based assessment of suitable habitat and patch connectivity for the Eurasian lynx. Ecol. Appl. 2002, 12, 1469–1483. [Google Scholar] [CrossRef]
- Sawyer, S.C.; Epps, C.W.; Brashares, J.S. Placing linkages among fragmented habitats: Do least-cost models reflect how animals use landscapes? J. Appl. Ecol. 2011, 48, 668–678. [Google Scholar] [CrossRef]
- Assis, J.C.; Giacomini, H.C.; Ribeiro, M.C. Road Permeability Index: Evaluating the heterogeneous permeability of roads for wildlife crossing. Ecol. Indic. 2019, 99, 365–374. [Google Scholar] [CrossRef]
- Schippers, P.; Verboom, J.; Knaapen, J.P.; Van Apeldoorn, R.C. Dispersal and habitat connectivity in complex heterogeneous landscapes: An analysis with a GIS-based random walk model. Ecography 1996, 19, 97–106. [Google Scholar] [CrossRef]
- Wierzchowski, J.; Kučas, A.; Balčiauskas, L. Application of Least-Cost Movement Modeling in Planning Wildlife Mitigation Measures along Transport Corridors: Case Study of Forests and Moose in Lithuania. Forests 2019, 10, 831. [Google Scholar] [CrossRef]
- Clevenger, A.P.; Wierzchowski, J. Maintaining and restoring connectivity in landscapes fragmented by roads. In Maintaining Connections for Nature; Crooks, K., Sanjayan, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 502–535. [Google Scholar]
- Chetkiewicz, C.L.B.; Boyce, M.S. Use of resource selection functions to identify conservation corridors. J. Appl. Ecol. 2009, 46, 1036–1047. [Google Scholar] [CrossRef]
- Kauth, R.J.; Thomas, G.S. The Tasselled Cap—A Graphic Description of the Spectral-Temporal Development of Agricultural Crops as Seen by LANDSAT. In LARS Symposia; Purdue University: West Lafayette, IN, USA, 1976; p. 159. Available online: http://docs.lib.purdue.edu/lars_symp/159 (accessed on 15 April 2005).
- Borowik, T.; Pettorelli, N.; Sönnichsen, L.; Jędrzejewska, B. Normalized difference vegetation index (NDVI) as a predictor of forage availability for ungulates in forest and field habitats. Eur. J. Wildl. Res. 2013, 59, 675–682. [Google Scholar] [CrossRef]
- Tellería, J.L.; Virgós, E. Distribution of an increasing roe deer population in a fragmented Mediterranean landscape. Ecography 1997, 20, 247–252. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Guinness, F.E.; Albon, S.D. Red deer: Behavior and ecology of two sexes; University of Chicago Press: Chicago, IL, USA, 1982. [Google Scholar]
- Tufto, J.; Andersen, R.; Linnell, J. Habitat Use and Ecological Correlates of Home Range Size in a Small Cervid: The Roe Deer. J. Anim. Ecol. 1996, 65, 715. [Google Scholar] [CrossRef]
- Howells, O.; Edwards-Jones, G. A feasibility study of reintroducing wild boar Sus scrofa to Scotland: Are existing woodlands large enough to support minimum viable populations. Biol. Conserv. 1997, 81, 77–89. [Google Scholar] [CrossRef]
- Saaty, T.L. A scaling method for priorities in hierarchical structures. J. Math. Psychol. 1977, 15, 234–281. [Google Scholar] [CrossRef]
- Bíl, M.; Andrášik, R.; Svoboda, T.; Sedoník, J. The KDE+ software: A tool for effective identification and ranking of animal-vehicle collision hotspots along networks. Landsc. Ecol. 2016, 31, 231–237. [Google Scholar] [CrossRef]
- Bíl, M.; Andrášik, R.; Nezval, V.; Bílová, M. Identifying locations along railway networks with the highest tree fall hazard. Appl. Geogr. 2017, 87, 45–53. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Data Science Textbook. 2020. Available online: https://docs.tibco.com/data-science/textbook (accessed on 15 March 2020).
