The Effect of Staphylococcus spp., Streptococcus spp. and Enterobacteriaceae on the Development of Whey Protein Levels and Oxidative Stress Markers in Cows with Diagnosed Mastitis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Analyses
2.2. Statistical Analyses
- 0 CFU/mL
- 0.1–1 CFU/mL
- >1.1 CFU/mL
- <200,000 cell/mL
- 200,000–400,000 cell/mL
- >400,000 cell/mL
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Lassa, H.; Kubiak, J.; Małkińska-Horodyska, M. Antibiotic susceptibility of the bacteria most often isolated from clinical mastitis in cows. Życ. Wet. 2013, 88, 651–653. [Google Scholar]
- Gröhn, Y.T.; Wilson, D.J.; González, R.N.; Hertl, J.A.; Schulte, H.; Bennett, G.; Schukken, Y.H. Effect of pathogen-specific clinical mastitis on milk yield in dairy cows. J. Dairy Sci. 2004, 87, 3358–3374. [Google Scholar] [CrossRef]
- Malinowski, E.; Lassa, H.; Kłossowska, A.; Markiewicz, H.; Kaczmarowski, M.; Smulski, S. Relationship between mastitis agents and somatic cell count in foremilk samples. Bull. Vet. Instit. Pulawy 2006, 50, 349–352. [Google Scholar]
- Wernicki, A.; Puchalski, A.; Urban-Chmiel, R.; Dec, M.; Stegierska, D.; Dudzic, A.; Wójcik, A. Antimicrobial properties of gold, silver, copper and platinum nanoparticles against selected microorganisms isolated from cases of mastitis in cattle. Med. Wet. 2014, 70, 564–567. [Google Scholar]
- Klaas, I.C.; Zadoks, R.N. An update on environmental mastitis: Challenging perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar] [CrossRef]
- Chaneton, J.; Pérez Sáez, M.; Bussmann, L.E. Antimicrobial activity of bovine β-lactoglobulin against mastitis-causing bacteria. J. Dairy Sci. 2011, 94, 138–145. [Google Scholar] [CrossRef]
- Chaterton, D.E.; Smithers, G.; Roupas, P.; Brodkorb, A. Bioactivity of β-lactoglobulin and α-lactalbumin- Technological implications for processing. Internat. Dairy J. 2006, 16, 1229–1240. [Google Scholar] [CrossRef]
- Schopen, G.C.B.; Heck, J.M.L.; Bovenhuis, H.; Visker, M.H.P.W.; VAN Valenberg, H.J.F.; VAN Arendonk, J.A.M. Genetic parameters for major milk proteins in Dutch Holstein-Friesians. J. Dairy Sci. 2009, 92, 1182–1191. [Google Scholar] [CrossRef]
- Brodziak, A.; Król, J. Właściwości prozdrowotne mleka, Cz. II. Białka mleka. J. NutriLife 2014, 1. Available online: http://www.NutriLife.pl/index.php?art=138 (accessed on 30 July 2020).
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of Animals, a review. J. Sci. Food. Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Cleary, T.G. Effect of lactoferrin on enteric pathogens. Biochimie 2009, 91, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Artym, J. Udział laktoferyny w gospodarce żelazem w organizmie. Część II. Działanie przeciwmikrobiologiczne i przeciwzapalne poprzez sekwestrację żelaza. Postęp. Hig. Medyc. Doświad. 2010, 64, 604–616. [Google Scholar]
- Brandenburg, K.; Jurgens, G.; Muller, M.; Fukuoka, S.; Koch, M.H. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol. Chem. 2001, 382, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Czyżewska-Dors, E.; Dors, A.; Pomorska-Mól, M. Właściwości biofilmu bakteryjnego warunkujące odporność na antybiotyki oraz metody jego zwalczania. Życie Wet. 2018, 93, 765–771. [Google Scholar]
- Leśnierowski, G.; Cegielska-Radziejewska, R. Nowe formy lizozymu możliwości produkcji i wykorzystania. Przem. Spożyw. 2016, 70, 27–29. [Google Scholar] [CrossRef]
- Bochnak, J.; Drabik, K.; Pluta, A.; Batkowska, J.; Budzyńska, A. Lizozym-budowa, funkcje, zastosowanie. Jakość Surow. Pochodz. Zwierzęc. 2017, 32–38. [Google Scholar]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Kapusta, A.; Kuczyńska, B.; Puppel, K.; Kamaszewski, M. The relationship between early stages of lactation and antioxidant capacity of milk and blood plasma of PHF cows. Anim. Sci. Pap. Rep. 2018, 36, 149–158. [Google Scholar]
- Kapusta, A.; Kuczyńska, B.; Puppel, K. Relationship between the degree of antioxidant protection and the level of malondialdehyde in high-performance Polish Holstein-Friesian cows in peak of lactation. PLoS ONE 2018, 13, e0193512. [Google Scholar] [CrossRef]
- Miller, J.; Brzezinska-Slebodzinska, E.; Madsen, F. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Gurer, H.; Ercal, N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med. 2000, 29, 927–945. [Google Scholar] [CrossRef]
- IBM Crop. Released IBM SPSS for Windows; Version 23.0; IBM Crop: Armonk, NY, USA, 2020; Available online: https://www.ibm.com/products/software (accessed on 20 July 2020).
