Effect of Dietary Supplementation with Coarse or Extruded Oat Hulls on Growth Performance, Blood Biochemical Parameters, Ceca Microbiota and Short Chain Fatty Acids in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Housing
2.2. Oat Hulls, Diets and Experimental Design
2.3. Measurements and Sampling
2.4. Chemical Analyses
2.4.1. Proximate Analysis
2.4.2. Blood Biochemistry Analysis
2.4.3. Short-Chain Fatty Acids and Total Bacterial Density
2.4.4. Quantitative Real-Time PCR Analyses of Digesta DNA Samples
2.5. Bioinformatics and Statistical Analyses
3. Results
3.1. Effect of Coarse and Extruded OH on Growth Performance, Organ Weights, Cecal Short-Chain Fatty Acids and Blood Biochemical Parameters in Broiler Chickens
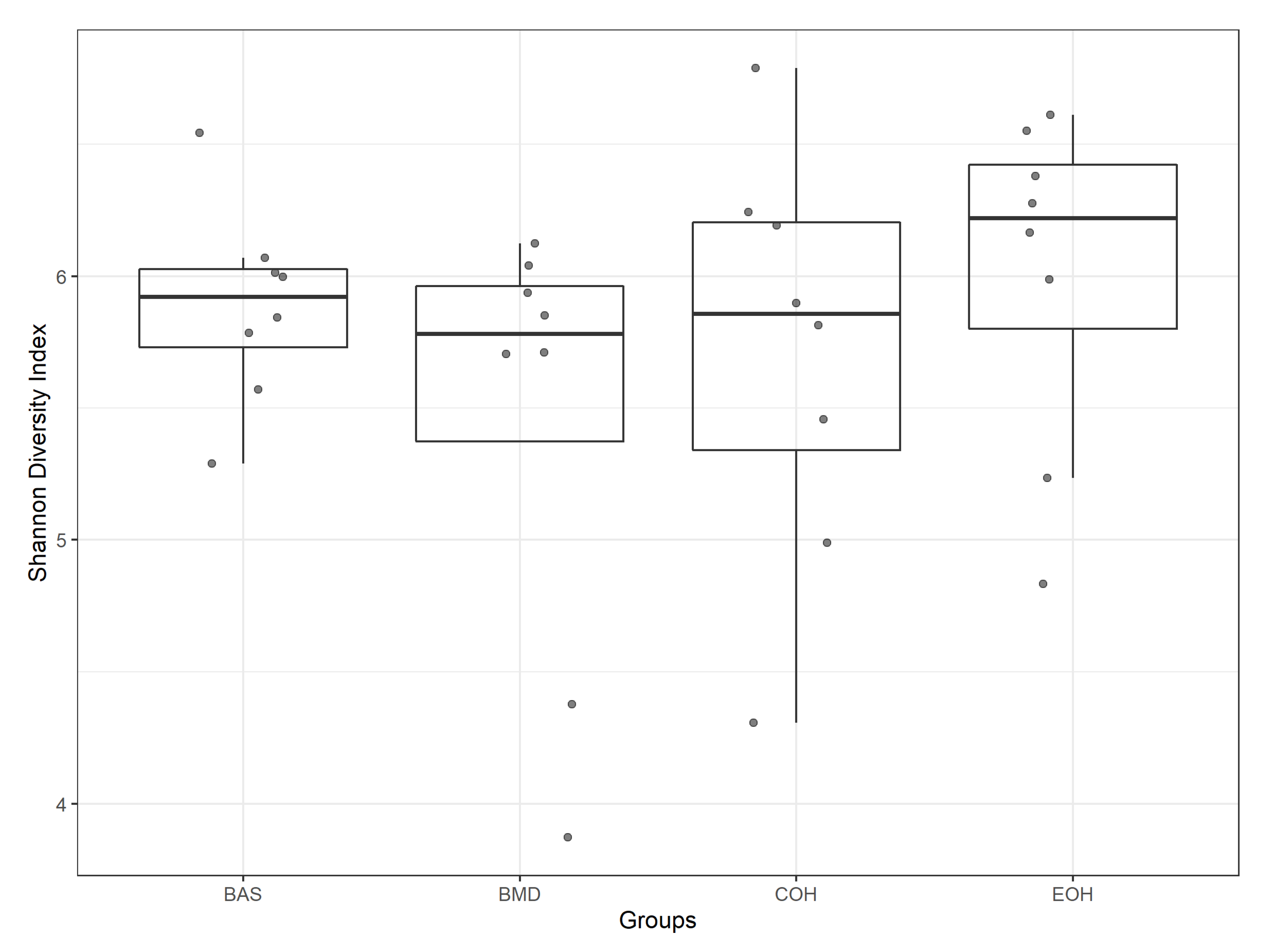
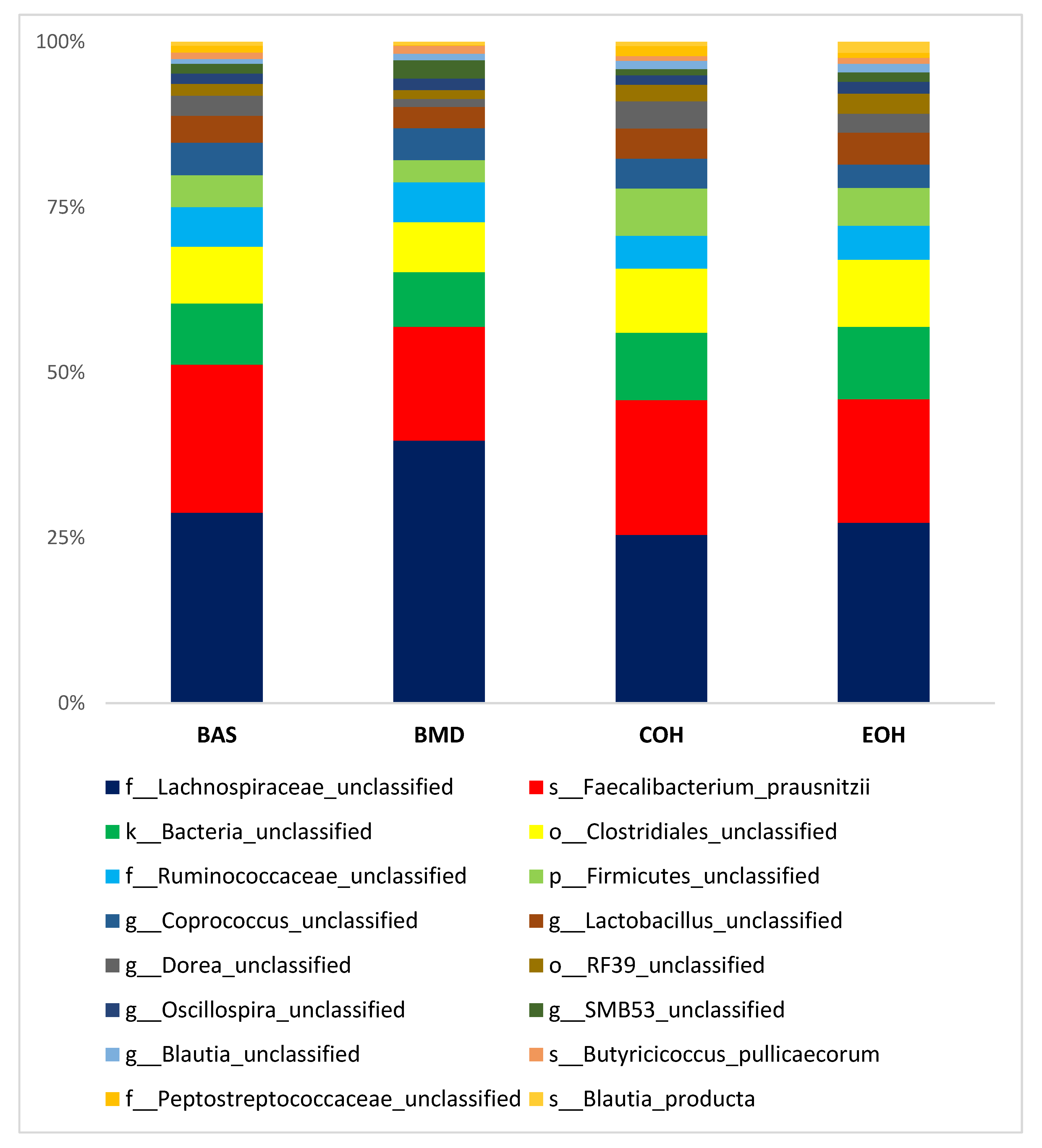
3.2. Effect of Coarse and Extruded OH on Gut Microbiota of Broiler Chickens
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.C.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Addition of oat hulls to an extruded rice-based diet for weaner pigs ameliorates the incidence of diarrhoea and reduces indices of protein fermentation in the gastrointestinal tract. Br. J. Nutr. 2008, 99, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, E.; González-Alvarado, J.M.; Lázaro, R.; Mateos, G.G. Effects of type of cereal, heat processing of the cereal, and fiber inclusion in the diet on gizzard pH and nutrient utilization in broilers at different ages. Poult. Sci. 2009, 88, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, E.; Frikha, M.; De Coca-Sinova, A.; García, J.; Mateos, G.G. Oat hulls and sugar beet pulp in diets for broilers 1. Effects on growth performance and nutrient digestibility. Anim. Feed Sci. Technol. 2013, 182, 33–43. [Google Scholar] [CrossRef]
- Jiménez-Moreno, E.; González-Alvarado, J.M.; De Coca-Sinova, A.; Lázaro, R.P.; Cámara, L.; Mateos, G.G. Insoluble fiber sources in mash or pellets diets for young broilers. Effects on gastrointestinal tract development and nutrient digestibility. Poult. Sci. 2019, 98, 2531–2547. [Google Scholar] [CrossRef]
- Torki, M.; Schokker, D.; Duijster-Lensing, M.; Van Krimpen, M.M. Effect of nutritional interventions with quercetin, oat hulls, β-glucans, lysozyme and fish oil on performance and health status related parameters of broilers chickens. Br. Poult. Sci. 2018, 59, 579–590. [Google Scholar] [CrossRef]
- Ndou, S.P.; Tun, H.M.; Kiarie, E.; Walsh, M.C.; Khafipour, E.; Nyachoti, C.M. Dietary supplementation with flaxseed meal and oat hulls modulates intestinal histomorphometric characteristics, digesta-and mucosa-associated microbiota in pigs. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Kheravii, S.K.; Swick, R.A.; Choct, M.; Wu, S.B. Effect of oat hulls as a free choice feeding on broiler performance, short chain fatty acids and microflora under a mild necrotic enteritis challenge. Anim. Nutr. 2018, 4, 65–72. [Google Scholar] [CrossRef]
