Consequences of Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation on the Development of Calf Muscle and Adipose Tissue
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Lipid Extraction and Fatty Acid Analysis
2.3. Muscle Cross-Sectional Area Measurement
2.4. Histology
2.5. Immunohistochemistry
2.6. Protein Extraction and Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Maternal FA Supplementation Changed FA Concentrations of the Calf Muscle Tissues
3.2. Maternal FA Supplementation Changed FA Composition of the Calf Adipose Tissues
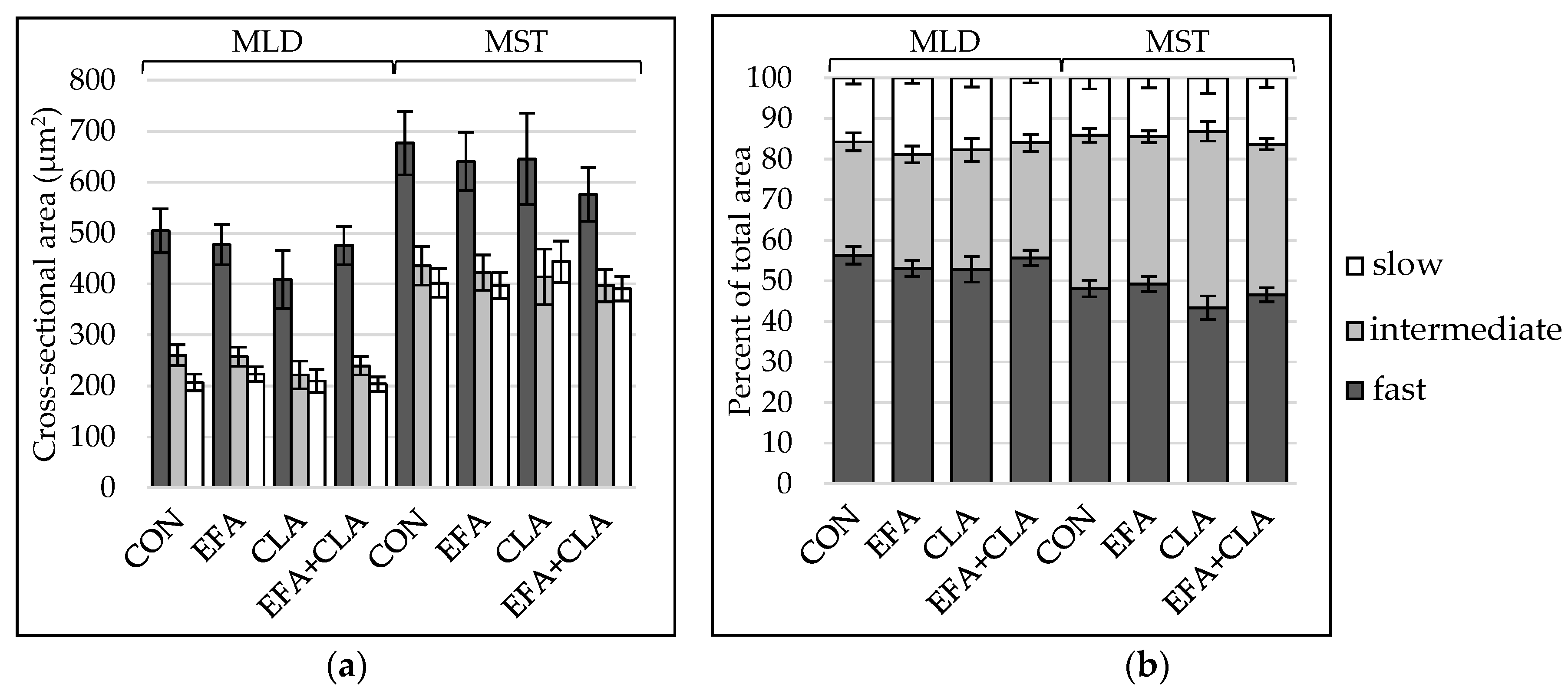
3.3. Muscle Fiber Type Composition Was Unchanged by Maternal FA Supplementation
3.4. Muscle Capillarization Was Not Affected by Maternal FA Supplementation
3.5. No Effects of Maternal FA Supplementation on Intramuscular Fat Deposition
3.6. Maternal FA Supplementation Changed the Fat Cell Size Distribution in Different Depots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chilliard, Y.; Ferlay, A.; Doreau, M. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids. Livest. Prod. Sci. 2001, 70, 31–48. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Mann, N.J.; Sinclair, A.J. Effect of feeding systems on omega-3 fatty acids, conjugated linoleic acid and trans fatty acids in Australian beef cuts: Potential impact on human health. Asia Pac. J. Clin. Nutr. 2006, 15, 21–29. [Google Scholar] [PubMed]
- Kliem, K.; Morgan, R.; Humphries, D.J.; Shingfield, K.J.; Givens, D.I. Effect of replacing grass silage with maize silage in the diet on bovine milk fatty acid composition. Animal 2008, 2, 1850–1858. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 145–150. [Google Scholar] [CrossRef]
- Gurr, M.I.; Harwood, J.L.; Frayn, K.N. Lipid Biochemistry; Blackwell Science: Oxford, UK, 2002. [Google Scholar] [CrossRef]
- Doreau, M.; Ferlay, A. Digestion and utilisation of fatty acids by ruminants. Anim. Feed Sci. Technol. 1994, 45, 379–396. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bernard, L.; Leroux, C.; Chilliard, Y. Role oftransfatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 2010, 4, 1140–1166. [Google Scholar] [CrossRef]
- Agatha, G.; Voigt, A.; Kauf, E.; Zintl, F. Conjugated linoleic acid modulation of cell membrane in leukemia cells. Cancer Lett. 2004, 209, 87–103. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary fats and membrane function: Implications for metabolism and disease. Biol. Rev. 2005, 80, 155–169. [Google Scholar] [CrossRef]
- Bezard, J.; Blond, J.; Bernard, A.; Clouet, P. The metabolism and availability of essential fatty acids in animal and human tissues. Reprod. Nutr. Dev. 1994, 34, 539–568. [Google Scholar] [CrossRef]
- Griinari, J.M.; Bauman, D.E. Biosynthesis of Conjugated Linoleic Acid and Its Incorporation into Meat and Milk in Ruminants. In Advances in Conjugated Linoleic Acid Research; Yurawecz, M.P., Mossoba, M.M., Kramer, J.K., Pariza, M.W., Nelson, G.J., Eds.; AOCS Press: Champaign, IL, USA, 1999; Volume 1, pp. 180–200. [Google Scholar]
- Bauman, D.E.; Harvatine, K.J.; Lock, A.L. Nutrigenomics, Rumen-Derived Bioactive Fatty Acids, and the Regulation of Milk Fat Synthesis. Annu. Rev. Nutr. 2011, 31, 299–319. [Google Scholar] [CrossRef]