- Jóźwik, A.; Poławska, E.; Krzyżewski, J.; Bagnicka, E.; Niemczuk, K.; Strzałkowska, N.; Wierzbicka, A.; Lipińska, P.; Horbańczuk, J.O. Relations between the oxidative status, mastitis, milk quality and disorders of reproductive functions in dairy cows—A review. Anim. Sci. Pap. Rep. 2012, 30, 297–307. [Google Scholar]
- Gill, J.J.; Sabour, P.M.; Leslie, K.E.; Griffiths, M.W. Bovine whey proteins inhibit the interaction of Staphylococcus aureus and bacteriophage K. J. App. Microbiol. 2006, 101, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wessely-Szponder, J.; Bobowiec, R.; Martelli, F.; Wojcik, M.; Kosior-Korzecka, U. Assessment of neutrophil components as markers of lung injury in the course of bovine respiratory tract infections. Pol. J. Vet. Sci. 2004, 7, 157–161. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, metabolism, and signaling mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Su, W.J.; Chang, C.J.; Peh, H.C.; Lee, S.L.; Huang, M.C.; Zhao, X. Apoptosis and oxidative stress of infiltrated neutrophils obtained from mammary glands of goats during various stages of lactation. Am. J. Vet. Res. 2002, 63, 241–246. [Google Scholar] [CrossRef][Green Version]
- Suriyasathaporn, W.; Vinitketkumnuen, U.; Chewonarin, T.; Boonyayatra, S.; Kreausukon, K.; Schukken, Y.H. Higher somatic cell counts resulted in higher malondialdehyde concentrations in raw cow’s milk. Int. Dairy J. 2006, 16, 1088–1091. [Google Scholar] [CrossRef]
- Kuczaj, M.; Preś, J.; Twardoń, J.; Orda, J.; Panek, P.; Wieliczko, A. Analiza związku liczby komórek somatycznych w mleku ze stanami zapalnymi gruczołu mlekowego i wskaźnikami produkcyjno-fizjologicznymi u krów mlecznych. Zesz. Nauk. UP Wroc. Biol. Hod. Zwierz. 2012, LXVI 590, 43–50. [Google Scholar]