- Adewole, D.; MacIsaac, J.; Fraser, G.; Rathgeber, B. Effect of Oat Hulls Incorporated in the Diet or Fed as Free Choice on Growth Performance, Carcass Yield, Gut Morphology, and Digesta Short Chain Fatty Acids of Broiler Chickens. Sustainability 2020, 12, 3744. [Google Scholar] [CrossRef]
- Gracia, M.I.; Sánchez, J.; Millán, C.; Casabuena, Ó.; Vesseur, P.; Martín, Á.; García-Peña, F.J.; Medel, P. Effect of feed form and whole grain feeding on gastrointestinal weight and the prevalence of Campylobacter jejuni in broilers orally infected. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Hetland, H.; Svihus, B. Effect of oat hulls on performance, gut capacity and feed passage time in broiler chickens. Br. Poult. Sci. 2001, 42, 354–361. [Google Scholar] [CrossRef]
- Sacranie, A.; Adiya, X.; Mydland, L.T.; Svihus, B. Effect of intermittent feeding and oat hulls to improve phytase efficacy and digestive function in broiler chickens. Br. Poult. Sci. 2017, 58, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Sunvold, G.D.; Fahey, G.C.; Merchen, N.R.; Reinhart, G.A. In vitro fermentation of selected fibrous substrates by dog and cat fecal inoculum: Influence of diet composition on substrate organic matter disappearance and short-chain fatty acid production. J. Anim. Sci. 1995, 73, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, R.; Ganguly, S. Extrusion technique in food processing and a review on its various technological parameters. Ind. J. Sci. Res. Tech. 2014, 2, 1–3. [Google Scholar]
- Rojas, O.J.; Stein, H.H. Processing of ingredients and diets and effects on nutritional value for pigs. J. Anim. Sci. Biotechnol. 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Ralet, M.C.; Della Valle, G.; Thibault, J.F. Raw and extruded fibre from pea hulls. Part I: Composition and physico-chemical properties. Carbohydr. Polym. 1993, 20, 17–23. [Google Scholar] [CrossRef]
- Sobota, A.; Rzedzicki, Z. Effect of the extrusion process of corn semolina and pea hulls blends on chemical composition and selected physical properties of the extrudate. Int. Agrophys. 2009, 23, 67–79. [Google Scholar]
- Zhong, Y.; Nyman, M.; Fak, F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076. [Google Scholar] [CrossRef]
- Diaz, D.; Morlacchini, M.; Masoero, F.; Moschini, M.; Fusconi, G.; Piva, G. Pea seeds (Pisum sativum), faba beans (Vicia faba var. minor) and lupin seeds (Lupinus albus var. multitalia) as protein sources in broiler diets: Effect of extrusion on growth performance. Ital. J. Anim. Sci. 2006, 5, 43–53. [Google Scholar] [CrossRef]
- Anjola, O.A.; Adejobi, M.A.; Tijani, L.A. Growth Performance and Blood Characteristics of Broilers ChicNen Fed on Diet Containing Brewer Spent Grain at Finisher Phase. Int. J. Anim. Vet. Sci. 2016, 10, 214–217. [Google Scholar]
