Assessment of DNA Methylation and Oxidative Changes in the Heart and Brain of Rats Receiving a High-Fat Diet Supplemented with Various Forms of Chromium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Forms of Chrome Used in the Experiment
2.2. Animals and Diets
2.3. Sample Collection and Analyses
2.4. Ex Vivo Analysis
2.4.1. Oxidative Status in the Plasma
2.4.2. Oxidative Status in the Brain and Heart
2.4.3. DNA Repair Enzymes in the Blood
2.4.4. Histological Examinations of the Brain
2.5. Statistical Analysis
3. Results
3.1. The Effect of a High-Fat Diet in Rats
3.2. The Effect of Different Forms of Cr in the Diet of Rats
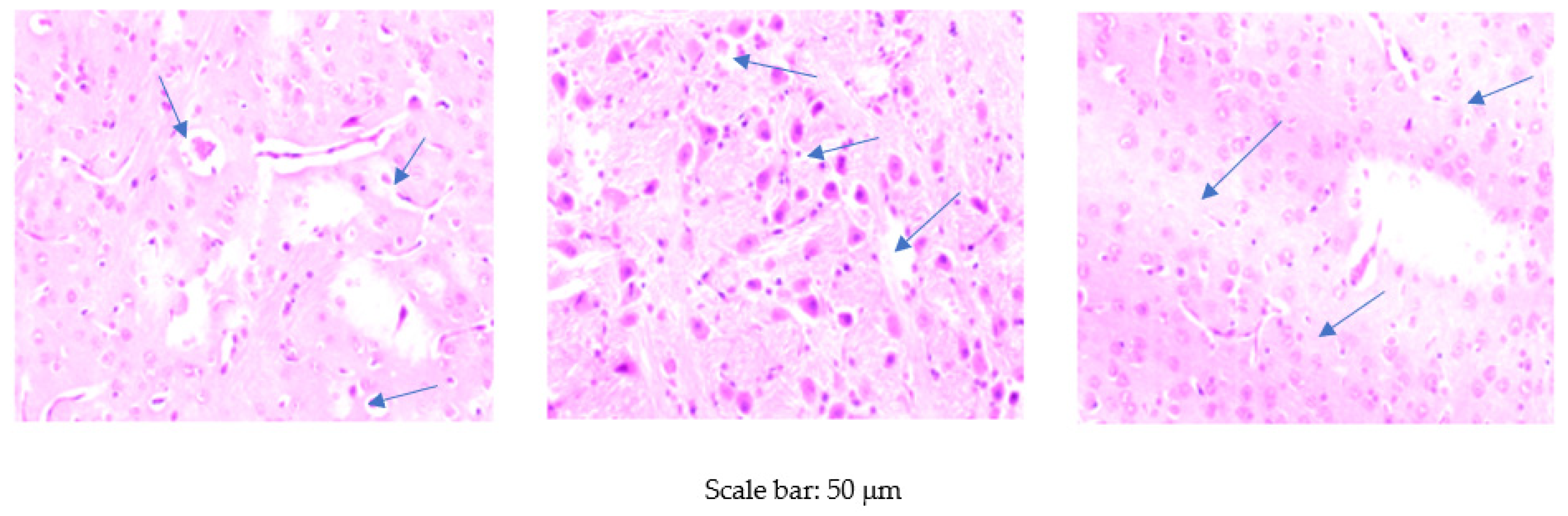
3.3. Histological Examination of the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thaker, V.V. Genetic and epigenetic causes of obesity. Adolesc. Med. State Art Rev. 2017, 28, 379–405. [Google Scholar] [PubMed]
- Haleem, D.J.; Mahmood, K. Brain serotonin in high-fat diet-induced weight gain, anxiety and spatial memory in rats. Nutr. Neurosci. 2019, 22, 1–10. [Google Scholar] [CrossRef]
- Leopoldo, A.S.; Sugizaki, M.M.; Lima-Leopoldo, A.P.; do Nascimento, A.F.; Luvizotto Rde, A.; de Campos, D.H.; Okoshi, K.; Dal Pai-Silva, M.; Padovani, C.R.; Cicogna, A.C. Cardiac remodeling in a rat model of diet-induced obesity. Can. J. Cardiol. 2010, 26, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Y.; Tang, J.; Guo, X.; Li, K.; Li, D. Dietary fat intake and risk of Alzheimer’s Disease and Dementia: A meta-analysis of cohort studies. Curr. Alzheimer Res. 2018, 15, 869–876. [Google Scholar] [CrossRef]
- Craft, S. The role of metabolic disorders in Alzheimer disease and vascular dementia: Two roads converged. Arch. Neurol. 2009, 66, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, F.; Dai, Y.; Han, L.; Chen, S. Serum TNF-α, GTH and MDA of high-fat diet-induced obesity and obesity resistant rats. Saudi Pharm. J. 2016, 24, 333–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pechova, A.; Pavlata, L. Chromium as an essential nutrient: A review. Vet. Med. (Praha) 2007, 52, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.Q.; Xu, Z.R. Effect of chromium nanoparticle on growth performance, carcass characteristics, pork quality and tissue chromium in finishing pigs. Asian-Aust. J. Anim. Sci. 2004, 17, 1118–1122. [Google Scholar] [CrossRef]
- Spears, J.W.; Whisnant, C.S.; Huntington, G.B.; Lloyd, K.E.; Fry, R.S.; Krafka, K.; Lamptey, A.; Hyda, J. Chromium propionate enhances insulin sensitivity in growing cattle. J. Dairy Sci. 2012, 95, 2037–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gültepe, E.E.; Uyarlar, C.; Bayram, I. Effects of dietary chromium on immune system. Kocatepe Vet. J. 2017, 10, 99–105. [Google Scholar]
- Krikorian, R.; Eliassen, J.; Boespflug, E.; Nash, T.; Shidler, M. Improved cognitive-cerebral function in older adults with chromium supplementation. Nutr. Neurosci. 2010, 13, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Chromium: Celebrating 50 years as an essential element? Dalton. Trans. 2010, 39, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.M.; Boohaker, J.G.; Sawyer, R.D.; Behling, J.E.; Rasco, J.F.; Jernigan, J.J.; Hood, R.D.; Vincent, J.B. Exposure of pregnant mice to chromium picolinate results in skeletal defects in their offspring. Birth Defects Res. B Dev. Reprod. Toxicol. 2006, 77, 244–249. [Google Scholar] [CrossRef]