- Anderson, D.R.; Burnham, K.P.; Thompson, W.L. Null hypothesis testing: Problems, prevalence, and an alternative. J. Wildl. Manag. 2000, 64, 912–923. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Schloss, A.L.; Kicklighter, D.W.; Kaduk, J.; Wittenberg, U. Comparing global models of terrestrial net primary productivity (NPP): Comparison of NPP to climate and the Normalized Difference Vegetation Index (NDVI). Glob. Change Biol. 1999, 5, 25–34. [Google Scholar] [CrossRef]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived normalized difference vegetation index (NDVI) to assess ecological effects of environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Pettorelli, N.; Gaillard, J.M.; Mysterud, A.; Duncan, P.; Stenseth, N.C.; Delorme, D.; Van Laere, G. Using a proxy of plant productivity (NDVI) to track animal performance: The case of roe deer. Oikos 2006, 112, 565–572. [Google Scholar] [CrossRef]
- Pettorelli, N.; Ryan, S.; Mueller, T.; Bunnefeld, N.; Jędrzejewska, B.; Lima, M.; Kausrud, K. The normalized difference vegetation index (NDVI): Unforeseen successes in animal ecology. Clim. Res. 2011, 46, 15–27. [Google Scholar] [CrossRef]
- Prins, H.H.; Fritz, H. Species diversity of browsing and grazing ungulates: Consequences for the structure and abundance of secondary production. In The Ecology of Browsing and Grazing; Springer: Berlin/Heidelberg, Germany, 2008; pp. 179–200. [Google Scholar]
- Ryan, S.J.; Cross, P.C.; Winnie, J.; Hay, C.; Bowers, J.; Getz, W.M. The utility of normalized difference index for predicting African buffalo forage quality. J. Wildl. Manag. 2012, 46, 1499–1508. [Google Scholar] [CrossRef]
- Muratet, A.; Lorrillière, R.; Clergeau, P.; Fontaine, C. Evaluation of landscape connectivity at community level using satellite-derived NDVI. Landscape Ecol. 2012, 28, 95–105. [Google Scholar] [CrossRef]
- Neumann, W.; Martinuzzi, S.; Estes, A.B.; Pidgeon, A.M.; Dettki, H.; Ericsson, G.; Radeloff, V.C. Opportunities for the application of advanced remotely-sensed data in ecological studies of terrestrial animal movement. Mov. Ecol. 2015, 3. [Google Scholar] [CrossRef]
- Forester, J.D.; Ives, A.R.; Turner, M.G.; Anderson, D.P.; Fortin, D.; Beyer, H.L.; Smith, D.W.; Boyce, M.S. State–space models link elk movement patterns to landscape characteristics in Yellowstone National Park. Ecol. Monogr. 2007, 77, 285–299. [Google Scholar] [CrossRef]
- Hamel, S.; Garel, M.; Festa-Bianchet, M.; Gaillard, J.M.; Côté, S.D. Spring Normalized Difference Vegetation Index (NDVI) predicts annual variation in timing of peak faecal crude protein in mountain ungulates. J. Appl. Ecol. 2009, 46, 582–589. [Google Scholar] [CrossRef]
- Stillfried, M.; Gras, P.; Börner, K.; Göritz, F.; Painer, J.; Röllig, K.; Wenzler, M.; Hofer, H.; Ortmann, S.; Kramer-Schadt, S. Secrets of success in a landscape of fear: Urban wild boar adjust risk perception and tolerate disturbance. Front. Ecol. Evol. 2017, 5, 157. [Google Scholar] [CrossRef]