- Behera, D.P.; Sethi, A.P.S.; Singh, C.; Singh, U.; Wadhwa, M. Effect of citrus waste on blood parameters of broiler birds with and without cocktail of enzymes. Vet. World. 2019, 12, 483–488. [Google Scholar] [CrossRef]
- Khoobani, M.; Hasheminezhad, S.H.; Javandel, F.; Nosrati, M.; Seidavi, A.; Kadim, I.T.; Laudadio, V.; Tufarelli, V. Effects of Dietary Chicory (Chicorium intybus L.) and Probiotic Blend as Natural Feed Additives on Performance Traits, Blood Biochemistry, and Gut Microbiota of Broiler Chickens. Antibiotics 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kwiecień, M.; Klebaniuk, R.; Szymańczyk, S.; Tomczyk, A.; Kowalik, S.; Milczarek, A.; Świetlicka, I. The influence of dietary replacement of soybean meal with high-tannin faba beans on gut-bone axis and metabolic response in broiler chickens. Ann. Anim. Sci. 2018, 18, 801–824. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Roth-Maier, D.A.; Kirchgessner, M. The effect of enzyme supplements and high amounts of white lupins on concentrations of lipids in serum and meat in fattening chickens. Arch. Anim. Nutr. 1996, 49, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M.S. Cholesterol-lowering effects of oats B-glucan: A meta-analysis of randomized control trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef]
- Canadian Council on Animal Care. Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing; Canadian Council on Animal Care: Ottawa, ON, Canada, 2009. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses: Apparatus, Reagents, Procedures, and Some Applications; Agricultural Research Service; United States Department of Agriculture: Washington, DC, USA, 1970; Volume 379.
- Hall, M.B.; Hoover, W.H.; Jennings, J.P.; Wesbster, T.K.M. A method for partitioning neutral detergent-soluble carbohydrates. J. Sci. Food Agric. 1999, 79, 2079–2086. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K.; Raatikainen, K.; Holder, V.; Moran, C.A. Conversion of branched-chain amino acids to corresponding isoacids-an in vitro tool for estimating ruminal protein degradability. Front. Vet. Sci. 2019, 6, 311. [Google Scholar] [CrossRef]
- Kozich, J.J.; Sarah, L.; Westcott, N.T.; Baxter Sarah, K.H.; Patrick, D.S. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Env. Microbiol. 2013, 01043–01113. [Google Scholar] [CrossRef]
- Schloss, P.D.; Sarah, L.W.; Thomas, R.; Justine, R.H.; Martin, H.; Emily, B.H.; Ryan, A.L. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Env. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Statistics Canada. 2019. Available online: https://grainscanada.gc.ca/en/grain-research/export-quality/cereals/oats/2019/preliminary/?wbdisable=true (accessed on 13 August 2020).
