Udder Morphometry and Its Relationship with Intramammary Infections and Somatic Cell Count in Serrana Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Management Conditions
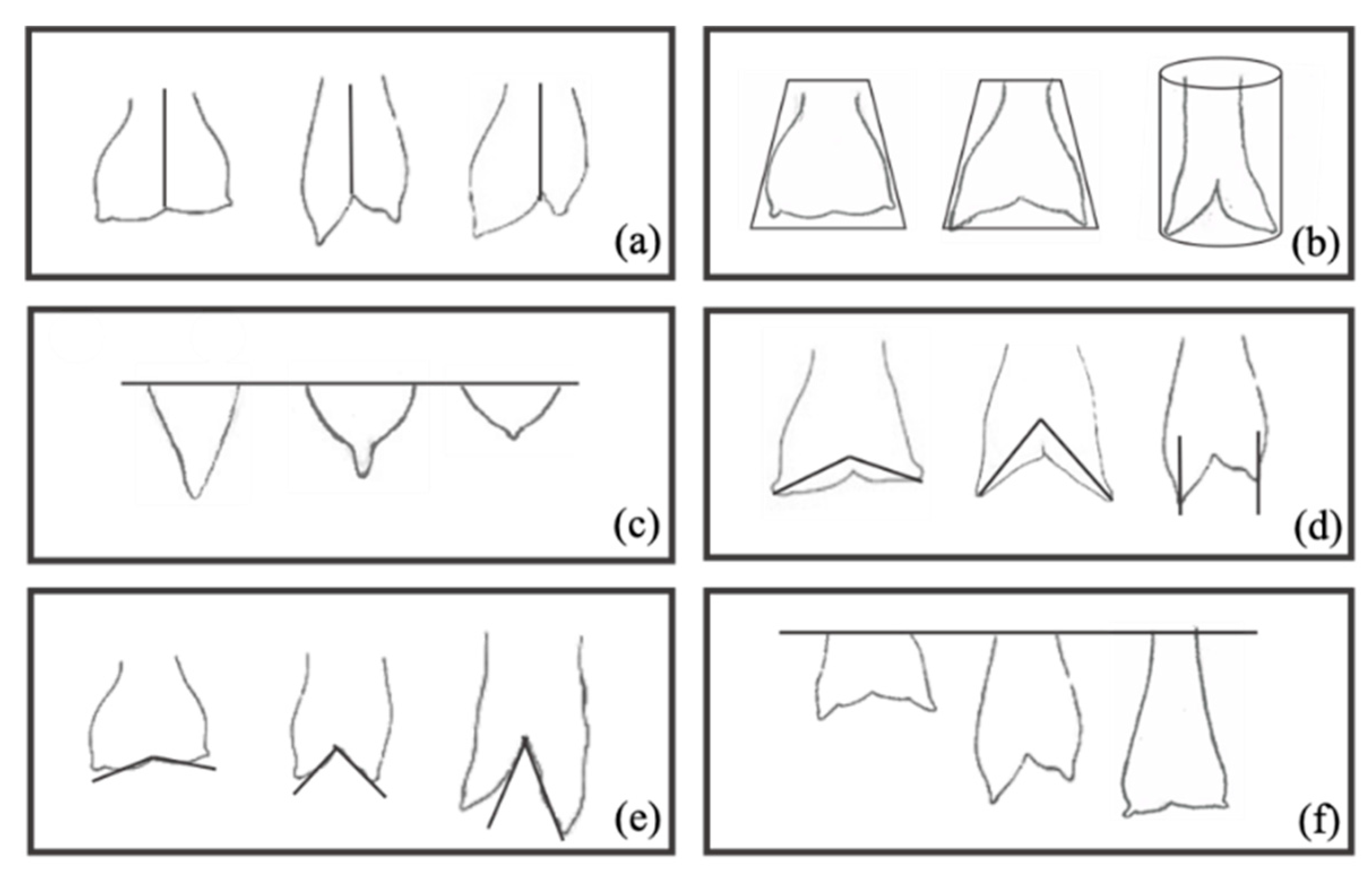
2.2. Udder Conformation
2.3. Milk Sampling, Somatic Cell Counts and Microbial Identification
2.4. Statistical Analysis
3. Results
3.1. Udder Conformation
3.2. Relationships Between Udder Traits and Intramammary Infections
3.3. Relationships Between Udder Traits and Somatic Cells
4. Discussion
4.1. Udder Conformation
4.2. Relationships Between Udder Traits and Intramammary Infections
4.3. Relationships Between Udder Traits and Somatic Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Margatho, G.; Rodríguez-Estévez, V.; Quintas, H.; Simões, J. The Effects of Reproductive Disorders, Parity and Litter Size on Milk Yield of Serrana Goats. Animals 2019, 9, 968. [Google Scholar] [CrossRef] [Green Version]
- SPOC (Sociedade Portuguesa de Ovinotecnia e Caprinotecnia). Available online: http://www.ovinosecaprinos.com/serrana.html (accessed on 19 August 2020).
