Bioavailability of Methionine-Coated Zinc Nanoparticles as a Dietary Supplement Leads to Improved Performance and Bone Strength in Broiler Chicken Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
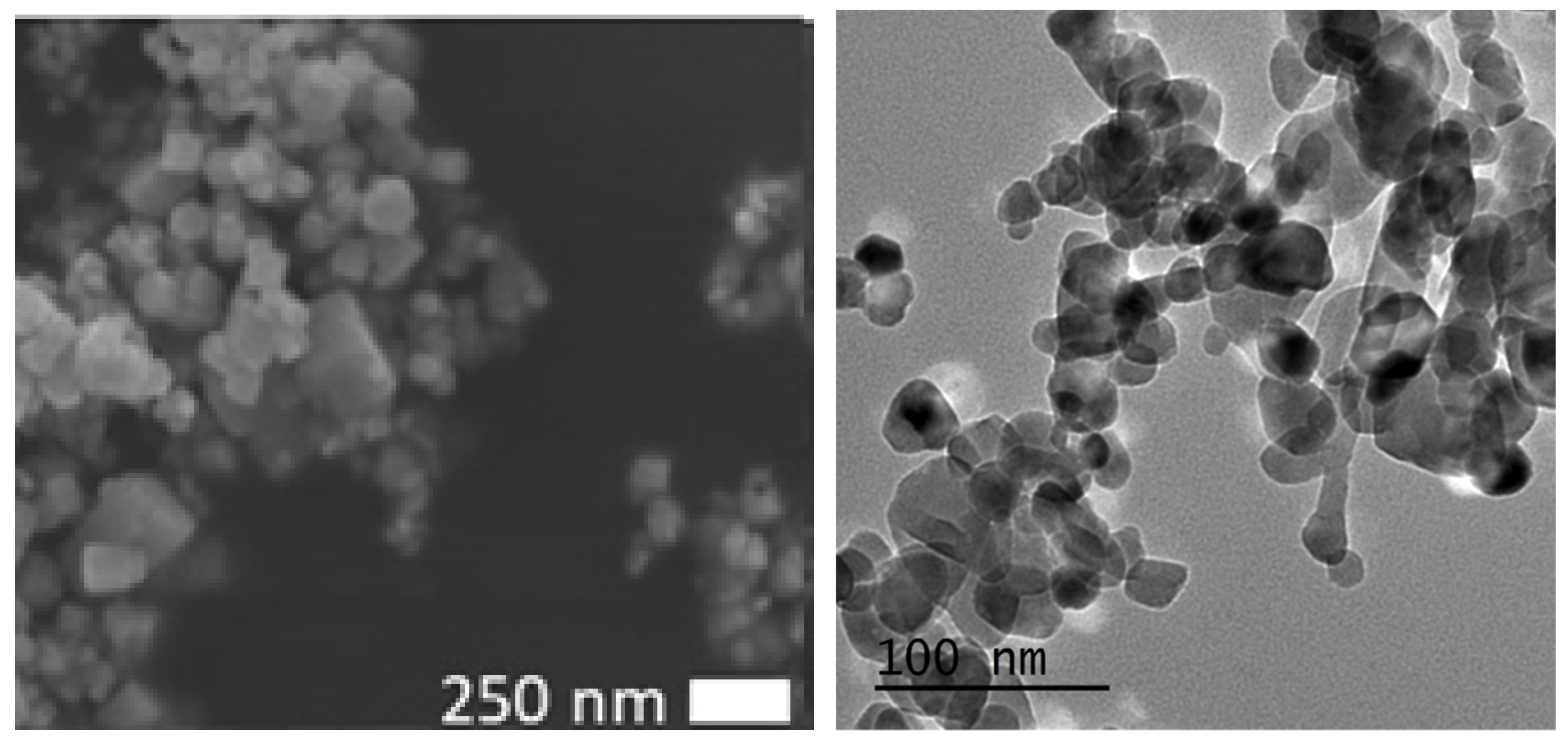
2.1. Zinc Oxide Nanoparticle Preparation
2.2. Nanoparticle Size Determination
2.3. Dietary Treatments
2.4. Birds And Husbandry
2.5. Diet Analysis
2.6. Blood And Tissue Zinc Measures
2.7. Tibia Measurements
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin Win, K.; Feng, S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Rajendran, D. Application of nano minerals in animal production system. Res. J. Biotechnol. 2013, 1, 517–530. [Google Scholar]
- Li, M.; Huang, J.; Tsai, Y.; Mao, S.; Fu, C.; Lien, T. Nanosize of zinc oxide and the effects on zinc digestibility, growth performances, immune response and serum parameters of weanling piglets. Anim. Sci. J. 2016, 87, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Amde, M.; Liu, J.; Tan, Z.; Bekana, D. Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environ. Pollut. 2017, 230, 250–267. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.; Alagawany, M.; Arif, M.; Chaudhry, M.; Emam, M.; Patra, A. Organic or inorganic zinc in poultry nutrition: A review. Worlds Poult. Sci. J. 2017, 73, 904–915. [Google Scholar] [CrossRef]
- Batal, A.; Parr, T.; Baker, D. Zinc bioavailability in tetrabasic zinc chloride and the dietary zinc requirement of young chicks fed a soy concentrate diet. Poult. Sci. 2001, 80, 87–90. [Google Scholar] [CrossRef]
- Kwiecień, M.; Winiarska-Mieczan, A.; Milczarek, A.; Klebaniuk, R. Biological Response of Broiler Chickens to Decreasing Dietary Inclusion Levels of Zinc Glycine Chelate. Biol. Trace Elem. Res. 2017, 175, 204–213. [Google Scholar] [CrossRef]
- Shao, Y.; Lei, Z.; Yuan, J.; Yang, Y.; Guo, Y.; Zhang, B. Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica serovar typhimurium. J. Microbiol. 2014, 52, 1002–1011. [Google Scholar] [CrossRef]