- Griinari, J.M.; Corl, B.A.; Lacy, S.H.; Chouinard, P.; Nurmela, K.V.V.; Bauman, D.E. Conjugated Linoleic Acid Is Synthesized Endogenously in Lactating Dairy Cows by Δ9-Desaturase. J. Nutr. 2000, 130, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, S.; Hurtaud, C.; Marnet, P.; Faverdin, P.; Peyraud, J. Composition of Milk Fat from Cows Selected for Milk Fat Globule Size and Offered Either Fresh Pasture or a Corn Silage-Based Diet. J. Dairy Sci. 2007, 90, 392–403. [Google Scholar] [CrossRef]
- Lahlou, M.N.; Kanneganti, R.; Massingill, L.J.; Broderick, G.A.; Park, Y.; Pariza, M.W.; Ferguson, J.D.; Wu, Z. Grazing increases the concentration of CLA in dairy cow milka. Animal 2014, 8, 1191–1200. [Google Scholar] [CrossRef]
- Du, M.; Ford, S.P.; Zhu, M.-J. Optimizing livestock production efficiency through maternal nutritional management and fetal developmental programming. Anim. Front. 2017, 7, 5–11. [Google Scholar] [CrossRef]
- Du, M.; Huang, Y.; Das, A.K.; Yang, Q.; Duarte, M.S.; Dodson, M.V.; Zhu, M.-J. Meat Science and Muscle Biology Symposium: Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J. Anim. Sci. 2013, 91, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.C.; Shand, J.H.; Drummond, J.T.; Moore, J.H. “Protected” Polyunsaturated Fatty Acid in the Diet of the Ewe and the Essential Fatty Acid Status of the Neonatal Lamb. J. Nutr. 1978, 108, 1868–1876. [Google Scholar] [CrossRef]
- Dänicke, S.; Kowalczyk, J.; Renner, L.; Pappritz, J.; Meyer, U.; Kramer, R.; Weber, E.-M.; Doll, S.; Rehage, J.; Jahreis, G. Effects of conjugated linoleic acids fed to dairy cows during early gestation on hematological, immunological, and metabolic characteristics of cows and their calves. J. Dairy Sci. 2012, 95, 3938–3953. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Odle, J. Nutritional Factors Influencing Intestinal Health of the Neonate12. Adv. Nutr. 2012, 3, 687–696. [Google Scholar] [CrossRef]
- Garcia, M.; Greco, L.F.; Favoreto, M.G.; Marsola, R.S.; Martins, L.T.; Bisinotto, R.S.; Shin, J.H.; Lock, A.L.; Block, E.; Thatcher, W.W.; et al. Effect of supplementing fat to pregnant nonlactating cows on colostral fatty acid profile and passive immunity of the newborn calf. J. Dairy Sci. 2014, 97, 392–405. [Google Scholar] [CrossRef]
- Garcia, M.; Greco, L.F.; Favoreto, M.G.; Marsola, R.S.; Wang, D.; Shin, J.H.; Block, E.; Thatcher, W.W.; Santos, J.E.; Staples, C.R. Effect of supplementing essential fatty acids to pregnant nonlactating Holstein cows and their preweaned calves on calf performance, immune response, and health. J. Dairy Sci. 2014, 97, 5045–5064. [Google Scholar] [CrossRef]
- Allen, R.E.; Luiten, L.S.; Dodson, M.V. Effect of Insulin and Linoleic Acid on Satellite Cell Differentiation 1. J. Anim. Sci. 1985, 60, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.S.; Flux, C.; Salter, A.M.; Brameld, J.M. Effects of fatty acids on skeletal muscle cell differentiation in vitro. Br. J. Nutr. 2006, 95, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Silvey, D.T.; Johnson, B.J.; Doumit, M.E.; Chung, K.Y.; Sawyer, J.E.; Go, G.W.; Smith, S.B. Conjugated linoleic acid (t-10, c-12) reduces fatty acid synthesis de novo, but not expression of genes for lipid metabolism in bovine adipose tissue ex vivo. Lipids 2014, 49, 15–24. [Google Scholar] [CrossRef]