- Gaudry, W.; Saïd, S.; Gaillard, J.-M.; Chevrier, T.; Loison, A.; Maillard, D.; Bonenfant, C. Partial migration or just habitat selection? Seasonal movements of roe deer in an Alpine population. J. Mammal. 2015, 96, 502–510. [Google Scholar] [CrossRef]
- Lendrum, P.E.; Anderson, C.R.; Monteith, K.L.; Jenks, J.A.; Bowyer, R.T. Relating the movement of a rapidly migrating ungulate to spatiotemporal patterns of forage quality. Mamm. Biol. 2014, 79, 369–375. [Google Scholar] [CrossRef]
- Colino-Rabanal, V.J.; Peris, S.J. Wildlife roadkills: Improving knowledge about ungulate distributions? Hystrix 2016, 27. [Google Scholar] [CrossRef]
- Sáenz-de-Santa-María, A.; Tellería, J.L. Wildlife-vehicle collisions in Spain. Eur. J. Wildl. Res. 2015, 61, 399–406. [Google Scholar] [CrossRef]
- Seiler, A.; Helldin, J.O. Mortality in wildlife due to transportation. In The ecology of Transportation: Managing Mobility for the Environment; Springer: Dordrecht, The Netherlands, 2006; pp. 165–189. [Google Scholar]
- Litvaitis, J.A.; Tash, J.P. An Approach Toward Understanding Wildlife-Vehicle Collisions. Environ. Manag. 2008, 42, 688–697. [Google Scholar] [CrossRef]
- Putzu, N.; Bonetto, D.; Civallero, V.; Fenoglio, S.; Meneguz, P.G.; Preacco, N.; Tizzani, P. Temporal patterns of ungulate-vehicle collisions in a subalpine Italian region. Ital. J. Zool. 2014, 81, 463–470. [Google Scholar] [CrossRef]
- Balčiauskas, L. The influence of roadkill on protected species and other wildlife in Lithuania. In Proceedings of the 2011 International Conference on Ecology and Transportation, Seattle, WA, USA, 21 August 2011; Wagner, P.J., Nelson, D., Murray, E., Eds.; North Carolina State University, Center for Transportation and the Environment: Raleigh, NC, USA, 2012; pp. 647–655. [Google Scholar]
- Bobek, B.; Boyce, M.S.; Kosobucka, M. Factors affecting red deer (Cervus elaphus) population density in southeastern Poland. J. Appl. Ecol. 1984, 21, 881–890. [Google Scholar] [CrossRef]
- Bobek, B.; Kosobucka, M.; Perzanowski, K.; Rebisz, S. Seasonal changes of the group size and sex ratio in various populations of red deer in southern Poland. In Global Trends in Wildl. Management; Świat Press: Krakow/Warszawa, Poland, 1992; Volume 2, pp. 185–192. [Google Scholar]
- Hudson, R.J.; Watkins, W.G.; Pauls, R.W. Seasonal bioenergetics of wapiti in western Canada. R. Soc. NZ Wellington Bull. 1985, 22, 447–452. [Google Scholar]
- Jeppesen, J.L. Seasonal variation in group size, and sex and age composition in a Danish red deer (Cervus elaphus) population under heavy hunting pressure. Medd. Vildtbiologisk Stn. (Denmark) 1987, 212, 19. [Google Scholar]
- Parker, J.L.; Thomas, J.W. Wildlife Habitats in Managed Forests: The Blue Mountains of Oregon and Washington (No. 553); US Department of Agriculture: Portland, OR, USA, 1979.