- Zimecki, M.; Artym, J. Właściwości terapeutyczne białek i peptydów z siary i mleka. Postęp. Hig. Med. Doświad. 2005, 59, 309–323. [Google Scholar]
- Waage, S.; Mork, T.; Roros, A.; Aasland, D.; Hunshamar, A.; Odegaard, S.A. Bacteria associated with clinical mastitis in dairy heifers. J. Dairy Sci. 1999, 82, 712–719. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, G.; Jensen, E.R.; Lenoy, E.; Schneewind, O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 2000, 97, 5510–5515. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, T.A.; Sacco, R.E.; Nonnecke, B.J.; Lippolis, J.D. Bovine milk proteome: Quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J. Proteom. 2013, 82, 141–154. [Google Scholar] [CrossRef]
- Jahani, S.; Shakiba, A.; Jahani, L. The Antimicrobial Effect of Lactoferrin on Gram-Negative and Gram-Positive Bacteria. Int. J. Infect. 2015, 2, e27954. [Google Scholar] [CrossRef]
- Andrzejczak, O. Lizozym-aktualne trendy w nauce i przemyśle. In Przegląd Wybranych prac z Zakresu Enzymologii; Książka w języku polskim: Lublin, Poland, 2016; pp. 59–72. ISBN 978-83-65272-34-8. [Google Scholar]
- Gasińska, I. Laktoferyna: Wielokierunkowe działanie w profilaktyce i leczeniu zakażeń u noworodków urodzonych przedwcześnie. Post. Neonatol. 2018, 24, 87–95. [Google Scholar] [CrossRef]
- Ellison, R.T.; Giehl, T.J. Killing of Gram-negative Bacteria by Lactoferrin and Lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Yamauchi, K.; Tomita, M.; Giehl, T.J.; Ellison, R.T., 3rd. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 1993, 61, 719–728. [Google Scholar] [CrossRef]
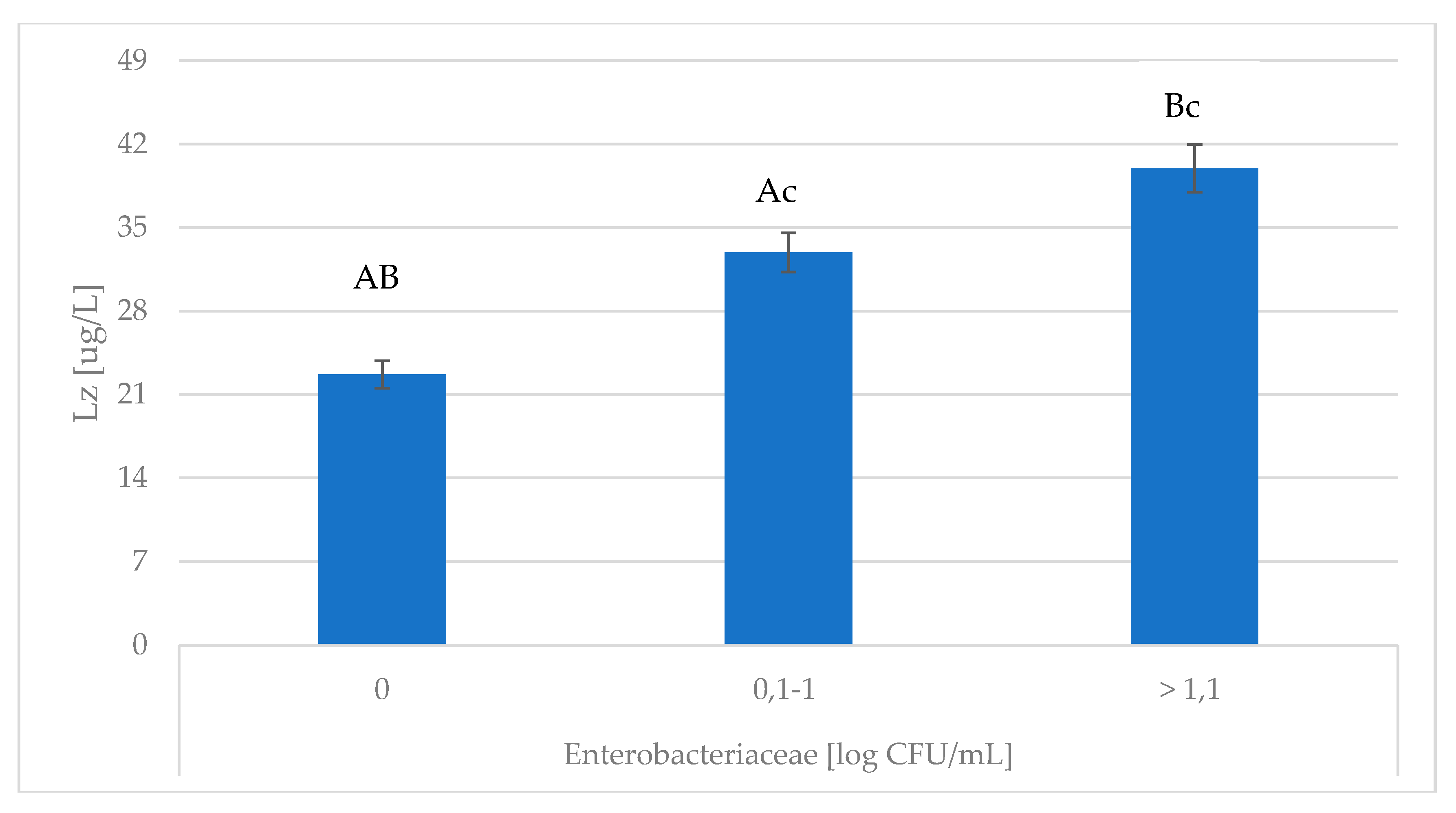

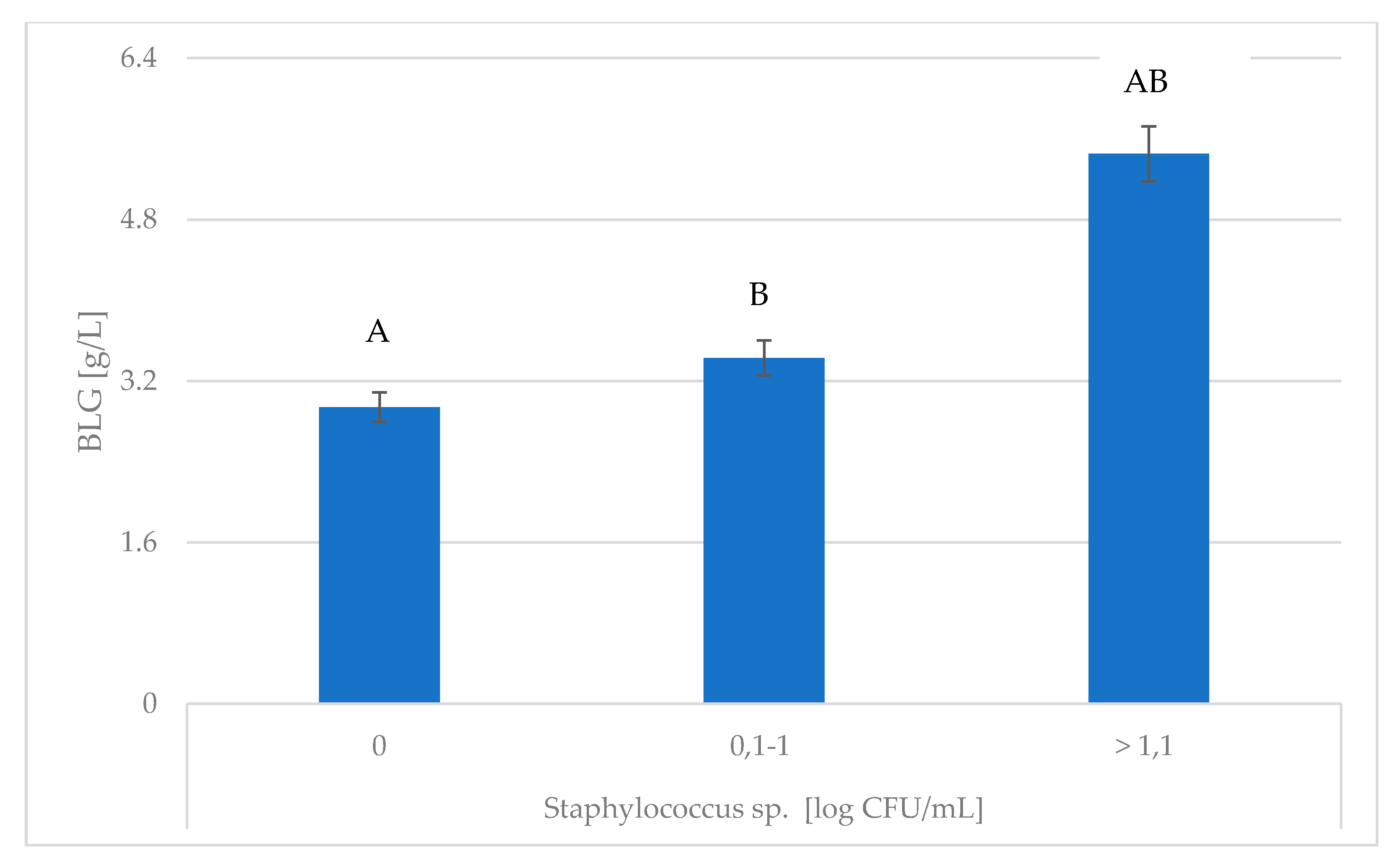

| SCC [103 cell/mL] | LSM | SEM | |