- Morgan, N.; Scholey, D.; Burton, E. Use of Zn concentration in the gastrointestinal tract as a measure of phytate susceptibility to the effect of phytase supplementation in broilers. Poult. Sci. 2017, 96, 1298–1305. [Google Scholar] [CrossRef]
- O’Dell, B.; Yohe, J.; Savage, J. Zinc availability in the chick as affected by phytate, calcium and ethylenediaminetetraacetate. Poult. Sci. 1964, 43, 415–419. [Google Scholar] [CrossRef]
- Nielsen, F. History of Zinc in Agriculture. Adv. Nutr. 2012, 3, 783–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Scientific opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J. 2014, 12, 1–77. [CrossRef] [Green Version]
- EFSA Scientific opinion on principles for deriving and applying dietary reference values. EFSA J. 2010, 8, 1–30. [CrossRef] [Green Version]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Idris, M.; Khalique, M.; Zia-Ur-Ra, Z.; Alhidary, I.; Abdelrahman, M.; Khan, R.; Chand, N.; Farooq, U.; Ahmad, S. The activity and use of zinc in poultry diets. Worlds Poult. Sci. J. 2016, 72, 159–167. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Koreleski, J.; Zhong, D. The bioavailability of zinc from inorganic and organic sources in broiler chickens as affected by addition of phytase. J. Anim. Feed Sci. 2001, 10, 317–328. [Google Scholar] [CrossRef]
- De Grande, A.; Leleu, S.; Delezie, E.; Rapp, C.; De Smet, S.; Goossens, E.; Haesebrouck, F.; Van Immerseel, F.; Ducatelle, R. Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult. Sci. 2020, 99, 441–453. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2002; ISBN 0070494398/9780070494398. [Google Scholar]
- Hanafy, B.; Cave, G.; Barnett, Y.; Pierscionek, B. Treatment of human lens epithelium with high levels of nanoceria leads to reactive oxygen species mediated apoptosis. Molecules 2020, 25, 441. [Google Scholar] [CrossRef] [Green Version]
- Cave, G.; Mudell, V. Coating Metal Oxide Particles. Available online: https://patents.google.com/patent/US10154628B2/en (accessed on 7 May 2020).