- Horak, F.; Kasing, J. An evaluation of the morphological properties of the udders of goats. Ziv. Vyrob. 1970, 15, 205–216. [Google Scholar]
- Mavrogenis, A.P.; Papachristoforou, C.; Lysandrides, P.; Roushias, A. Environmental and genetic effects on udder characteristics and milk production in Damascus goats. Small Rumin. Res. 1989, 2, 333–343. [Google Scholar] [CrossRef]
- Wang, P.Q. Udder characteristics in Toggenburg dairy goats. Small Rumin. Res. 1989, 2, 181–190. [Google Scholar] [CrossRef]
- Charon, K. Morphological characteristics of udders asselection criteria for improvement of mammarygland health and productivity of sheep.1. Variability and genetic parameters of uddermorphological traits. J. Anim. Feed Sci. 1993, 2, 105–116. [Google Scholar] [CrossRef]
- Szymanowska, A.; Patkowski, K.; Miduch, A.; Milerski, M. Correlation between mammary gland morphology and gland cistern size to lactation milk yield in goat. Ann. UMCS Zootech. 2010, 28. [Google Scholar] [CrossRef]
- Contreras, A.; Sierra, D.; Sánchez, A.; Corrales, J.C.; Marco, J.C.; Paape, M.J.; Gonzalo, C. Mastitis in small ruminants. Small Rumin. Res. 2007, 68, 145–153. [Google Scholar] [CrossRef]
- White, J.M.; Vinson, W.E. Relationships among udder characteristics, milk yield, and nonyield traits. J. Dairy Sci. 1975, 58, 729–738. [Google Scholar] [CrossRef]
- Jatsch, O.; Sagi, R. Machine milkability as related to dairy yield and its fractions in dairy ewes. Ann. Zootech. 1979, 28, 251–260. [Google Scholar] [CrossRef]
- De Cremoux, R.; Lagriffoul, G.; Allain, C.; Alaoui-Sossé, L.; Astruc, J.-M.; Batut, E.; Bergonier, D.; Brun-Lafleur, L.; Clément, V.; Couzy, C.; et al. Mamovicap-Vers des outils innovants d’intervention et d’aide à la décision pour la maîtrise des mammites en élevage de petits ruminants laitiers. Innov. Agron. 2018, 63, 99–114. [Google Scholar] [CrossRef]
- Schutz, M.M.; Hansen, L.B.; Steuernagel, G.R.; Reneau, J.K.; Kuck, A.L. Genetic Parameters for Somatic Cells, Protein, and Fat in Milk of Holsteins. J. Dairy Sci. 1990, 73, 494–502. [Google Scholar] [CrossRef]
- Santos, D.S.; Lima, M.G.B.; Noznica, C.F.; Lima, D.M.; Batista, C.F.; Gomes, R.C.; Bertagnon, H.G.; Santos, B.P.; Libera, A.M.M.P.D. Conformação de úbere de caprinos da raça Saanen: Parâmetros estéticos ou funcionais? Arq. Bras. Med. Vet. Zootec. 2015, 67, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Montaldo, H.; Martínez-Lozano, F.J. Phenotypic relationships between udder and milking characteristics, milk production and California mastitis test in goats. Small Rumin. Res. 1993, 12, 329–337. [Google Scholar] [CrossRef]