- Vogel, L.; Gnott, M.; Kröger-Koch, C.; Dannenberger, D.; Tuchscherer, A.; Tröscher, A.; Kienberger, H.; Rychlik, M.; Starke, A.; Bachmann, L.; et al. Effects of abomasal infusion of essential fatty acids together with conjugated linoleic acid in late and early lactation on performance, milk and body composition, and plasma metabolites in dairy cows. J. Dairy Sci. 2020, 103, 7431–7450. [Google Scholar] [CrossRef] [PubMed]
- Kalbe, C.; Priepke, A.; Nürnberg, G.; Dannenberger, D. Effects of long-term microalgae supplementation on muscle microstructure, meat quality, and fatty acid composition in growing pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 574–582. [Google Scholar] [CrossRef]
- Dannenberger, D.; Nuernberg, G.; Nuernberg, K.; Will, K.; Schauer, N.; Schmicke, M. Effects of diets supplemented with n–3 or n–6 PUFA on pig muscle lipid metabolites measured by non-targeted LC–MS lipidomic profiling. J. Food. Compost. Anal. 2017, 56, 47–54. [Google Scholar] [CrossRef]
- Wegner, J.; Albrecht, E.; Fiedler, I.; Teuscher, F.; Papstein, H.J.; Ender, K. Growth-and breed-related changes of muscle fiber characteristics in cattle. J. Anim. Sci. 2000, 78, 1485–1496. [Google Scholar] [CrossRef]
- Szentkuti, L.; Eggers, A. Eine zuverlässige Modifikation der Myosin-ATPase-Reaktion zur histochemischen Darstellung von drei Fasertypen in der Skelettmuskulatur von Schweinen. Fleischwirtsch 1985, 65, 1398–1404. [Google Scholar]
- Albrecht, E.; Gotoh, T.; Ebara, F.; Xu, J.X.; Viergutz, T.; Nurnberg, G.; Maak, S.; Wegner, J. Cellular conditions for intramuscular fat deposition in Japanese Black and Holstein steers. Meat Sci. 2011, 89, 13–20. [Google Scholar] [CrossRef]
- Spannhof, L. Einführung in die Praxis der Histochemie; VEB Gustav-Fischer-Verlag: Jena, Germany, 1967. [Google Scholar]
- Zitnan, R.; Albrecht, E.; Kalbe, C.; Miersch, C.; Revajova, V.; Levkut, M.; Röntgen, M. Muscle characteristics in chicks challenged with Salmonella Enteritidis and the effect of preventive application of the probiotic Enterococcus faecium. Poult. Sci. 2019, 98, 2014–2025. [Google Scholar] [CrossRef]
- Hammon, H.M.; Uken, K.L.; Vogel, L.; Gnott, M.; Tuchscherer, A.; Tröscher, A.; Dannenberger, D. Effect of maternal supplementation with essential fatty acids and conjugated linoleic acid on fatty acid status in neonatal calves. In Proceedings of the Annual Meeting of the American Dairy Science Association, Knoxville, TN, USA, 24–27 June 2018. [Google Scholar]
- Moallem, U.; Zachut, M. Short communication: The effects of supplementation of various n-3 fatty acids to late-pregnant dairy cows on plasma fatty acid composition of the newborn calves. J. Dairy Sci. 2012, 95, 4055–4058. [Google Scholar] [CrossRef] [PubMed]