- Chang, M.; Liu, H.; Tai, C. Preparation of copper oxide nanoparticles and its application in nanofluid. Powder Technol. 2011, 207, 378–386. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Prasad, S.; Gambhir, I.S. Nanotechnology in Medicine and Antibacterial Effect of Silver Nanoparticles. Dig. J. Nanomater. Biostruct. 2008, 3, 115–122. [Google Scholar]
- Jo, Y.-K.; Kim, B.H.; Jung, G. Antifungal Activity of Silver Ions and Nanoparticles on Phytopathogenic Fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parak, W.J.; Simmel, F.C.; Holleitner, A.W. Top-Down Versus Bottom-Up. In Nanotechnology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; Volume 46, pp. 41–71. [Google Scholar]
- Kilkenny, C.; Browne, W.; Cuthill, I.; Emerson, M.; Altman, D. Animal research: Reporting in vivo experiments: The arrive guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Short, F.; Gorton, P.; Wiseman, J.; Boorman, K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Morgan, N.; Scholey, D.; Burton, E. A comparison of two methods of determining titanium dioxide marker content in broiler digestibility studies. Animal 2014, 8, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Scholey, D.; Burton, E.; Morgan, N.; Sanni, C.; Madsen, C.; Dionisio, G.; Brinch-Pedersen, H. P and Ca digestibility is increased in broiler diets supplemented with the high-phytase HIGHPHY wheat. Animal 2017, 11, 1457–1463. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- UK-Gov. A Month in UK Climate Statistics. Available online: https://www.metoffice.gov.uk/about-us/press-office/news/weather-and-climate/2019/july-statistics (accessed on 13 July 2020).
- Syafwan, S.; Kwakkel, R.; Verstegen, M. Heat stress and feeding strategies in meat-type chickens. Worlds Poult. Sci. J. 2011, 67, 653–674. [Google Scholar] [CrossRef] [Green Version]
- Salim, H.; Jo, C.; Lee, B. Zinc in broiler feeding and nutrition. Avian Biol. Res. 2008, 1, 5–18. [Google Scholar] [CrossRef]
- Bartlett, J.; Smith, M. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 2003, 82, 1580–1588. [Google Scholar] [CrossRef]
- Tsai, Y.; Mao, S.; Li, M.; Huang, J.; Lien, T. Effects of nanosize zinc oxide on zinc retention, eggshell quality, immune response and serum parameters of aged laying hens. Anim. Feed Sci. Technol. 2016, 213, 99–107. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, H.; Jalal, M.; AL-Titi, H.; Souad, A. Effect of Sources and Levels of Dietary Zinc on the Performance, Carcass Traits and Blood Parameters of Broilers. Rev. Bras. Ciência Avícola 2017, 19, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; Alagawany, M.; Salah, A.S.; Abdel-Latif, M.A.; Farghly, M.F.A. Effects of Dietary Supplementation of Zinc Oxide and Zinc Methionine on Layer Performance, Egg Quality, and Blood Serum Indices. Biol. Trace Elem. Res. 2018, 184, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yamaguchi, R. Action of zinc on bone metabolism in rats. Biochem. Pharmacol. 1986, 35, 773–777. [Google Scholar] [CrossRef]
- Mendieta-Araica, B.; Spörndly, E.; Reyes-Sánchez, N.; Salmerón-Miranda, F.; Halling, M. Biomass production and chemical composition of Moringa oleifera under different planting densities and levels of nitrogen fertilization. Agrofor. Syst. 2013, 87, 81–92. [Google Scholar] [CrossRef]
- Seo, H.; Cho, Y.; Kim, T.; Shin, H.; Kwun, I. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Choct, M.; Iji, P.; Bruerton, K. Optimal dietary inclusion of organically complexed zinc for broiler chickens. Br. Poult. Sci. 2009, 50, 95–102. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kwiecień, M.; Winiarska-Mieczan, A.; Świetlicka, I.; Wawrzyniak, A. Effect of zinc level and source (zinc oxide vs. zinc glycine) on bone mechanical and geometric parameters, and histomorphology in male Ross 308 broiler chicken. Rev. Bras. Ciência Avícola 2017, 19, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Ohki, K. Zinc nutrition related to critical deficiency and toxicity levels for sorghum. Agron. J. 1984, 76, 253–256. [Google Scholar] [CrossRef]
- Giordano, P.; Mortvedt, J.; Mays, D. Effect of municipal wastes on crop yields and uptake of heavy metals. J. Environ. Qual. 1975, 4, 394–399. [Google Scholar] [CrossRef]
- Lapkin, A.; Plucinski, P. Engineering Factors for Efficient Flow Processes in Chemical Industries. In Chemical Reactions and Processes Under Flow Conditions; Luis, S., García-Verdugo, E., Eds.; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 18–23. [Google Scholar]
- Davies, K. Biofortification of Potato (Solanum tuberosum) Using Metal Oxide Nanoparticles. Ph.D. Thesis, Nottingham Trent University, Nottingham, UK, 2018. Available online: http://irep.ntu.ac.uk/ (accessed on 6 August 2020).