- Sagi, R.; Morag, M. Udder conformation, milk yield and milk fractionation in the dairy ewe. Ann. Zootech. 1974, 23, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Rovai, M.; Such, X.; Piedrafita, J.; Caja, G. Evolution of mammary morphology traits during lactation and its relationship with milk yield of Manchega and Lacaune dairy sheep. In Proceedings of the Milking and Milk Production of Dairy Sheep and Goats, Athens, Greece, 26 September–1 October 1998; Barillet, F., Zervas, N.P., Eds.; Wageningen Pers: Wageningen, The Netherlands, 1999; Volume 95, pp. 107–109. [Google Scholar]
- Epstein, H. The Awassi Sheep with Special Reference to the Improved Dairy Type; FAO: Rome, Italy, 1985. [Google Scholar]
- Casu, S.; Pernazza, I.; Carta, A. Feasibility of a Linear Scoring Method of Udder Morphology for the Selection Scheme of Sardinian Sheep. J. Dairy Sci. 2006, 89, 2200–2209. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, A.; Sierra, D.; Luengo, C.; Corrales, J.C.; Morales, C.T.; Contreras, A.; Gonzalo, C. Influence of Storage and Preservation on Fossomatic Cell Count and Composition of Goat Milk. J. Dairy Sci. 2005, 88, 3095–3100. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.; Paape, M.J.; Miller, R.H. Prevalence of subclinical intramammary infection caused by Staphylococcus epidermidis in a commercial dairy goat herd. Small Rumin. Res. 1999, 31, 203–208. [Google Scholar] [CrossRef]
- James, I.J.; Osinowo, O.A.; Adegbasa, O.I. Evaluation of udder traits of west african dwarf (wad) goats and sheep in Ogun state, Nigeria. J. Agric. Sci. Environ. 2009, 9, 75–87. [Google Scholar]
- Almendra, L. A cabra Serrana transmontana—Origem, caracterização da raça e sistemas de produção. Available online: http://www.ovinosecaprinos.com/ (accessed on 3 July 2020).
- Le Du, J.; Benmederbel, B. Aptitude des chevres de race Saanen a la traite mecanique. Relations avec les caracteristiques physiques du trayon (Machine milkability of Saanen breed. Relationships with teat physical traits). Ann. Zootech. 1984, 33, 375–384. [Google Scholar] [CrossRef]
- Mendonça, Á.; Nunes, M.A.; Valentim, R.; Correia, T.M.; Trigo, M.; Maurício, R.; Costa, C.; Coelho, A. Mastitis diagnosis in dairy goats through somatic cell counts and California mastitis test. Preliminary Results. In Proceedings of the Future of the Sheep and Goat Dairy Sectors; CIHEAM: Zaragoza, Spain, 2004. [Google Scholar]