- Herdmann, A.; Martin, J.; Nürnberg, G.; Dannenberger, D.; Nürnberg, K. Effect of Dietary n-3 and n-6 PUFA on Lipid Composition of Different Tissues of German Holstein Bulls and the Fate of Bioactive Fatty Acids during Processing. J. Agric. Food Chem. 2010, 58, 8314–8321. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Dannenberger, D.; Nürnberg, K.; Richardson, I.; MacKintosh, S.; Hocquette, J.-F.; Moloney, A. Enhancing the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2014, 97, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Hiller, B.; Herdmann, A.; Nuernberg, K. Dietary n-3 fatty acids significantly suppress lipogenesis in bovine muscle and adipose tissue: A functional genomics approach. Lipids 2011, 46, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, A.K. Cellular uptake of long-chain fatty acids: Role of membrane-associated fatty-acid-binding/transport proteins. Cell. Mol. Life. Sci. 2000, 57, 1360–1372. [Google Scholar] [CrossRef]
- Rodemann, H.P.; Goldberg, A.L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J. Biol. Chem. 1982, 257, 1632–1638. [Google Scholar]
- Veliça, P.; Khanim, F.L.; Bunce, C.M. Prostaglandin D2 inhibits C2C12 myogenesis. Mol. Cell. Endocrinol. 2010, 319, 71–78. [Google Scholar] [CrossRef]
- Briolay, A.; Jaafar, R.; Nemoz, G.; Bessueille, L. Myogenic differentiation and lipid-raft composition of L6 skeletal muscle cells are modulated by PUFAs. Biochim. Biophys. Acta Biomembr. 2013, 1828, 602–613. [Google Scholar] [CrossRef]
- Abreu, P.; Leal-Cardoso, J.H.; Ceccatto, V.M.; Hirabara, S.M. Regulation of muscle plasticity and trophism by fatty acids: A short review. Rev. Assoc. Med. Bras. 2017, 63, 148–155. [Google Scholar] [CrossRef]
- Abbott, S.K.; Else, P.L.; Hulbert, A.J. Membrane fatty acid composition of rat skeletal muscle is most responsive to the balance of dietary n-3 and n-6 PUFA. Br. J. Nutr. 2010, 103, 522–529. [Google Scholar] [CrossRef]
- O’Rourke, P.K.; Entwistle, K.W.; Arman, C.; Esdale, C.R.; Burns, B.M. Fetal development and gestational changes in Bostaurus and Bosindicus genotypes in the tropics. Theriogenology 1991, 36, 839–853. [Google Scholar] [CrossRef]
- Mao, W.H.; Albrecht, E.; Teuscher, F.; Yang, Q.; Zhao, R.Q.; Wegner, J. Growth-and breed-related changes of fetal development in cattle. Asian-Australas. J. Anim. Sci. 2008, 21, 640–647. [Google Scholar] [CrossRef]
- Luff, A.R.; Goldspink, G. Total number of fibers in muscles of several strains of mice. J. Anim. Sci. 1970, 30, 891–893. [Google Scholar] [CrossRef]
- Rubinstein, N.A.; Kelly, A.M. Development of muscle fiber specialization in the rat hindlimb. J. Cell Biol. 1981, 90, 128–144. [Google Scholar] [CrossRef]
- Kugelberg, E. Adaptive transformation of rat soleus motor units during growth: Histochemistry and contraction speed. J. Neurol. Sci. 1976, 27, 269–289. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A.; Ohmori, H.; Katsuta, S. Nonuniform changes in fiber types in the soleus muscle of the developing rat. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 132–137. [Google Scholar] [CrossRef]
- Butler-Browne, G.S.; Whalen, R.G. Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev. Biol. 1984, 102, 324–334. [Google Scholar] [CrossRef]
- Staron, R.S.; Pette, D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry 1986, 86, 19–23. [Google Scholar] [CrossRef]