- Crosbie, G.B.; Tarr, A.W.; Portmann, P.A.; Rowe, J.B. Variation in hull composition and digestibility among oat genotypes. Crop Sci. 1984, 25, 678–680. [Google Scholar] [CrossRef]
- Perruzza, A.L. Exploring pretreatment methods and enzymatic Hydrolysis of oat hulls. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2010. [Google Scholar]
- Adewole, D.I.; Rogiewicz, A.; Dyck, B.; Slominski, B.A. Chemical and nutritive characteristics of canola meal from Canadian processing facilities. Anim. Feed Sci. Technol. 2016, 222, 17–30. [Google Scholar] [CrossRef]
- Miao, Y.; Amari, M.; Yoshizaki, S. Mechanism of spontaneous heating of hay part 2—chemical changes in spontaneous heated hay. Trans. Am. Soc. Agric. Eng. 1994, 37, 1567–1570. [Google Scholar] [CrossRef]
- Reece, F.N.; Lott, B.D.; Deaton, J.W. The effects of feed form, grinding method, energy level, and gender on broiler performance in a moderate (21 C) environment. Poult. Sci. 1985, 64, 1834–1839. [Google Scholar] [CrossRef]
- Parsons, A.S.; Buchanan, N.P.; Blemings, K.P.; Wilson, M.E.; Moritz, J.S. Effect of corn particle size and pellet texture on broiler performance in the growing phase. J. Appl. Poult. Res. 2006, 15, 245–255. [Google Scholar] [CrossRef]
- Van der Klis, J.D.; Kwakernaak, C.; Jansman, A.; Blok, M. Energy in poultry diets: Adjusted AME or net energy. Proc. Aust. Poult. Sci. 2010, 21, 44–49. [Google Scholar]
- Abdollahi, M.R.; Zaefarian, F.; Hunt, H.; Anwar, M.N.; Thomas, D.G.; Ravindran, V. Wheat particle size, insoluble fiber sources, and whole wheat feeding influence gizzard musculature and nutrient utilization to different extents in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 146–161. [Google Scholar] [CrossRef]
- Qaisrani, S.N.; Van Krimpen, M.M.; Kwakkel, R.P.; Verstegen, M.W.A.; Hendriks, W.H. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poult. Sci. 2015, 94, 2152–2164. [Google Scholar] [CrossRef]
- Xu, Y.; Stark, C.R.; Ferket, P.R.; Williams, C.M.; Brake, J. Effects of feed form and dietary coarse ground corn on broiler live performance, body weight uniformity, relative gizzard weight, excreta nitrogen, and particle size preference behaviors. Poult. Sci. 2015, 94, 549–1556. [Google Scholar] [CrossRef]
- Svihus, B. Limitations to wheat starch digestion in growing broiler chickens: A brief review. Anim. Prod. Sci. 2011, 51, 583–589. [Google Scholar] [CrossRef]
- Husvéth, F.; Pál, L.; Galamb, E.; Ács, K.C.; Bustyaházai, L.; Wágner, L.; Dublecz, F.; Dublecz, K. Effects of whole wheat incorporated into pelleted diets on the growth performance and intestinal function of broiler chickens. Anim. Feed Sci. Technol. 2015, 210, 144–151. [Google Scholar] [CrossRef]
- Amerah, A.M.; Ravindran, V.; Lentle, R.G.; Thomas, D.G. Influence of feed particle size and feed form on the performance, energy utilization, digestive tract development, and digesta parameters of broiler starters. Poult. Sci. 2007, 86, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Behnke, K.C. Factors influencing pellet quality. Feed Technol. 2001, 5, 19–22. [Google Scholar]
- Razdan, A.; Pettersson, D. Effect of chitin and chitosan on nutrient digestibility and plasma lipid concentrations in broiler chickens. Br. J. Nutr. 1994, 72, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Ghoujegh, D.; Gharejeh, A.M. Effects of dietary chitosan on nitrogen metabolite levels in mice. Arch. Iran. Med. 2001, 4, 96–98. [Google Scholar]
- Sarikhan, M.; Shahryar, H.A.; Nazer-Adl, K.; Gholizadeh, B.; Behesht, B. Effects of insoluble fiber on serum biochemical characteristics in broiler. Int. J. Agric. Biol. 2009, 11, 73–76. [Google Scholar]
- McNaughton, J.L. Effect of dietary fiber on egg yolk, liver, and plasma cholesterol concentrations of the laying hen. J. Nutr. 1978, 108, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, S. Defatted rice bran nonstarch polysaccharides in broiler diets: Effects of supplements on nutrient digestibilities. J. Poult. Sci. 2002, 39, 67–76. [Google Scholar]
- Mathlouthi, N.; Lalles, J.P.; Lepercq, P.; Juste, C.; Larbier, M. Xylanase and β-glucanase supplementation improve conjugated bile acid fraction in intestinal contents and increase villus size of small intestine wall in broiler chickens fed a rye-based diet. J. Anim. Sci. 2002, 80, 2773–2779. [Google Scholar] [CrossRef]