|---|---|---|---|
| MDA [nM/mL] | <200 | 19.369 AB | 0.151 |
| 200–400 | 21.091 AC | 0.184 | |
| >400 | 28.749 BC | 0.169 | |
| TAS [mmol/L] | <200 | 0.808 aB | 0.027 |
| 200–400 | 0.717 aC | 0.028 | |
| >400 | 0.471 BC | 0.027 | |
| Lz [µg/L] | <200 | 30.112 A | 1.270 |
| 200–400 | 30.024 B | 1.272 | |
| >400 | 34.076 AB | 1.278 | |
| Lf [g/L] | <200 | 0.239 A | 0.016 |
| 200–400 | 0.245 B | 0.018 | |
| >400 | 0.469 AB | 0.016 | |
| BLG [g/L] | <200 | 3.239 A | 0.123 |
| 200–400 | 3.368 B | 0.103 | |
| >400 | 5.333 AB | 0.139 |
| Enterobacteriaceae [log CFU/mL] | LSM | SEM | |
|---|---|---|---|
| MDA [nM/mL] | 0 | 20.630 AB | 1.469 |
| 0.1–1 | 25.006 AC | 1.028 | |
| >1.1 | 29.611 BC | 1.124 | |
| TAS [mmol/L] | 0 | 0.806 | 0.041 |
| 0.1–1 | 0.787 | 0.039 | |
| >1.1 | 0.668 | 0.047 | |
| BLG [g/L] | 0 | 3.383 | 0.176 |
| 0.1–1 | 3.569 | 0.128 | |
| >1.1 | 3.193 | 0.144 |
| Staphylococcus spp. [log CFU/mL] | LSM | SEM | |
|---|---|---|---|
| MDA [nM/mL] | 0 | 26.224 AB | 1.570 |
| 0.1–1 | 22.034 A | 1.794 | |
| >1.1 | 22.000 B | 1.418 | |
| TAS [mmol/L] | 0 | 0.865 | 0.060 |
| 0.1–1 | 0.868 | 0.060 | |
| >1.1 | 0.765 | 0.044 | |
| Lz [µg/L] | 0 | 34.310 A | 0.503 |
| 0.1–1 | 29.489 AB | 0.240 | |
| >1.1 | 31.112 B | 0.263 | |
| Lf [g/L] | 0 | 0.294 | 0.038 |
| 0.1–1 | 0.243 | 0.022 | |
| >1.1 | 0.247 | 0.022 |
| Streptococcus spp. [log CFU/mL] | LSM | SEM | |
|---|---|---|---|
| TAS [mmol/L] | 0 | 0.899 | 0.057 |
| 0.1–1 | 0.716 | 0.045 | |
| >1.1 | 0.867 | 0.060 | |
| Lz [µg/L] | 0 | 30.165 A | 1.221 |
| 0.1–1 | 30.662 B | 1.797 | |
| >1.1 | 42.118 AB | 1.451 | |
| Lf [g/L] | 0 | 0.265 A | 0.019 |
| 0.1–1 | 0.228 B | 0.026 | |
| >1.1 | 0.474 AB | 0.038 | |
| BLG [g/L] | 0 | 4.576 AB | 0.209 |