- Staron, R.S.; Pette, D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibers. Rabbit soleus muscle. Biochem. J. 1987, 243, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Men, X.M.; Deng, B.; Xu, Z.W.; Tao, X.; Qi, K.K. Age-related changes and nutritional regulation of myosin heavy-chain composition in longissimus dorsi of commercial pigs. Animal 2013, 7, 1486–1492. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Tompkins, G.; Doerr, L. Postnatal development of muscle fiber types in domestic animals. J. Anim. Sci. 1972, 34, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, G.D.; Jeong, J.Y.; Hur, S.J.; Joo, S.T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010, 86, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, M.; Murray, M.; Petrovic, N. Modulation of angiogenesis by ω-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood 2008, 111, 3514–3521. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.; Stephen Lee, K.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef] [PubMed]
- Fève, B. Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef]
- Gnanalingham, M.G.; Mostyn, A.; Symonds, M.E.; Stephenson, T. Ontogeny and nutritional programming of adiposity in sheep: Potential role of glucocorticoid action and uncoupling protein-2. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R1407–R1415. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Duffield, J.A.; McMillen, I.C. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology 2007, 148, 878–885. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Lenhard, J.M.; Willson, T.M.; Patel, I.; Morris, D.C.; Lehmann, J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 1995, 83, 813–819. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Baker, P.R.S.; Giles, G.; Chumley, P.; Batthyany, C.; Crawford, J.; Patel, R.P.; Hogg, N.; Branchaud, B.P.; Lancaster, J.R.; et al. Fatty acid transduction of nitric oxide signaling Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J. Biol. Chem. 2005, 280, 19289–19297. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.A.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Chung, K.Y.; Choi, C.B.; Kawachi, H.; Yano, H.; Smith, S.B. Trans-10, cis-12 conjugated linoleic acid down-regulates arginine-promoted differentiation of bovine preadipocytes. Adipocytes 2006, 2, 93–100. [Google Scholar]
- Kadegowda, A.K.G.; Burns, T.A.; Pratt, S.L.; Duckett, S.K. Inhibition of stearoyl-CoA desaturase 1 reduces lipogenesis in primary bovine adipocytes. Lipids 2013, 48, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Yanting, C.; Yang, Q.Y.; Ma, G.L.; Du, M.; Harrison, J.H.; Block, E. Dose-and type-dependent effects of long-chain fatty acids on adipogenesis and lipogenesis of bovine adipocytes. J. Dairy Sci. 2018, 101, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Robelin, J. Growth of adipose tissues in cattle; partitioning between depots, chemical composition and cellularity. A review. Livest. Prod. Sci. 1986, 14, 349–364. [Google Scholar] [CrossRef]
- Dugan, M.E.R.; Aalhus, J.L.; Jeremiah, L.E.; Kramer, J.K.G.; Schaefer, A.L. The effects of feeding conjugated linoleic acid on subsequent pork quality. Can. J. Anim. Sci. 1999, 79, 45–51. [Google Scholar] [CrossRef]