- Bailey, M.M.; Boohaker, J.G.; Jernigan, P.L.; Townsend, M.B.; Sturdivant, J.; Rasco, J.F.; Vincent, J.B.; Hood, R.D. Effects of pre- and postnatal exposure to chromium picolinate or picolinic acid on neurological development in CD-1 mice. Biol. Trace Elem. Res. 2008, 124, 70–82. [Google Scholar] [CrossRef]
- Whittaker, P.; San, R.H.C.; Clarke, J.J.; Seifried, H.E.; Dunkel, V.C. Mutagenicity of chromium picolinate and its components in Salmonella typhimurium and L5178Y mouse lymphoma cells. Food. Chem. Toxicol. 2005, 43, 1619–1625. [Google Scholar] [CrossRef]
- Król, E.; Krejpcio, Z.; Okulicz, M.; Śmigielska, H. Chromium(III) glycinate complex supplementation improves the blood glucose level and attenuates the tissular copper to zinc ratio in rats with mild hyperglycaemia. Biol. Trace Elem. Res. 2020, 2193, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.Y.; Xiao, Q.G.; Xu, H.B.; Zhang, Y. Hypoglycemic activity and acute oral toxicity of chromium methionine complexes in mice. J. Trace. Elem. Med. Biol. 2014, 29, 136–144. [Google Scholar] [CrossRef]
- El-Nagar, S.H.; Helal, M.A.; Mahmoud, S.; Dillard, S.L. Comparative histopathological changes of liver, kidney and appendix of rabbits treated with inorganic nano chromium to ameliorate heat stress effect. Slov. Vet. Res. 2019, 56, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Gubajdullina, I.Z.; Gavrish, I.A.; Lebedev, S.V. Effect of metallic nanoparticles on exchange of chemical elements in broiler chickens. IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012169. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament and The Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. OJEU 2010, 276, 33–79. [Google Scholar]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838–841. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for chromium. EFSA J. 2014, 12, 3845. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, R.; Zhu, D.L.; Bi, Y.; Yang, D.H.; Wang, Y.P. Anti-oxidative effect of apocynin on insulin resistance in high-fat diet mice. Ann. Clin. Lab. Sci. 2011, 41, 236–243. [Google Scholar]
- Khairunnuur, F.A.; Zulkhairi, A.; Hairuszah, I.; Azrina, A.; Nursakinah, I.; Fazali, F.; Kamal, M.N.H.; Zamree, M.S.; Kamilah, K.A.K. Hypolipemic and weight reducing properties from Tamarindus indica L. pulp extract in diet-induced obese rats. Int. J. Pharm. 2010, 6, 216–223. [Google Scholar]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet-induced insulin resistance. Oxid. Med. Cell. Longev. 2018, 6, 6940515. [Google Scholar] [CrossRef] [Green Version]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Cell Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Fatima, R.; Akhtar, K.; Hossain, M.M.; Ahmad, R. Chromium oxide nanoparticle-induced biochemical and histopathological alterations in the kidneys and brain of Wistar rats. Toxicol. Ind. Health 2017, 33, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzan, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S. Mechanistic investigation of toxicity of chromium oxide nanoparticles in murine fibrosarcoma cells. Int. J. Nanomed. 2016, 11, 1253–1259. [Google Scholar]