| 0.1–1 | 3.195 A | 0.243 | |
| >1.1 | 3.032 B | 0.285 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puppel, K.; Kalińska, A.; Kot, M.; Slósarz, J.; Kunowska-Slósarz, M.; Grodkowski, G.; Kuczyńska, B.; Solarczyk, P.; Przysucha, T.; Gołębiewski, M. The Effect of Staphylococcus spp., Streptococcus spp. and Enterobacteriaceae on the Development of Whey Protein Levels and Oxidative Stress Markers in Cows with Diagnosed Mastitis. Animals 2020, 10, 1591. https://doi.org/10.3390/ani10091591
Puppel K, Kalińska A, Kot M, Slósarz J, Kunowska-Slósarz M, Grodkowski G, Kuczyńska B, Solarczyk P, Przysucha T, Gołębiewski M. The Effect of Staphylococcus spp., Streptococcus spp. and Enterobacteriaceae on the Development of Whey Protein Levels and Oxidative Stress Markers in Cows with Diagnosed Mastitis. Animals. 2020; 10(9):1591. https://doi.org/10.3390/ani10091591
Chicago/Turabian StylePuppel, Kamila, Aleksandra Kalińska, Magdalena Kot, Jan Slósarz, Małgorzata Kunowska-Slósarz, Grzegorz Grodkowski, Beata Kuczyńska, Paweł Solarczyk, Tomasz Przysucha, and Marcin Gołębiewski. 2020. "The Effect of Staphylococcus spp., Streptococcus spp. and Enterobacteriaceae on the Development of Whey Protein Levels and Oxidative Stress Markers in Cows with Diagnosed Mastitis" Animals 10, no. 9: 1591. https://doi.org/10.3390/ani10091591
APA StylePuppel, K., Kalińska, A., Kot, M., Slósarz, J., Kunowska-Slósarz, M., Grodkowski, G., Kuczyńska, B., Solarczyk, P., Przysucha, T., & Gołębiewski, M. (2020). The Effect of Staphylococcus spp., Streptococcus spp. and Enterobacteriaceae on the Development of Whey Protein Levels and Oxidative Stress Markers in Cows with Diagnosed Mastitis. Animals, 10(9), 1591. https://doi.org/10.3390/ani10091591