- Huang, J.X.; Qi, R.L.; Chen, X.L.; You, X.Y.; Liu, X.Q.; Yang, F.Y.; Liu, Z.H. Improvement in the carcass traits and meat quality of growing-finishing Rongchang pigs by conjugated linoleic acid through altered gene expression of muscle fiber types. Genet. Mol. Res. 2014, 13, 7061–7069. [Google Scholar] [CrossRef]
- Huang, F.R.; Zhan, Z.P.; Luo, J.; Liu, Z.X.; Peng, J. Duration of dietary linseed feeding affects the intramuscular fat, muscle mass and fatty acid composition in pig muscle. Livest. Sci. 2008, 118, 132–139. [Google Scholar] [CrossRef]
- Cooke, R.F.; Bohnert, D.W.; Moriel, P.; Hess, B.W.; Mills, R.R. Effects of polyunsaturated fatty acid supplementation on ruminal in situ forage degradability, performance, and physiological responses of feeder cattle. J. Anim. Sci. 2011, 89, 3677–3689. [Google Scholar] [CrossRef]
- Fehrman, C.; Rode, H.; Grubbs, J.; Blair, A.; Underwood, K. Rumen Protected Long Chain Fatty Acid Supplementation Effects on Beef Carcass Traits and Composition. Meat Muscle Biol. 2019, 1, 95. [Google Scholar] [CrossRef]
- Mandell, I.B.; Buchanan-Smith, J.G.; Holub, B.J.; Campbell, C.P. Effects of fish meal in beef cattle diets on growth performance, carcass characteristics, and fatty acid composition of longissimus muscle. J. Anim. Sci. 1997, 75, 910–919. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, X.; Wang, Z.; Zhou, A.; Peng, Q.; Zou, H.; Xue, B.; Wang, L. Dietary conjugated linoleic acids increase intramuscular fat deposition and decrease subcutaneous fat deposition in Yellow Breed× Simmental cattle. Anim. Sci. J. 2016, 87, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Teuscher, F.; Ender, K.; Wegner, J. Growth-and breed-related changes of marbling characteristics in cattle. J. Anim. Sci. 2006, 84, 1067–1075. [Google Scholar] [CrossRef] [PubMed]





| Fatty Acid | Supplementation Group | Effect (p-Value) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | EFA | CLA | EFA + CLA | EFA | CLA | EFA × CLA | |||||
| C18:2 c-9, t-11 (CLA) | 1.10 | ±0.10 b | 0.85 | ±0.09 b | 1.85 | ±0.14 a | 1.65 | ±0.08 a | 0.036 | <0.001 | 0.815 |
| C18:3 n-3 (ALA) | 2.03 | ±1.04 c | 11.22 | ±0.91 b | 1.99 | ±1.47 c | 15.19 | ±0.86 a | <0.001 | 0.073 | 0.066 |
| C20:5 n-3 (EPA) | 1.89 | ±0.78 b | 8.73 | ±0.69 a | 1.67 | ±1.08 b | 10.24 | ±0.66 a | <0.001 | 0.409 | 0.265 |
| C22:5 n-3 (DPA) | 8.72 | ±1.07 b | 16.55 | ±0.98 a | 8.64 | ±1.54 b | 19.32 | ±0.91 a | <0.001 | 0.250 | 0.232 |
| C22:6 n-3 (DHA) | 5.79 | ±0.95 b | 9.44 | ±0.86 a | 4.96 | ±1.27 b | 9.65 | ±0.82 a | <0.001 | 0.718 | 0.552 |
| Sum n-3 PUFA 1 | 18.55 | ±3.14 b | 46.15 | ±2.78 a | 17.44 | ±4.45 b | 54.97 | ±2.62 a | <0.001 | 0.233 | 0.128 |
| C18:2 n-6 (LA) | 63.30 | ±6.43 | 63.51 | ±5.70 | 75.05 | ±9.11 | 78.58 | ±5.39 | 0.781 | 0.048 | 0.798 |
| C20:4 n-6 (ARA) | 67.29 | ±4.20 ab | 57.23 | ±3.80 b | 75.42 | ±5.59 a | 62.91 | ±3.64 ab | 0.008 | 0.077 | 0.749 |
| C22:4 n-6 (ADA) | 19.58 | ±1.33 a | 13.53 | ±1.20 b | 22.27 | ±1.79 a | 15.07 | ±1.14 b | <0.001 | 0.093 | 0.642 |
| Sum n-6 PUFA 2 | 181.82 | ±12.68 | 163.08 | ±11.31 | 204.08 | ±17.71 | 188.74 | ±10.71 | 0.196 | 0.066 | 0.893 |
| Sum PUFA 3 | 202.77 | ±14.83 | 211.39 | ±13.21 | 225.11 | ±20.81 | 246.61 | ±12.49 | 0.329 | 0.062 | 0.666 |