- Crump, R.E.; Cooper, S.; Smith, E.M.; Grant, C.; Green, L.E. Heritability of phenotypic udder traits to improve resilience to mastitis in Texel ewes. Animal 2019, 13, 1570–1575. [Google Scholar] [CrossRef] [Green Version]
- Rupp, R.; Clément, V.; Piacere, A.; Robert-Granié, C.; Manfredi, E. Genetic parameters for milk somatic cell score and relationship with production and udder type traits in dairy Alpine and Saanen primiparous goats. J. Dairy Sci. 2011, 94, 3629–3634. [Google Scholar] [CrossRef] [Green Version]
- Mello, A.A.; Silva, E.R.; Vasconcellos, I.M.A. Udder morphometry in goats: Correlation between milk production, milking rate and mastitis incidence. Arq. Bras. Med. Vet. Zootec 1998, 50, 469–472. [Google Scholar]

| Mean ± SD | Range | |
|---|---|---|
| UP | 31.3 ± 9.1 | 16–54 |
| UC | 21.6 ± 8.0 | 6–38 |
| UD | 6.4 ± 4.0 | 3–15 |
| TP | 19.9 ± 7.2 | 5–38 |
| TL | 9.5 ± 3.1 | 5–20 |
| DBT | 11.7 ± 3.4 | 5–19 |
| TDG | 24.3 ± 5.8 | 11–36 |
| Ushape | 1.9 ± 0.8 | 1–3 |
| Symm | 0.7 ± 0.8 | 0–2 |
| Dsusp | 2 ± 0.8 | 1–3 |
| Dsep | 1.6 ± 0.8 | 1–3 |
| Tshape | 1.8 ± 0.9 | 1–3 |
| Tangle | 1.9 ± 0.6 | 1–3 |
| UP | UC | UD | TP | TL | DBT | TDG | |
|---|---|---|---|---|---|---|---|
| UP | 1.000 | 0.892 ** | −0.419 ** | 0.777 ** | 0.724 ** | 0.757 ** | −0.640 ** |
| UC | 0.892 ** | 1.000 | −0.390 ** | 0.746 ** | 0.714 ** | 0.674 ** | −0.613 ** |
| UD | −0.419 ** | −0.390 ** | 1.000 | −0.403 ** | −0.445 ** | −0.416 ** | 0.611 ** |
| TP | 0.777 ** | 0.746 ** | −0.403 ** | 1.000 | 0.773 ** | 0.638 ** | −0.660 ** |
| TL | 0.724 ** | 0.714 ** | −0.445 ** | 0.773 ** | 1.000 | 0.640 ** | −0.743 ** |
| DBT | 0.757 ** | 0.674 ** | −0.416 ** | 0.638 ** | 0.640 ** | 1.000 | −0.685 ** |
| TDG | −0.640 ** | −0.613 ** | 0.611 ** | −0.660 ** | −0.743 ** | −0.685 ** | 1.000 |
| Lact n° | NS | 0.290 * | −0.596 ** | 0.426 ** | 0.333 ** | NS | NS |
| Lact stage | 0.392 ** | 0.381 ** | NS | NS | NS | 0.562 ** | −0.268 * |
| Traits | Groups | ||||
|---|---|---|---|---|---|
| Negative (n = 9) | Yeasts (n = 11) | S. aureus (n = 12) | Streptococcus spp. (n = 12) | CNS (n = 11) | |
| UP | 30.7 ± 3.7 ab | 35.3 ± 6.7 a | 34.9 ± 10.5 a | 34.4 ± 9.5 a | 26.1 ± 5.6 b |
| UC | 20.4 ± 3.8 a | 24.9 ± 5.1 a | 25.4 ± 6.8 a | 26.1 ± 9.9 a | 14.4 ± 3.1b |
| TP | 19 ± 3.1 ab | 20.8 ± 5.8 a | 24.2 ± 7.5 a | 22.6 ± 8.4 a | 17.3 ± 6.4 b |
| DBT | 13.9 ± 3.9 a | 13 ± 2.6 a | 12.2 ± 2.6 a | 11.2 ± 3.2 ab | 9.4 ± 1.8 b |
| Ushape | 1.5 ± 0.8 a | 1.5 ± 0.5 a | 2.5 ± 0.7 b | 2 ± 0.9 ab | 1.9 ± 1.1 a |