- Thomas, J.W.; Miller, R.J.; Black, H.; Rodiek, J.E.; Maser, C. Guidelines for maintaining and enhancing wildlife habitat in forest management in the Blue Mountains of Oregon and Washington. In Transactions of the North American Wildl. and Natural Resources Conference; Wildlife Management Institute: Washington, DC, USA, 1976; Volume 41, pp. 452–476. [Google Scholar]
- Strangaard, H. The roe deer population at Kalo and the factors regulating its size. Dan. Rev. Game Biol. 1972, 7, 1–205. [Google Scholar]
- Pielowski, Z. Some aspects of population structure and longevity of field roe deer. Acta Theriol. 1984, 29, 17–33. [Google Scholar] [CrossRef]
- Bobek, B. Summer food as the factor limiting roe deer population size. Nature 1977, 268, 47–49. [Google Scholar] [CrossRef]
- Perzanowski, K. Environmental factors effecting variability in the weight of roe deer antlers in Poland. Wildl. Conserv. Jpn. 1997, 2, 51–60. [Google Scholar]
- Kaluzinski, J. Roe deer mortality due to mechanization of work in agrocenoses. Acta Theriol. 1982, 27, 449–455. [Google Scholar] [CrossRef]
- Kaluzinski, J. The occurrence and distribution of field ecotype of roe-deer in Poland. Acta Theriol. 1974, 19, 14–25. [Google Scholar] [CrossRef]
- Sempere, A. Territorial behavior of the roe buck as determined by radio tracking: Qualitative and quantitative analysis of territorial movements. In A Handbook on Biotelemetry and Radio Tracking; Amlaner, C.J., Macdonald, D.W., Eds.; Pergamon Press: Oxford, UK, 1980; pp. 679–684. [Google Scholar]
- Pielowski, Z. Sarna (Capreolus capreolus L.). In Lowiectwo; Fruzinski, B., Krupka, J., Pielowski, Z., Pucek, Z., Eds.; PWRiL: Warszawa, Poland, 1989; 528p. [Google Scholar]
- Mysterud, A. Bed-site selection by adult roe deer Capreolus capreolus in southern Norway during summer. Wildl. Biol. 1996, 2, 101–106. [Google Scholar] [CrossRef]
- Perzanowski, K. The effect of winter food composition on roe deer energy budget. Acta Theriol. 1978, 23, 451–467. [Google Scholar] [CrossRef]
- Drożdż, A. Seasonal intake and digestibility of natural foods by roe-deer. Acta Theriol. 1979, 24, 137–170. [Google Scholar] [CrossRef]
- Fruziński, B. Wild Boar; Cedrus: Warszawa, Poland, 1992; 248p. [Google Scholar]
- Spitz, F.; Janeau, G. Spatial strategies: An attempt to classify daily movements of wild boar. Acta Theriol. 1990, 35, 129–149. [Google Scholar] [CrossRef]
- Wlazełko, M.; Łabudzki, L. Über die Nahrungskomponenten und die trophische Stellung des Schwarzwildes im Forschungsgebiet Zielonka. Z. Jagdwiss. 1992, 38, 81–87. (In German) [Google Scholar] [CrossRef]
- Bouldoire, J.L.; Vassant, J. Le Sanglier. Collection Faune Sauvage; Hatier: Paris, France, 1989; 228p. (In French) [Google Scholar]







| Feature | Comment | Red Deer | Roe Deer | Wild Boar |
|---|---|---|---|---|
| Roads and Railways | Except for forest and local field roads | 0.00 | 0.00 | 0.00 |