| C18:1 c-9 (OA) | 270.83 | ±12.51 ab | 240.65 | ±11.59 b | 297.36 | ±15.71 a | 247.38 | ±11.24 b | 0.001 | 0.099 | 0.321 |
| C18:1 t-11 (VA) | 0.45 | ±0.15 | 0.32 | ±0.13 | 0.48 | ±0.21 | 0.39 | ±0.13 | 0.474 | 0.749 | 0.892 |
| Sum MUFA 4 | 349.86 | ±15.98 ab | 313.24 | ±14.79 b | 383.29 | ±20.10 a | 320.03 | ±14.34 b | 0.001 | 0.120 | 0.299 |
| C16:0 (PA) | 147.14 | ±9.41 | 128.36 | ±8.81 | 148.85 | ±11.51 | 134.83 | ±8.59 | 0.029 | 0.552 | 0.731 |
| C18:0 (SA) | 93.25 | ±5.49 | 87.85 | ±4.89 | 104.17 | ±7.64 | 104.22 | ±4.64 | 0.633 | 0.017 | 0.616 |
| Sum SFA 5 | 257.48 | ±14.36 | 230.10 | ±13.26 | 269.91 | ±18.16 | 255.19 | ±12.85 | 0.086 | 0.111 | 0.585 |
| Total fat content (%) | 0.81 | ±0.04 | 0.76 | ±0.04 | 0.87 | ±0.05 | 0.82 | ±0.04 | 0.143 | 0.066 | 0.997 |
| Fatty Acid | Supplementation Group | Effect (p-Value) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | EFA | CLA | EFA + CLA | EFA | CLA | EFA × CLA | |||||
| C18:2 c-9, t-11 (CLA) | 0.12 | ±0.02 b | 0.09 | ±0.01 b | 0.20 | ±0.02 a | 0.17 | ±0.01 a | 0.102 | <0.001 | 0.958 |
| C18:3 n-3 (ALA) | 0.10 | ±0.05 b | 0.35 | ±0.04 a | 0.06 | ±0.07 b | 0.47 | ±0.04 a | <0.001 | 0.484 | 0.167 |
| C20:5 n-3 (EPA) | 0.01 | ±0.01 b | 0.03 | ±0.00 a | NA | 0.03 | ±0.00 a | <0.001 | 0.898 | 0.716 | |
| C22:5 n-3 (DPA) | 0.08 | ±0.01 b | 0.14 | ±0.01 a | NA | 0.13 | ±0.01 ab | <0.001 | 0.339 | 0.642 | |
| C22:6 n-3 (DHA) | 0.03 | ±0.01 | 0.04 | ±0.00 | NA | 0.02 | ±0.00 | 0.067 | 0.049 | 0.686 | |
| Sum n-3 PUFA 1 | 0.22 | ±0.07 b | 0.57 | ±0.06 a | 0.10 | ±0.09 b | 0.68 | ±0.06 a | <0.001 | 0.986 | 0.121 |
| C18:2 n-6 (LA) | 1.64 | ±0.15 | 1.78 | ±0.14 | 1.68 | ±0.22 | 1.78 | ±0.13 | 0.491 | 0.910 | 0.915 |
| C20:4 n-6 (ARA) | 0.30 | ±0.03 | 0.25 | ±0.03 | 0.24 | ±0.04 | 0.21 | ±0.03 | 0.218 | 0.164 | 0.795 |
| C22:4 n-6 (ADA) | 0.11 | ±0.01 a | 0.08 | ±0.01 ab | 0.09 | ±0.02 ab | 0.07 | ±0.01 b | 0.031 | 0.240 | 0.659 |
| Sum n-6 PUFA 2 | 2.32 | ±0.19 | 2.37 | ±0.17 | 2.33 | ±0.27 | 2.30 | ±0.16 | 0.971 | 0.898 | 0.849 |
| Sum PUFA 3 | 2.71 | ±0.25 | 3.07 | ±0.23 | 2.79 | ±0.36 | 3.19 | ±0.22 | 0.169 | 0.705 | 0.938 |
| C18:1 c-9 (OA) | 35.71 | ±0.90 | 33.82 | ±0.82 | 34.88 | ±1.29 | 33.97 | ±0.78 | 0.161 | 0.731 | 0.622 |
| C18:1 t-11 (VA) | 0.17 | ±0.03 | 0.14 | ±0.03 | 0.21 | ±0.04 | 0.18 | ±0.03 | 0.425 | 0.184 | 0.973 |
| Sum MUFA 4 | 42.57 | ±0.93 | 40.72 | ±0.85 | 41.72 | ±1.34 | 40.27 | ±0.81 | 0.113 | 0.523 | 0.844 |
| C16:0 (PA) | 36.94 | ±0.80 | 38.01 | ±0.73 | 37.05 | ±1.14 | 37.35 | ±0.69 | 0.434 | 0.747 | 0.663 |
| C18:0 (SA) | 12.23 | ±0.34 b | 13.08 | ±0.30 ab | 12.77 | ±0.44 ab | 13.56 | ±0.29 a | 0.013 | 0.096 | 0.901 |
| Sum SFA 5 | 54.72 | ±0.99 | 56.21 | ±0.91 | 55.49 | ±1.43 | 56.53 | ±0.86 | 0.248 | 0.614 | 0.839 |
| Total fat content (g/100 g tissue) | 16.09 | ±3.99 | 16.21 | ±3.52 | 16.31 | ±5.70 | 19.95 | ±3.40 | 0.659 | 0.633 | 0.666 |
| Fatty Acid | Supplementation Group | Effect (p-Value) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | EFA | CLA | EFA + CLA | EFA | CLA | EFA × CLA | |||||
| C18:2 c-9, t-11 (CLA) | 49.92 | ±6.30 ab | 35.58 | ±5.78 b | 68.13 | ±9.07 a | 62.58 | ±5.32 a | 0.154 | 0.002 | 0.527 |