- Lewicki, S.; Zdanowski, R.; Krzyżowska, M.; Lewicka, A.; Dębski, B.; Niemcewicz, M.; Goniewicz, M. The role of Chromium III in the organism and its possible use in diabetes and obesity treatment. Ann. Agric. Environ. Med. 2014, 21, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Driessen, M.D.; Mues, S.; Vennemann, A.; Hellack, B.; Bannuscher, A.; Vimalakanthan, V.; Riebeling, C.; Ossig, R.; Wiemann, M.; Schnekenburger, J.A.; et al. Proteomic analysis of protein carbonylation: A useful tool to unravel nanoparticle toxicity mechanisms. Part. Fibre Toxicol. 2015, 12, 36. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid Redox. Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative stress, amyloid-β Peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [Green Version]
- Djuric, Z.; Lewis, S.M.; Lu, M.H.; Mayhugh, M.; Tang, N.; Hart, R.W. Effect of varying dietary fat levels on rat growth and oxidative DNA damage. Nutr. Cancer 2001, 39, 214–219. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair (Amst) 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keleher, M.R.; Zaidi, R.; Hicks, L.; Shah, S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J.M. A high-fat diet alters genome-wide DNA methylation and gene expression in SM/J mice. BMC Genom. 2018, 19, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccarone, F.; Castelli, S.; Ioannilli, L.; Ciriolo, M.R. High dietary fat intake affects DNA methylation/hydroxymethylation in mouse heart: Epigenetic hints for obesity-related cardiac dysfunction. Mol. Nutr. Food. Res. 2019, 63, e1800970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim. Nutr. 2015, 1, 144–151. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in human obesity and type 2 diabetes. Cell. Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [Green Version]
- Unnikrishnan, A.; Freeman, W.M.; Jackson, J.; Wren, J.D.; Porter, H.; Richardson, A. The role of DNA methylation in epigenetics of aging. Pharm. Ther. 2018, 195, 172–185. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [Green Version]
- Pei, D.; Jia, P.; Luo, J.; Liu, W.; Strauss, P.R. AP endonuclease 1 (Apex1) influences brain development linking oxidative stress and DNA repair. Cell. Death Dis. 2019, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Sarkar, B.; Cholia, R.; Gautam, N.; Dhiman, M.; Mantha, A.K. APE1/Ref-1 as an emerging therapeutic target for various human diseases: Phytochemical modulation of its functions. Exp. Mol. Med. 2014, 46, e106. [Google Scholar] [CrossRef]