| Built-up areas | As barriers (various buffers) | 0.00 | 0.00 | 0.00 |
| Other barriers (mines, pits, etc.) | As barriers | 0.00 | 0.00 | 0.00 |
| Lakes and rivers (major) | Obstacle but not barrier | 0.05 | 0.01 | 0.01 |
| Marshes | Obstacle but not barrier | 0.05 | 0.05 | 0.05 |
| Agriculture (fields) | >400 m from forest edge | 0.10 | 0.40 | 0.30 |
| Agriculture (fields) | 0–400 m from forest edge | 0.15 | 0.60 | 0.35 |
| Grasslands/pasture | >400 m from forest edge | 0.40 | 0.65 | 0.50 |
| Berry/fruit tree plantations | 0.40 | 0.50 | 0.45 | |
| Grasslands/pasture | 0–400 m from forest edge | 0.45 | 0.70 | 0.55 |
| Agriculture with significant natural areas | >400 m from forest edge | 0.45 | 0.65 | 0.40 |
| Agriculture(fields) | 0–250 m from forest edge | 0.50 | 0.65 | 0.50 |
| Agriculture with significant natural areas | 0–400 m from forest edge | 0.55 | 0.70 a | 0.55 |
| Agriculture with significant natural areas | 0–250 m from forest edge | 0.60 | 0.75 | 0.60 |
| Transitional wood/shrub lands | 0.60 | 0.80 | 0.50 | |
| Coniferous forests <1250 ha | 0.65 | 0.90 | 0.55 | |
| Deciduous forests <1250 ha | 0.70 | 1.00 | 0.95 | |
| Riparian vegetation | Defined as >average NDVI * (50 m buffer around rivers and streams) | 0.70 | 0.80 | 0.60 |
| Riparian vegetation | Within coniferous forests > 1250 ha | 0.65 b | ||
| Mixed forests <1250 ha | 0.75 | 1.00 | 0.80 | |
| Grasslands/pasture | 0–250 m from forest edge | 0.80 | 0.75 | 0.60 |
| Coniferous forests >1250 ha | 0.85 | 0.90 | 0.60 | |
| Peat bogs | 0.95 | 0.95 | 0.70 | |
| Deciduous forests >1250 ha | 0.95 | 1.00 | 1.00 | |
| Mixed forests >1250 ha | 1.00 | 1.00 | 0.80 |
| Species | Small Towns and Villages | Large Towns | Cemeteries | Unvegetated Wasteland | Outlying Industrial | Quarries/Pits |
|---|---|---|---|---|---|---|
| Red deer | 250 m | 1000 m | B | B | B | B |
| Roe deer | footprint | 100 m | B | B | ||
| Wild boar | 100 m | 2000 m | B | B | B |
| Attribute | Red Deer | Roe Deer, Wild Boar | All Species |
|---|---|---|---|
| Number of crossed roads | 0.0247 | 0.0247 | |
| Habitat quality | 0.2233 | 0.2644 | 0.18 |
| Distance to built-up areas | 0.1555 | 0.155 | 0.32 |
| Distance to hiding cover | 0.12 | 0.12 | 0.50 |
| Proportion of the path within hiding cover | 0.3121 | 0.27 | |
| Path length ratio to minimum distance between entry and exit points | 0.0654 | 0.0654 | |
| Path length ratio to distance between entry and exit locations | 0.1 | 0.1 |
| Zone | KM | F | Red Deer | Roe Deer | Wild Boar | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACF | PSM | APR | ACF | PSM | APR | ACF | PSM | APR | |||
| A1-1 | 1–100 | Y | 33 | 14.3 | 0.62 ± 0.03 | 47 | 18.3 | 0.54 ± 0.14 | 44 | 19.9 | 0.53 ± 0.10 |
| A1-2 | 100–170 | N | 6 | 32.2 | 0.60 ± 0.04 | 62 | 12.8 | 0.56 ± 0.09 | 27 | 20.5 | 0.39 ± 0.24 |
| A1-3 | 170–230 | N | 22 | 7.1 | 0.53 ± 0.03 | 34 | 21.1 | 0.44 ± 0.09 | 32 | 18.8 | 0.35 ± 0.16 |