- Ciurescu, G.; Vasilachi, A.; Grigore, D.; Grosu, H. Growth performance, carcass traits, and blood biochemistry of broiler chicks fed with low-fibre sunflower meal and phytase. S. Afr. J. Anim. Sci. 2019, 49, 735–745. [Google Scholar] [CrossRef]
- Kimiaeitalab, M.V.; Cámara, L.; Mirzaie Goudarzi, S.; Jiménez-Moreno, E.; Mateos, G.G. Effects of the inclusion of sunflower hulls in the diet on growth performance and digestive tract traits of broilers and pullets fed a broiler diet from zero to 21 d of age. A comparative study. Poult. Sci. 2017, 96, 581–592. [Google Scholar] [CrossRef]
- Leung, H.; Arrazola, A.; Torrey, S.; Kiarie, E. Utilization of soy hulls, oat hulls, and flax meal fiber in adult broiler breeder hens. Poult. Sci. 2018, 97, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H. Gut microbiota-mediated drug-antibiotic interactions. Drug Metab. Dispos. 2015, 43, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Knarreborg, A.; Engberg, R.M.; Jensen, S.K.; Jensen, B.B. Quantitative determination of bile salt hydrolase activity in bacteria isolated from the small intestine of chickens. Appl. Env. Microbiol. 2002, 68, 6425–6428. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007, 61, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Diaz Carrasco, J.M.; Redondo, E.A.; Pin Viso, N.D.; Redondo, L.M.; Farber, M.D.; Fernandez Miyakawa, M.E. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. Biomed. Res. Int. 2018, 1879168. [Google Scholar] [CrossRef]
- Neijat, M.; Habtewold, J.; Shirley, R.B.; Welsher, A.; Barton, J.; Thiery, P.; Kiarie, E. Bacillus subtilis DSM29784 modulates cecal microbiome, short chain fatty acids concentration, and apparent retention of dietary components in Shaver Whites during grower, developer and laying phases. Appl. Environ Microbiol. 2019, 85, e00402–e00419. [Google Scholar] [CrossRef]
- Qu, A.; Brulc, J.M.; Wilson, M.K.; Law, B.F.; Theoret, J.R.; Joens, L.A.; Konkel, M.E.; Angly, F.; Dinsdale, E.A.; Edwards, R.A.; et al. Comparative metagenomics reveals host specific metavirulomes and horizontal gene transfer elements in the chicken cecum microbiome. PLoS ONE 2008, 3, e2945. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic Growth Promoters in Agriculture: History and Mode of Action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Fung, S.; Rempel, H.; Forgetta, V.; Dewar, E.T.K.; Diarra, M.S. Ceca Microbiome of Mature Broiler Chickens Fed with or without Salinomycin. In Proceedings of the Keystone Symposia on Molecular and Cellular Biology, Gut Microbiome: The Effector/Regulatory Immune Network Conference (B3), Taos, NM, USA, 10–15 February 2013. [Google Scholar]
- Lin, J.; Hunkapiller, A.A.; Layton, A.C.; Chang, Y.J.; Robbins, K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog. Dis. 2013, 10, 331–337. [Google Scholar] [CrossRef]
- Dumonceaux, T.J.; Hill, J.E.; Hemmingsen, S.M.; Van Kessel, A.G. Characterization of Intestinal Microbiota and Response to Dietary Virginiamycin Supplementation in the Broiler Chicken. Appl. Env. Microbiol. 2006, 72, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, J.L.; Kim, H.B.; Isaacson, R.E.; Tu, Z.J.; Johnson, T.J. Modulations of the Chicken 551 Cecal Microbiome and Metagenome in Response to Anticoccidial and Growth Promoter Treatment. PLoS ONE 2011, 6, e27949. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Ho Hong, Y.; Lee, S.H.; Jang, S.I.; Park, M.S.; Bautista, D.A.; Ritter, G.D.; Jeong, W.; Jeoung, H.Y.; An, D.J.; et al. Effects of Anticoccidial and Antibiotic Growth Promoter Programs on Broiler Performance and Immune Status. Res. Vet. Sci. 2012, 93, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gong, J.; Brisbin, J.T.; Yu, H.; Sanei, B.; Sabour, P.; Sharif, S. Appropriate Chicken Sample Size for Identifying the Composition of Broiler Intestinal Microbiota Affected by Dietary Antibiotics, Using the Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis Technique. Poult. Sci. 2007, 86, 2541–2549. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Nandha, N.K.; Kiarie, E.; Nyachoti, C.M.; Khafipour, E. Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poult. Sci. 2016, 95, 528–540. [Google Scholar] [CrossRef]