- Ding, G.; Chen, Y.; Pan, H.; Qiu, H.; Tang, W.; Chen, S. Association between apurinic/apyrimidinic endonuclease 1 rs1760944 T > G polymorphism and susceptibility of cancer: A meta-analysis involving 21764 subjects. Biosci. Rep. 2019, 39, BSR20190866. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA damage, and development of obesity-related diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Lillis, A.P.; Van Duyn, L.B.; Murphy-Ullrich, J.E.; Strickland, D.K. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008, 88, 887–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Han, D.; Shi, M.; Sun, Z.; Xie, J.; Zhu, J.; Liu, Y. Effects of high-fat diet on the morphological characteristics of cerebral microvasculature without hyperlipidemia in Wistar rat. Int. J. Clin. Exp. Pathol. 2016, 9, 11752–11759. [Google Scholar]
- Song, J.; Kang, S.M.; Kim, E.; Kim, C.H.; Song, H.T.; Lee, J.E. Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: An in vivo and in vitro study. Cell Death Dis. 2015, 6, e1844. [Google Scholar] [CrossRef]
| Ingredient/Group | S | F |
|---|---|---|
| Casein a | 20.0 | 20.0 |
| DL-methionine | 0.3 | 0.3 |
| Celullose b | 5.0 | 3.00 |
| Sucrose | 10.0 | 10.0 |
| Rapeseed oil | 8.0 | 8.0 |
| Lard | - | 17.0 |
| Vitamin mixture c | 1.0 | 1.0 |
| Mineral mixture d | 3.5 | 3.5 |
| Choline chlorid e | 0.2 | 0.2 |
| Cholesterol | 0.3 | 0.3 |
| Corn starch f | 51.7 | 36.7 |
| Heart | Brain | |||||||
|---|---|---|---|---|---|---|---|---|
| DNA Methylation, % | PC nmoL/mg Protein | 8-OHdG ng/g Tissue | MDA μmoL/kg Tissue | DNA Methylation, % | PC nmoL/mg Protein | 8-OHdG ng/g Tissue | MDA μmoL/kg Tissue | |
| Diet (D) | ||||||||
| S | 4.985 ± 0.052 b | 1.013 ± 0.012 | 1.919 ± 0.047 | 4.112 ± 0.046 b | 8.164 ± 0.068 b | 1.606 ± 0.059 | 1.727 ± 0.061 | 5.501 ± 0.049 b |
| F | 5.123 ± 0.051 a | 1.083 ± 0.013 | 1.970 ± 0.048 | 4.384 ± 0.052 a | 8.796 ± 0.063 a | 1.609 ± 0.063 | 1.792 ± 0.057 | 8.183 ± 0.058 a |
| Cr source (Cr) | ||||||||
| Without | 5.0085 ± 0.056 | 0.9825 ± 0.007 | 1.867 ± 0.036 b | 4.091 ± 0.055 b | 8.329 ± 0.071 b | 1.569 ± 0.059 | 1.661 ± 0.067 b | 6.780 ± 0.057 b |
| Cr-Pic | 5.1245 ± 0.058 | 1.0785 ± 0.013 | 1.937 ± 0.041 a,b | 4.264 ± 0.049 a,b | 8.368 ± 0.069 b | 1.630 ± 0.062 | 1.838 ± 0.063 a | 6.845 ± 0.052 b |
| Cr-Met | 5.0155 ± 0.046 | 1.0825 ± 0.015 | 1.835 ± 0.031 b | 4.194 ± 0.052 a,b | 8.519 ± 0.068 a,b | 1.603 ± 0.058 | 1.678 ± 0.073 b | 6.677 ± 0.053 b |
| Cr-NPs | 5.0685 ± 0.051 | 1.0485 ± 0.014 | 2.138 ± 0.036 a | 4.443 ± 0.054 ± a | 8.705 ± 0.070 a | 1.629 ± 0.066 | 1.861 ± 0.059 a | 7.067 ± 0.059 a |
| p-Value | ||||||||
| D effect | 0.013 | 0.874 | 0.463 | 0.048 | 0.045 | 0.239 | 0.147 | <0.001 |
| Cr effect | 0.158 | 0.269 | 0.047 | 0.014 | 0.009 | 0.057 | 0.034 | <0.001 |
| D × Cr interaction | 0.158 | 0.587 | 0.355 | 0.478 | 0.884 | 0.043 | 0.034 | 0.962 |
| DNA Methylation, % | PC nmoL/mg | 8-OHdG ng/mL | MDA µmol/mL | Beta-Amyloid pg/mL | |
|---|---|---|---|---|---|
| Diet (D) | |||||
| S | 23.65 ± 1.93 | 1.536 ± 0.023 | 9.871 ± 0.097 b | 1.113 ± 0.036 b | 6099.2 ± 27.46 |
| F | 25.04 ± 1.97 | 1.542 ± 0.021 | 11.07 ± 0.094 a | 2.405 ± 0.042 a | 5949.7 ± 28.92 |
| Cr source (Cr) | |||||
| Without | 23.93 ± 1.81 | 1.260 ± 0.017 b | 9.767 ± 0.087 b | 1.754 ± 0.046 a,b | 6689.6 ± 31.76 a |