- EU. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32015R2283 (accessed on 6 August 2020).
- FDA. Guidance for Industry #220: Use of Nanomaterials in Food for Animals. Available online: https://www.fda.gov/media/88828/download (accessed on 6 August 2020).
- Peters, R.; Brandhoff, P.; Weigel, S.; Marvin, H.; Bouwmeester, H.; Aschberger, K.; Rauscher, H.; Amenta, V.; Arena, M.; Botelho Moniz, F.; et al. Inventory of Nanotechnology applications in the agricultural, feed and food sector. EFSA Support. Publ. 2014, 11, 621E. [Google Scholar] [CrossRef]
- EFSA. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, 4501. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Public Consultation on the Draft EFSA Guidance on Technical Requirements for Regulated Food and Feed Product Applications to Establish the Presence of Small Particles Including Nanoparticles. Available online: https://www.efsa.europa.eu/sites/default/files/consultation/consultation/Draft-Nano-Technical-Guidance-For-Public-Consultation.pdf (accessed on 6 August 2020).
| Starter | Grower | |
|---|---|---|
| Ingredient composition (g/Kg) | ||
| Wheat (10.5% protein) | 598.09 | 646.21 |
| Extracted Soya, (48% protein) | 336.79 | 283.04 |
| Soya oil | 32.50 | 41.82 |
| Limestone | 11.20 | 9.49 |
| Salt | 2.02 | 2.26 |
| Sodium bicarbonate | 1.80 | 1.52 |
| Monocalcium phosphate, HCl | 6.48 | 6.50 |
| Lysine HCl | 2.39 | 1.48 |
| Methionine | 3.11 | 2.41 |
| Threonine | 1.42 | 1.07 |
| Econase XT 25 powder | 0.10 | 0.10 |
| Quantum Blue phytase | 0.10 | 0.10 |
| Zinc-free vitamin/mineral premix * | 4.00 | 4.00 |
| Analysed chemical composition | ||
| Dry matter (g/Kg DM) | 874 | 878 |
| Ash (g/Kg DM) | 48.3 | 52.6 |
| Protein (g/Kg DM) | 25.0 | 21.6 |
| Fat (g/Kg DM) | 35.7 | 60.75 |
| P (g/Kg DM) | 7.81 | 5.13 |
| Ca (g/Kg DM) | 9.52 | 8.13 |
| Gross energy (MJ/Kg DM) | 15.9 | 16 |
| Control | M-Zn 1 | Nano-ZnO 1 | M-Nano-ZnO 1 | SEM 2 | p Value | |
|---|---|---|---|---|---|---|
| Zn COD 3 d21 | 0.635 b,c | 0.685 a,b | 0.607 c | 0.702 a | 0.021 | 0.003 |
| Zn digested d21 (g/kg) | 52.7 c | 67.8 a | 44.3 d | 63.0 b | 1.83 | <0.001 |
| Zn COD d35 | 0.662 a,b | 0.632 b,c | 0.596 c | 0.691 a | 0.018 | 0.003 |
| Zn digested d35 (g/kg) | 53.6 a | 49.8 a | 35.2 b | 51.1 a | 1.40 | <0.001 |
| Control | M-Zn 1 | Nano-ZnO 1 | M-Nano-ZnO 1 | SEM 2 | p Value | |
|---|---|---|---|---|---|---|
| Starter (d0–21) | ||||||
| Bird weight gain (g) | 846 c | 900 b | 914 b | 954 a | 14.5 | <0.001 |
| Feed intake (g) | 1156 | 1182 | 1205 | 1214 | 18.2 | 0.071 |
| Feed conversion ratio | 1.35 | 1.32 | 1.33 | 1.29 | 0.024 | 0.375 |
| Grower (d21–35) | ||||||
| Bird weight gain (g) | 1341 | 1343 | 1372 | 1397 | 24.3 | 0.49 |
| Feed intake (g) | 2060 | 2076 | 2124 | 2182 | 29.6 | 0.068 |