- Bosshard, P.P.; Zbinden, R.; Altwegg, M. Turicibacter sanguinis gen. nov., sp. nov., a novel anaerobic, Gram-positive bacterium. Int. J. Syst. Evol. Micr. 2002, 52, 1263–1266. [Google Scholar]
- Cuív, P.Ó.; Klaassens, E.S.; Durkin, A.S.; Harkins, D.M.; Foster, L.; McCorrison, J.; Torralba, M.; Nelson, K.E.; Morrison, M. Draft genome sequence of Turicibacter sanguinis PC909, isolated from human feces. J. Bacteriol. 2011, 193, 1288–1289. [Google Scholar]
- Siegerstetter, S.C.; Schmitz-Esser, S.; Magowan, E.; Wetzels, S.U.; Zebeli, Q.; Lawlor, P.G.; O’Connell, N.E.; Metzler-Zebeli, B.U. Intestinal microbiota profiles associated with low and high residual feed intake in chickens across two geographical locations. PLoS ONE 2017, 12, e0187766. [Google Scholar] [CrossRef]
- Rubio, L.A.; Peinado, M.J.; Ruiz, R.; Suárez-Pereira, E.; Ortiz Mellet, C.; Garcia Fernandez, J.M. Correlations between changes in intestinal microbiota composition and performance parameters in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2015, 99, 418–423. [Google Scholar] [CrossRef]
- Oh, J.K.; Pajarillo, E.A.B.; Chae, J.P.; Kim, I.H.; Yang, D.S.; Kang, D.K. Effects of Bacillus subtilis CSL2 on the composition and functional diversity of the faecal microbiota of broiler chickens challenged with Salmonella Gallinarum. J. Anim. Sci. Biotechnol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Liu, J.; Xu, T.; Zhu, W.; Mao, S. High-grain feeding alters caecal bacterial microbiota composition and fermentation and results in caecal mucosal injury in goats. Br. J. Nutr. 2014, 112, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Fang, Z.; Wahlqvist, M.L.; Hodgson, J.M.; Johnson, S.K. Extrusion cooking increases soluble dietary fibre of lupin seed coat. LWT Food Sci. Technol. 2019, 99, 547–554. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Miller, M.E.B.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Wahyudi, A.; Cahyant, M.N.; Soejono, M.; Bachruddin, Z. Potency of lignocellulose degrading bacteria isolated from buffalo and horse gastrointestinal tract and elephant dung for feed fiber degradation. J. Indones. Trop. Anim. Agric. 2010, 35, 34–41. [Google Scholar] [CrossRef]
- ALjahdali, N.; Carbonero, F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 2019, 59, 474–487. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Helou, C.; Denis, S.; Spatz, M.; Marier, D.; Rame, V.; Alric, M.; Tessier, F.J.; Gadonna-Widehem, P. Insights into bread melanoidins: Fate in the upper digestive tract and impact on the gut microbiota using in vitro systems. Food Funct. 2015, 6, 3737–3745. [Google Scholar] [CrossRef]
- Borda-Molina, D.; Seifert, J.; Camarinha-Silva, A. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput. Struct. Biotec. 2018, 16, 131–139. [Google Scholar] [CrossRef]
- Ishiguro, E.; Haskey, E.; Campbell, K. Gut Microbiota: Interactive Effects on Nutrition and Health; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Richards, P.; Wigley, P.; Fothergill, J.; Bernardeau, M. Development of the caecal microbiota in three broiler breeds. Front. Vet. Sci. 2019, 6, 201. [Google Scholar] [CrossRef]
- Biddle, A.S.; Black, S.J.; Blanchard, J.L. An in vitro model of the horse gut microbiome enables identification of lactate-utilizing bacteria that differentially respond to starch induction. PLoS ONE 2013, 8, e77599. [Google Scholar] [CrossRef] [PubMed]
- Torok, V.A.; Allison, G.E.; Percy, N.J.; Ophel-Keller, K.; Hughes, R.J. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl. Environ. Microbiol. 2011, 77, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.P.; Suen, G. Differences in major bacterial populations in the intestines of mature broilers after feeding virginiamycin or bacitracin methylene disalicylate. J. Appl. Microbiol. 2015, 119, 1515–1526. [Google Scholar] [CrossRef]
- Singh, P.; Karimi, A.; Devendra, K.; Waldroup, P.W.; Cho, K.K.; Kwon, Y.M. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poult. Sci. 2013, 92, 272–276. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Newman, M.M.; Stough, J.M.; Liles, M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016, 95, 247–260. [Google Scholar] [CrossRef]