| Symm | 0.6 ± 0.5 a | 0.09 ± 0.3 b | 0.9 ± 0.8 a | 1.1 ± 1 a | 0.6 ± 0.5 ab |
| Dsep | 1.3 ± 0.5 ac | 1 ± 0 a | 2.1 ± 0.8 b | 1.9 ± 0.7 c | 1.7 ± 1 ab |
| Tshape | 1.1 ± 0.4 a | 1.7 ± 1.0 a | 1.8 ± 0.6 a | 2.2 ± 0.9 b | 2.4 ± 1 c |
| SCC | 1218.4 ± 837.2 ab | 610.2 ± 259.1 a | 4477.5 ± 3605.2 c | 4984.9 ± 6680.9 cb | 11,322.3 ± 14,866.3 cb |
| (×103SCC/mL) | <1300 | >1300 and <6000 | >6000 |
|---|---|---|---|
| UP | 33.48 ± 6.56 a | 28.43 ± 6.2 b | 37.6 ± 11.3 a |
| UD | 7.5 ± 3.6 a | 6.6 ± 2.9 ab | 3.6 ± 4.5 b |
| Ushape | 1.4 ± 0.5 a | 2.2 ± 0.9 b | 2.5 ± 0.8 b |
| Sym | 0.2 ± 0.4 a | 1.2 ± 0.7 b | 1.2 ± 0.9 b |
| Dsusp | 1.7 ± 0.6 a | 2.2 ± 0.7 b | 2.4 ± 0.7 b |
| Dsep | 1.1 ± 0.3 a | 2.1 ± 0.6 b | 2 ± 0.9 b |
| Parameters | Profiles | % (n = 60) | Mean ± SD of SCC |
|---|---|---|---|
| Symmetry | Symmetrical | 50% | 3729.7 ± 9059.3 a |
| Moderate | 30% | 3125.6 ± 2852.4 ab | |
| Asymmetric | 20% | 6759.7 ± 5841.4 b | |
| Udder shape | Globular | 40% | 2001.5 ± 3602.8 a |
| Pear-shaped | 30% | 1585.3 ± 2198.1 a | |
| Cylindrical Pendulous | 30% | 9058.1 ± 10,250.6 b | |
| Degree separation | Slight | 56.7% | 2051.3 ± 3681.8 a |
| Moderate | 26.7% | 4618.2 ± 5518.9 ab | |
| Severe | 16.6% | 10,452.3 ± 12,934.2 b | |
| Degree suspension | Attached | 30% | 1561.8 ± 2154.7 a |
| Intermediate | 43.3% | 3128.3 ± 4834.5 a | |
| Extremely loose | 26.6% | 8967.3 ± 11,352.4 a | |
| Teat shape | Funnel | 50% | 3928.2 ± 5237.4 a |
| Bottle | 23.3% | 2842.7 ± 3450.8 a | |
| Balloon | 26.7% | 5478.3 ± 10,840.1 a | |
| Teat angle | 160°–180° | 26.7% | 6615.8 ± 11,249.4 a |
| 120°–160° | 43.3% | 1632.2 ± 2296.5 a | |
| 90°–120° | 30% | 5870.1 ± 6356.4 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margatho, G.; Quintas, H.; Rodríguez-Estévez, V.; Simões, J. Udder Morphometry and Its Relationship with Intramammary Infections and Somatic Cell Count in Serrana Goats. Animals 2020, 10, 1534. https://doi.org/10.3390/ani10091534
Margatho G, Quintas H, Rodríguez-Estévez V, Simões J. Udder Morphometry and Its Relationship with Intramammary Infections and Somatic Cell Count in Serrana Goats. Animals. 2020; 10(9):1534. https://doi.org/10.3390/ani10091534
Chicago/Turabian StyleMargatho, Gisele, Hélder Quintas, Vicente Rodríguez-Estévez, and João Simões. 2020. "Udder Morphometry and Its Relationship with Intramammary Infections and Somatic Cell Count in Serrana Goats" Animals 10, no. 9: 1534. https://doi.org/10.3390/ani10091534