| Feed conversion ratio | 1.53 | 1.55 | 1.55 | 1.56 | 0.019 | 0.609 |
| Overall (d0–35) | ||||||
| Bird weight d35 (g) | 2232 b | 2282 a,b | 2324 a | 2363 a | 33.1 | 0.044 |
| Bird weight gain (g) | 2193 b | 2244 a,b | 2286 a | 2325 a | 32.8 | 0.043 |
| Feed intake (g) | 3237 b | 3259 b | 3312 b | 3442 a | 40.7 | 0.006 |
| Feed conversion ratio | 1.45 | 1.45 | 1.46 | 1.45 | 0.013 | 0.825 |
| Control | M-Zn 1 | Nano-ZnO 1 | M-Nano-ZnO 1 | SEM 2 | p Value | |
|---|---|---|---|---|---|---|
| Day 21 | ||||||
| Tibia length (mm) | 72.3 b | 73.5 a | 72.6 b | 74.0 a | 0.31 | <0.001 |
| Tibia weight (g) | 7.18 c | 7.77 a | 7.33 b,c | 7.58 a,b | 0.12 | 0.004 |
| Tibia width (mm) | 5.46 b | 5.74 a | 5.45 b | 5.60 a,b | 0.06 | 0.001 |
| Tibia strength (N) | 181.6 b | 197.7 a | 178.5 b | 188.2 a,b | 3.77 | 0.001 |
| Day 35 | ||||||
| Tibia length (mm) | 98.3 c | 99.1 b,c | 100.5 a | 99.4 a,b | 0.43 | 0.001 |
| Tibia weight (g) | 17.79 | 17.73 | 18.15 | 18.12 | 0.2 | 0.365 |
| Tibia width (mm) | 7.44 | 7.47 | 7.47 | 7.48 | 0.06 | 0.992 |
| Tibia strength (N) | 284.2 | 282.1 | 298 | 285.2 | 5.01 | 0.084 |
| Control | M-Zn 1 | Nano-ZnO 1 | M-Nano-ZnO 1 | SEM 2 | p Value | |
|---|---|---|---|---|---|---|
| Serum Zn d21 | 76.4 | 73.3 | 76.8 | 94.0 | 8.73 | 0.404 |
| Serum Zn d35 | 90.9 | 106.7 | 92.3 | 112.7 | 8.88 | 0.067 |
| Liver d21 | 99.9 | 112.7 | 108.5 | 121.4 | 10.16 | 0.133 |
| Liver d35 | 82.0 | 83.5 | 81.7 | 93.6 | 4.06 | 0.173 |
| Spleen d21 | 92.5 | 90.6 | 92.8 | 93.4 | 6.23 | 0.995 |
| Spleen d35 | 93.3 | 94.5 | 92.6 | 91.8 | 4.00 | 0.958 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhtib, A.; Scholey, D.; Carter, N.; Cave, G.W.V.; Hanafy, B.I.; Kempster, S.R.J.; Mekapothula, S.; Roxborough, E.T.; Burton, E.J. Bioavailability of Methionine-Coated Zinc Nanoparticles as a Dietary Supplement Leads to Improved Performance and Bone Strength in Broiler Chicken Production. Animals 2020, 10, 1482. https://doi.org/10.3390/ani10091482
Alkhtib A, Scholey D, Carter N, Cave GWV, Hanafy BI, Kempster SRJ, Mekapothula S, Roxborough ET, Burton EJ. Bioavailability of Methionine-Coated Zinc Nanoparticles as a Dietary Supplement Leads to Improved Performance and Bone Strength in Broiler Chicken Production. Animals. 2020; 10(9):1482. https://doi.org/10.3390/ani10091482
Chicago/Turabian StyleAlkhtib, Ashraf, Dawn Scholey, Nicholas Carter, Gareth W.V. Cave, Belal I. Hanafy, Siani R.J. Kempster, Subbareddy Mekapothula, Eve T. Roxborough, and Emily J. Burton. 2020. "Bioavailability of Methionine-Coated Zinc Nanoparticles as a Dietary Supplement Leads to Improved Performance and Bone Strength in Broiler Chicken Production" Animals 10, no. 9: 1482. https://doi.org/10.3390/ani10091482