- Stanley, D.; Hughes, R.J.; Geier, M.S.; Moore, R.J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: Challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 2016, 7, 187. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, W.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef]
- Eeckhaut, V.; Van Immerseel, F.; Croubels, S.; De Baere, S.; Haesebrouck, F.; Ducatelle, R.; Louis, P.; Vandamme, P. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb. Biotechnol. 2011, 4, 503–512. [Google Scholar] [CrossRef]





| Coarse Oat Hulls | Extruded Oat Hulls | |
|---|---|---|
| Dry matter | 90.3 | 92.5 |
| Crude protein | 2.52 | 2.92 |
| Fat | 0.50 | 0.55 |
| Acid detergent fiber | 42.3 | 40.3 |
| Neutral detergent fiber | 76.2 | 73.9 |
| Lignin | 6.62 | 9.29 |
| Ash | 5.45 | 4.99 |
| Simple sugars | 1.10 | 0.63 |
| Starch | 4.37 | 2.70 |
| Non-structural carbohydrates | 7.68 | 6.72 |
| Calcium | 0.07 | 0.07 |
| Potassium | 0.27 | 0.28 |
| Magnesium | 0.07 | 0.07 |
| Phosphorus | 0.06 | 0.07 |
| Item | Production Period (Days of Age) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–14 | 14–24 | 24–36 | ||||||||||
| Basal | BMD | COH | EOH | Basal | BMD | COH | EOH | Basal | BMD | COH | EOH | |
| Corn | 41.3 | 41.2 | 38.0 | 38.0 | 44.3 | 44.2 | 40.9 | 40.9 | 48.5 | 48.4 | 45.1 | 45.1 |
| Soybean Meal | 40.2 | 40.2 | 40.5 | 40.5 | 36.5 | 36.5 | 36.9 | 36.9 | 31.5 | 31.5 | 31.9 | 31.9 |
| Wheat | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Oat Hulls | - | - | 3.00 | 3.00 | - | - | 3.00 | 3.00 | - | - | 3.00 | 3.00 |
| Animal/Vegetable Fat | 3.44 | 3.47 | 3.40 | 3.40 | 4.59 | 4.63 | 4.59 | 4.59 | 5.67 | 5.70 | 5.67 | 5.67 |
| Limestone | 1.80 | 1.80 | 1.79 | 1.79 | 1.65 | 1.65 | 1.64 | 1.64 | 1.52 | 1.52 | 1.51 | 1.51 |
| Dicalcium Phosphate | 1.23 | 1.23 | 1.24 | 1.24 | 1.06 | 1.06 | 1.07 | 1.07 | 0.93 | 0.93 | 0.94 | 0.94 |
| DL-Methionine Premix Z | 0.61 | 0.61 | 0.62 | 0.62 | 0.53 | 0.53 | 0.54 | 0.54 | 0.49 | 0.49 | 0.50 | 0.50 |
| Lysine HCl | 0.03 | 0.03 | 0.02 | 0.02 | - | - | - | - | 0.01 | 0.009 | 0.003 | 0.003 |
| Iodized Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.37 | 0.37 | 0.37 | 0.37 | 0.38 | 0.38 | 0.38 | 0.38 |
| Pellet Binding Agent Y | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| BMD 110 G X | - | 0.05 | - | - | - | 0.05 | - | - | - | 0.05 | - | - |
| Vitamin/Mineral Premix W, V | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Calculated Composition | ||||||||||||
| Crude Protein (%) | 23.0 | 23.0 | 23.0 | 23.0 | 21.5 | 21.5 | 21.5 | 21.5 | 19.5 | 19.5 | 19.5 | 19.5 |
| Metabolizable Energy (MJ·kg−1) | 12.6 | 12.6 | 12.2 | 12.2 | 13.0 | 13.0 | 12.6 | 12.6 | 13.4 | 13.4 | 13.0 | 13.0 |
| Calcium (%) | 0.96 | 0.96 | 0.96 | 0.96 | 0.87 | 0.87 | 0.87 | 0.87 | 0.78 | 0.78 | 0.78 | 0.78 |
| Available Phosphorus (%) | 0.48 | 0.48 | 0.48 | 0.48 | 0.44 | 0.44 | 0.44 | 0.44 | 0.39 | 0.39 | 0.39 | 0.39 |
| Digestible Lysine | 1.28 | 1.28 | 1.28 | 1.28 | 1.16 | 1.16 | 1.17 | 1.17 | 1.02 | 1.02 | 1.03 | 1.03 |
| Digestible Methionine + Cystine (%) | 0.95 | 0.95 | 0.95 | 0.95 | 0.87 | 0.87 | 0.87 | 0.87 | 0.80 | 0.80 | 0.80 | 0.80 |
| Sodium (%) | 0.19 | 0.19 | 0.19 | 0.19 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Analysed composition u | ||||||||||||
| Crude Protein (%) | 24.6 | 23.9 | 24.9 | 24.0 | 21.8 | 22.1 | 22.6 | 21.3 | 20.4 | 20.2 | 20.9 | 20.4 |
| ADF (%) | 3.74 | 4.82 | 5.85 | 5.08 | 3.15 | 3.70 | 4.41 | 4.83 | 3.87 | 3.84 | 5.02 | 5.84 |
| NDF (%) | 7.26 | 7.33 | 9.16 | 7.64 | 6.81 | 6.69 | 8.59 | 8.86 | 7.44 | 7.27 | 8.17 | 9.79 |
| Sodium (%) | 0.20 | 0.19 | 0.21 | 0.20 | 0.19 | 0.18 | 0.17 | 0.17 | 0.18 | 0.19 | 0.19 | 0.18 |
| Crude Fat (%) | 5.55 | 5.35 | 5.50 | 5.44 | 6.90 | 6.93 | 7.02 | 6.85 | 7.61 | 7.91 | 7.96 | 7.91 |
| Treatment 1 | SEM | p Value | ||||
|---|---|---|---|---|---|---|
| Basal | BMD | COH | EOH | |||
| Feed intake, g/bird | ||||||
| D 1–14 | 542 a | 548 a | 489 b | 515 ab | 11.22 | 0.0052 |
| D 14–28 | 1890 | 1857 | 1818 | 1821 | 32.37 | 0.3644 |
| D 28–36 | 1165 | 1153 | 1206 | 1185 | 21.61 | 0.3439 |
| D 1–36 | 3585 | 3587 | 3397 | 3522 | 77.07 | 0.2819 |
| Body weight gain, g/bird | ||||||
| D 1–14 | 447 a | 455 a | 408 b | 413 b | 6.880 | <0.0001 |
| D 14–28 | 1168 ab | 1177 a | 1106 bc | 1104 c | 16.62 | 0.0038 |