| C18:3 n-3 (LA) | 37.86 | ±21.49 c | 120.27 | ±19.72 ab | 24.25 | ±30.95 bc | 179.10 | ±18.15 a | <0.001 | 0.334 | 0.133 |
| C18:4 n-3 (SDA) | 2.48 | ±0.27 a | 1.51 | ±0.25 ab | 1.58 | ±0.39 ab | 1.31 | ±0.23 b | 0.044 | 0.070 | 0.244 |
| C20:5 n-3 (EPA) | 3.47 | ±0.82 b | 9.08 | ±0.73 a | 2.21 | ±1.17 b | 10.68 | ±0.69 a | <0.001 | 0.842 | 0.100 |
| C22:5 n-3 (DPA) | 24.36 | ±2.50 b | 38.88 | ±2.30 a | 15.36 | ±3.61 b | 45.30 | ±2.11 a | <0.001 | 0.635 | 0.009 |
| C22:6 n-3 (DHA) | 13.54 | ±2.18 | 19.78 | ±1.92 | 12.59 | ±3.20 | 20.00 | ±1.81 | 0.008 | 0.874 | 0.798 |
| Sum n-3 PUFA 1 | 84.62 | ±23.39 b | 200.15 | ±21.46 a | 58.14 | ±33.69 b | 270.20 | ±19.75 a | <0.001 | 0.391 | 0.069 |
| C18:2 n-6 (LA) | 571.82 | ±56.81 | 594.53 | ±52.12 | 549.65 | ±81.81 | 663.59 | ±47.97 | 0.274 | 0.702 | 0.467 |
| C20:4 n-6 (ARA) | 113.92 | ±8.34 | 99.50 | ±7.65 | 118.28 | ±12.01 | 107.55 | ±7.04 | 0.173 | 0.492 | 0.841 |
| C22:4 n-6 (ADA) | 45.67 | ±4.96 | 38.90 | ±4.55 | 49.34 | ±7.14 | 44.30 | ±4.19 | 0.279 | 0.400 | 0.874 |
| Sum n-6 PUFA 2 | 884.34 | ±71.73 | 890.02 | ±65.81 | 862.91 | ±103.30 | 978.00 | ±60.57 | 0.441 | 0.668 | 0.490 |
| Sum PUFA 3 | 1035 | ±88 | 1142 | ±81 | 1006 | ±127 | 1326 | ±75 | 0.033 | 0.420 | 0.277 |
| C18:1 c-9 (OA) | 22614 | ±957 | 23,365 | ±839 | 22,018 | ±1374 | 23,662 | ±795 | 0.246 | 0.880 | 0.648 |
| C18:1 t-11 (VA) | 58.18 | ±8.34 | 44.99 | ±7.66 | 50.03 | ±12.02 | 57.02 | ±7.05 | 0.733 | 0.829 | 0.277 |
| Sum MUFA 4 | 29,499 | ±1161 | 30750 | ±1028 | 28,989 | ±1621 | 29,717 | ±977 | 0.408 | 0.504 | 0.820 |
| C16:0 (PA) | 20,678 | ±917 | 19976 | ±829 | 19,867 | ±1211 | 20,328 | ±796 | 0.886 | 0.778 | 0.479 |
| C18:0 (SA) | 6301 | ±485 | 6071 | ±442 | 6591 | ±628 | 6821 | ±426 | 1.000 | 0.214 | 0.580 |
| Sum SFA 5 | 29,546 | ±1294 | 28193 | ±1194 | 28,950 | ±1632 | 29,329 | ±1158 | 0.649 | 0.793 | 0.406 |
| Total fat content (%) | 60.32 | ±1.87 | 60.09 | ±1.72 | 59.06 | ±2.36 | 60.46 | ±1.67 | 0.707 | 0.764 | 0.587 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahl, N.; Albrecht, E.; Dannenberger, D.; Uken, K.L.; Hammon, H.M.; Maak, S. Consequences of Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation on the Development of Calf Muscle and Adipose Tissue. Animals 2020, 10, 1598. https://doi.org/10.3390/ani10091598
Dahl N, Albrecht E, Dannenberger D, Uken KL, Hammon HM, Maak S. Consequences of Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation on the Development of Calf Muscle and Adipose Tissue. Animals. 2020; 10(9):1598. https://doi.org/10.3390/ani10091598
Chicago/Turabian StyleDahl, Nina, Elke Albrecht, Dirk Dannenberger, Katrin L. Uken, Harald M. Hammon, and Steffen Maak. 2020. "Consequences of Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation on the Development of Calf Muscle and Adipose Tissue" Animals 10, no. 9: 1598. https://doi.org/10.3390/ani10091598
APA StyleDahl, N., Albrecht, E., Dannenberger, D., Uken, K. L., Hammon, H. M., & Maak, S. (2020). Consequences of Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation on the Development of Calf Muscle and Adipose Tissue. Animals, 10(9), 1598. https://doi.org/10.3390/ani10091598