| Cr-Pic | 24.45 ± 1.90 | 1.382 ± 0.0122 b | 11.53 ± 1.008 a | 1.525 ± 0.041 b | 6425.2 ± 29.64 a |
| Cr-Met | 25.22 ± 1.87 | 1.376 ± 0.022 b | 10.60 ± 0.095 a,b | 1.774 ± 0.051 b | 5540.6 ± 27.57 b |
| Cr-NPs | 23.78 ± 1.73 | 2.138 ± 0.019 a | 9.985 ± 0.085 b | 1.983 ± 0.057 a | 5442.6 ± 26.88 b |
| p-Value | |||||
| D effect | 0.064 | 0.369 | 0.027 | 0.006 | 0.078 |
| Cr effect | 0.564 | 0.038 | 0.044 | 0.029 | 0.007 |
| D × Cr interaction | 0.987 | 0.052 | 0.392 | 0.477 | 0.039 |
| TDG ng/mL | APE-1 ng/mL | ANPG ng/mL | LRP-1 ng/mL | SOD U/g Hb | GPx U/g Hb | CAT U/g Hb | |
|---|---|---|---|---|---|---|---|
| Diet (D) | |||||||
| S | 12.96 ± 0.077 | 23.66 ± 0.099 a | 0.476 ± 0.009 b | 0.080 ± 0.005 | 49.39 ± 3.65 | 1939.8 ± 37.75 | 1886.9 ± 38.58 |
| F | 12.49 ± 0.072 | 19.13 ± 0.089 b | 0.589 ± 0.008 a | 0.079 ± 0.004 | 57.79 ± 3.86 | 2015.4 ± 39.65 | 1995.6 ± 35.97 |
| Cr source (Cr) | |||||||
| Without | 13.92 ± 0.078 b | 26.04 ± 1.061 a | 0.602 ± 0.007 a | 0.084 ± 0.005 a | 48.03 ± 4.01 c | 1902.3 ± 35.62 b | 1972.5 ± 32.63 a,b |
| Cr-Pic | 14.53 ± 0.074 a | 24.91 ± 1.024 b | 0.492 ± 0.009 b | 0.075 ± 0.003 b | 48.92 ± 3.78 c | 1929.5 ± 35,93 b | 1842.8 ± 34.58 b |
| Cr-Met | 9.304 ± 0.077 c | 15.81 ± 0.092 c | 0.507 ± 0.008 b | 0.077 ± 0.006 b | 57.05 ± 3.99 b | 1954.1 ± 37.765 b | 1908.8 ± 38.92 a,b |
| Cr-NPs | 13.13 ± 0.069 b | 18.82 ± 0.094 c | 0.529 ± 0.008 a,b | 0.083 ± 0.006 a | 60.36 ± 4.21 a | 2124.6 ± 39.68 a | 2041.0 ± 39.48 a |
| p-Value | |||||||
| D effect | 0.124 | 0.029 | 0.044 | 0.369 | 0.067 | 0.097 | 0.758 |
| Cr effect | <0.001 | <0.001 | 0.002 | 0.007 | <0.001 | 0.049 | 0.033 |
| D × Cr interaction | 0.028 | 0.019 | 0.052 | 0.067 | 0.092 | 0.074 | 0.064 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dworzański, W.; Cholewińska, E.; Fotschki, B.; Juśkiewicz, J.; Listos, P.; Ognik, K. Assessment of DNA Methylation and Oxidative Changes in the Heart and Brain of Rats Receiving a High-Fat Diet Supplemented with Various Forms of Chromium. Animals 2020, 10, 1470. https://doi.org/10.3390/ani10091470
Dworzański W, Cholewińska E, Fotschki B, Juśkiewicz J, Listos P, Ognik K. Assessment of DNA Methylation and Oxidative Changes in the Heart and Brain of Rats Receiving a High-Fat Diet Supplemented with Various Forms of Chromium. Animals. 2020; 10(9):1470. https://doi.org/10.3390/ani10091470
Chicago/Turabian StyleDworzański, Wojciech, Ewelina Cholewińska, Bartosz Fotschki, Jerzy Juśkiewicz, Piotr Listos, and Katarzyna Ognik. 2020. "Assessment of DNA Methylation and Oxidative Changes in the Heart and Brain of Rats Receiving a High-Fat Diet Supplemented with Various Forms of Chromium" Animals 10, no. 9: 1470. https://doi.org/10.3390/ani10091470
APA StyleDworzański, W., Cholewińska, E., Fotschki, B., Juśkiewicz, J., Listos, P., & Ognik, K. (2020). Assessment of DNA Methylation and Oxidative Changes in the Heart and Brain of Rats Receiving a High-Fat Diet Supplemented with Various Forms of Chromium. Animals, 10(9), 1470. https://doi.org/10.3390/ani10091470