| D 28–36 | 817 | 828 | 794 | 815 | 15.73 | 0.5042 |
| D 1–36 | 2449 ab | 2460 a | 2308 b | 2332 b | 28.06 | 0.0008 |
| Feed conversion ratio | ||||||
| D 1–14 | 1.21 | 1.21 | 1.19 | 1.25 | 0.022 | 0.2207 |
| D 14–28 | 1.62 | 1.58 | 1.65 | 1.65 | 0.024 | 0.1198 |
| D 28–36 | 1.44 | 1.40 | 1.53 | 1.45 | 0.038 | 0.0956 |
| D 1–36 | 1.50 | 1.46 | 1.47 | 1.51 | 0.018 | 0.1881 |
| Item | Treatment 1 | SEM | p Value | |||
|---|---|---|---|---|---|---|
| Basal | BMD | COH | EOH | |||
| Slaughter weight, kg | 2.80 | 2.82 | 2.73 | 2.90 | 0.096 | 0.6677 |
| Bursa, g/kg body weight | 1.82 | 1.69 | 1.74 | 1.92 | 0.182 | 0.8283 |
| Spleen, g/kg body weight | 0.95 | 0.85 | 0.88 | 0.78 | 0.069 | 0.4405 |
| Gizzard, g/kg body weight 2 | 11.7 b | 11.7 b | 14.4 a | 11.7 b | 0.601 | 0.0050 |
| Ceca, g/kg body weight 3 | 3.62 | 4.20 | 3.59 | 3.98 | 0.251 | 0.2660 |
| Item | Treatment 1 | SEM | p Value | |||
|---|---|---|---|---|---|---|
| Basal | BMD | COH | EOH | |||
| Acetic acid | 51.5 | 51.2 | 55.6 | 51.1 | 4.330 | 0.8578 |
| Propionic acid | 4.29 | 5.26 | 4.15 | 4.36 | 0.459 | 0.3275 |
| Butyric acid | 12.2 | 12.3 | 13.5 | 10.4 | 1.328 | 0.4380 |
| Valeric acid | 0.94 | 1.06 | 1.03 | 0.97 | 0.081 | 0.7299 |
| Lactic acid | 3.00 | 1.58 | 2.33 | 2.38 | 0.615 | 0.2166 |
| Total SCFA | 74.2 | 71.3 | 78.6 | 71.0 | 5.672 | 0.7648 |
| BCFA 2 | 2.23 | 1.38 | 2.01 | 1.81 | 0.227 | 0.4822 |
| VFA 3 | 71.2 | 69.8 | 76.3 | 68.6 | 5.745 | 0.7925 |
| Total Eubacteria | 12.0 | 11.9 | 11.8 | 11.9 | 0.109 | 0.8495 |
| Treatment 1 | SEM | p Value | ||||
|---|---|---|---|---|---|---|
| Basal | BMD | COH | EOH | |||
| Electrolytes and minerals, mmol·L−1 | ||||||
| Sodium | 152 | 154 | 159 | 164 | 9.484 | 0.8073 |
| Potassium | 5.76 | 5.09 | 5.56 | 5.65 | 0.356 | 0.5622 |
| Chloride | 111 | 112 | 116 | 120 | 7.982 | 0.8451 |
| Calcium | 2.68 | 2.76 | 2.89 | 2.92 | 0.108 | 0.3696 |
| Phosphorus | 1.77 | 1.59 | 1.68 | 1.70 | 0.143 | 0.8640 |
| Magnesium | 0.83 | 0.82 | 0.80 | 0.75 | 0.053 | 0.7413 |
| Metabolites, mmol·L−1 | ||||||
| Urea | 0.34 | 0.26 | 0.30 | 0.28 | 0.031 | 0.3451 |
| Creatinine | 2.00 | 2.75 | 2.00 | 2.25 | 1.096 | 0.9570 |
| Glucose | 14.7 | 15.1 | 15.9 | 15.9 | 0.835 | 0.6450 |
| Cholesterol | 2.94 | 3.41 | 3.13 | 3.01 | 0.236 | 0.6009 |
| Iron | 17.9 | 23.0 | 20.3 | 17.3 | 2.584 | 0.3977 |
| Bile acids | 17.0 | 14.0 | 17.9 | 17.4 | 2.481 | 0.6887 |
| Uric acid | 401 | 447 | 407 | 350 | 58.62 | 0.9169 |
| Total Bilirubin | 0.63 | 0.38 | 0.38 | 0.38 | 0.183 | 0.7079 |
| Enzymes, U·L−1 | ||||||
| Amylase | 410 | 483 | 516 | 417 | 67.72 | 0.4481 |
| Alkaline phosphatase | 3243 | 2722 | 2820 | 3316 | 599.9 | 0.6884 |
| Creatine kinase | 19,820 | 19784 | 18888 | 27667 | 4524 | 0.3751 |
| Aspartate Aminotransferase | 324 | 308 | 327 | 378 | 48.29 | 0.6416 |
| Gamma-Glutamyl Transferase | 10.0 | 11.9 | 13.5 | 10.9 | 1.329 | 0.3016 |
| Lipase | 22.0 | 17.8 | 19.1 | 17.0 | 3.143 | 0.6908 |
| Proteins, g·L−1 | ||||||
| Total protein | 28.0 | 28.4 | 29.4 | 28.3 | 2.215 | 0.9733 |
| Albumin | 12.0 | 12.1 | 12.5 | 11.8 | 0.917 | 0.9499 |
| Globulin | 16.0 | 16.3 | 16.9 | 16.5 | 1.344 | 0.9718 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adewole, D. Effect of Dietary Supplementation with Coarse or Extruded Oat Hulls on Growth Performance, Blood Biochemical Parameters, Ceca Microbiota and Short Chain Fatty Acids in Broiler Chickens. Animals 2020, 10, 1429. https://doi.org/10.3390/ani10081429
Adewole D. Effect of Dietary Supplementation with Coarse or Extruded Oat Hulls on Growth Performance, Blood Biochemical Parameters, Ceca Microbiota and Short Chain Fatty Acids in Broiler Chickens. Animals. 2020; 10(8):1429. https://doi.org/10.3390/ani10081429
Chicago/Turabian StyleAdewole, Deborah. 2020. "Effect of Dietary Supplementation with Coarse or Extruded Oat Hulls on Growth Performance, Blood Biochemical Parameters, Ceca Microbiota and Short Chain Fatty Acids in Broiler Chickens" Animals 10, no. 8: 1429. https://doi.org/10.3390/ani10081429
APA StyleAdewole, D. (2020). Effect of Dietary Supplementation with Coarse or Extruded Oat Hulls on Growth Performance, Blood Biochemical Parameters, Ceca Microbiota and Short Chain Fatty Acids in Broiler Chickens. Animals, 10(8), 1429. https://doi.org/10.3390/ani10081429