| A1-4 | 230–311 | Y | 8 | 46.4 | 0.72 ± 0.04 | 349 | 47.8 | 0.71 ± 0.14 | 67 | 40.7 | 0.62 ± 0.14 |
| A2-1 | 1–74 | Y | 69 | 29.0 | 0.57 ± 0.03 | 65 | 47.5 | 0.56 ± 0.09 | 8 | 37.0 | 0.48 ± 0.10 |
| A2-2 | 74–130 | Y | 53 | 71.0 | 0.71 ± 0.08 | 49 | 52.5 | 0.70 ± 0.10 | 65 | 63.0 | 0.60 ± 0.17 |
| Period | Highway | Species | N | Species-Related Clusters | ||
|---|---|---|---|---|---|---|
| n | Length | Strength | ||||
| 2002–2009 | A1 | Red deer | 2 | - | - | - |
| Roe deer | 136 | 10 | 126.0 ± 13.7 (116.2–135.8) | 0.364 ± 0.127 (0.273–0.455) | ||
| Wild boar | 49 | 3 | 144.3 ± 21.6 (90.7–198.0) | 0.526 ± 0.111 (0.251–0.801) | ||
| All three | 187 | 14 | 129.5 ± 16.4 (120.0–139.0) | 0.388 ± 0.142 (0.307–0.470) | ||
| 2002–2009 | A2 | Red deer | 8 | - | - | - |
| Roe deer | 187 | 28 | 147.5 ± 35.8 (133.6–161.4) | 0.453 ± 0.090 (0.418–0.488) | ||
| Wild boar | 50 | 4 | 138.7 ± 46.1 (65.3–212.1) | 0.422 ± 0.081 (0.294–0.551) | ||
| All three | 245 | 42 | 163.6 ± 64.0 (143.6–183.5) | 0.396 ± 0.102 (0.365–0.428) | ||
| 2010–2017 | A1 | Red deer | 10 | - | - | - |
| Roe deer | 286 | 39 | 134.5 ± 23.3 (126.9–142.0) | 0.445 ± 0.120 (0.407–0.484) | ||
| Wild boar | 61 | 4 | 132.8 ± 26.8 (90.2–175.3) | 0.482 ± 0.141 (0.258–0.706) | ||
| All three | 357 | 57 | 141.9 ± 35.0 (132.6–151.2) | 0.464 ± 0.129 (0.430–0.500) | ||
| 2010–2017 | A2 | Red deer | 6 | - | - | - |
| Roe deer | 156 | 26 | 146.6 ± 43.1 (129.1–164.0) | 0.431 ± 0.111 (0.386–0.476) | ||
| Wild boar | 25 | 1 | 101.0 | 0.334 | ||
| All three | 187 | 30 | 153.0 ± 46.6 (135.6–170.4) | 0.445 ± 0.117 (0.401–0.489) | ||
| Year | Abundance According National Survey 1 | Number of Roadkilled Animals 2 | L, km 3 | AADT 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Red Deer | Roe Deer | Wild Boar | Red Deer | Roe Deer | Wild Boar | |||
| 2002 | 11,098 | 69,276 | 24,050 | 5 | 150 | 23 | 81 | 5035 |
| 2009 | 18,978 | 112,091 | 50,126 | 13 | 527 | 101 | 142 | 7278 |
| 2017 | 41,266 | 143,433 | 19,141 | 33 | 1591 | 111 | 804 | 9413 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Wierzchowski, J.; Kučas, A.; Balčiauskienė, L. Habitat Suitability Based Models for Ungulate Roadkill Prognosis. Animals 2020, 10, 1345. https://doi.org/10.3390/ani10081345
Balčiauskas L, Wierzchowski J, Kučas A, Balčiauskienė L. Habitat Suitability Based Models for Ungulate Roadkill Prognosis. Animals. 2020; 10(8):1345. https://doi.org/10.3390/ani10081345
Chicago/Turabian StyleBalčiauskas, Linas, Jack Wierzchowski, Andrius Kučas, and Laima Balčiauskienė. 2020. "Habitat Suitability Based Models for Ungulate Roadkill Prognosis" Animals 10, no. 8: 1345. https://doi.org/10.3390/ani10081345
APA StyleBalčiauskas, L., Wierzchowski, J., Kučas, A., & Balčiauskienė, L. (2020). Habitat Suitability Based Models for Ungulate Roadkill Prognosis. Animals, 10(8), 1345. https://doi.org/10.3390/ani10081345








