A Camera-Trap Home-Range Analysis of the Indian Leopard (Panthera pardus fusca) in Jaipur, India
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
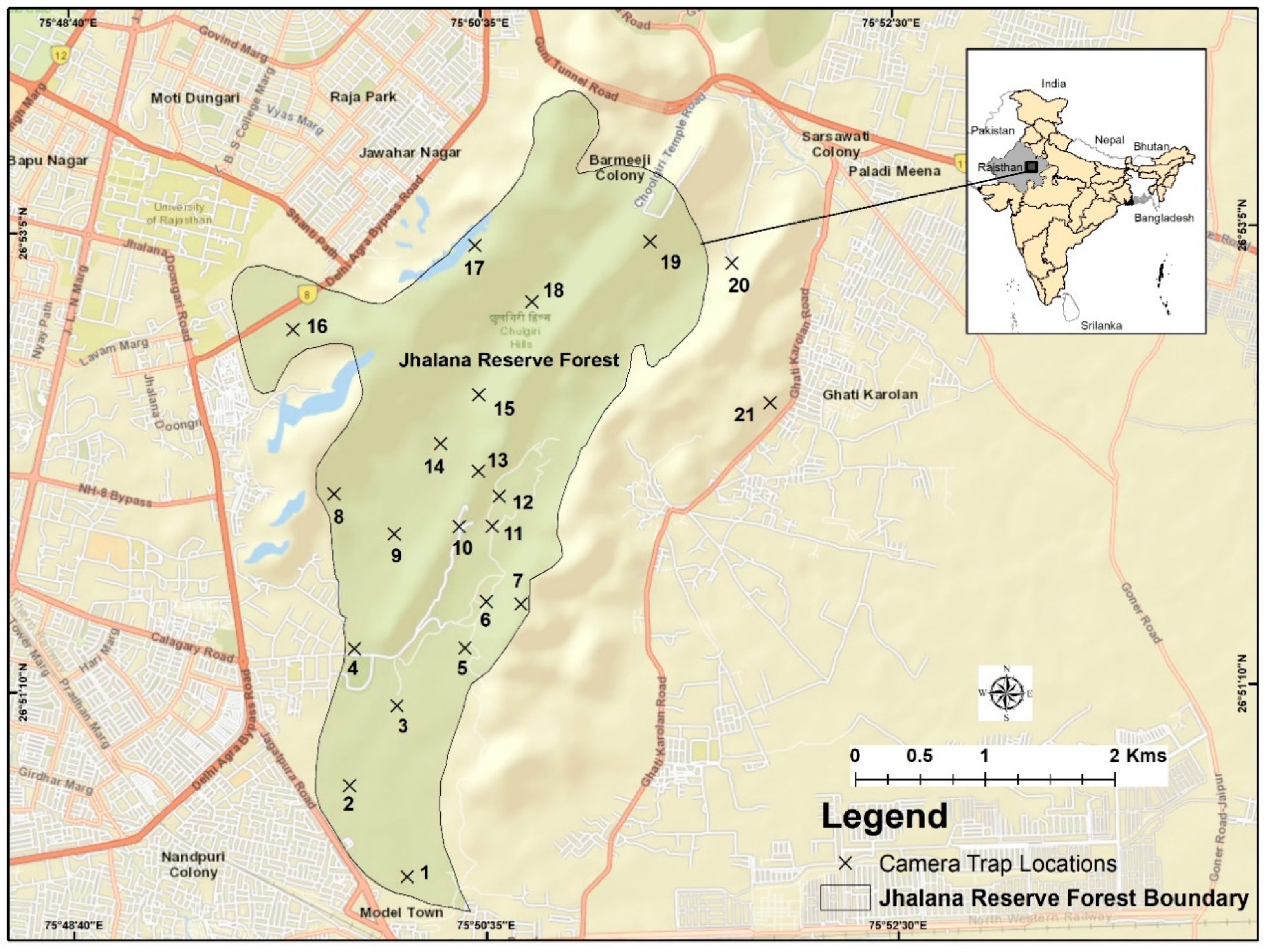
2.2. Study Area
2.3. Field Methods
2.4. Data Analyses
3. Results
3.1. Home Ranges and Core Areas of Male Leopards
3.2. Home Ranges and Core Areas of Female Leopards
3.3. Familial Overlap
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pallemaerts, L.; Adul, I.P.; Jeffers, K.A.; Macdonald, D.W.; Cheyne, S.M. Bornean clouded leopard minimum home range analysis. Indonesian Borneo. Cat News 2019, 70, 32–35. [Google Scholar]
- Burt, W. Territoriality and home range concepts as applied to mammals. Mammal 1943, 24, 346–352. [Google Scholar] [CrossRef]
- Börger, L.; Dalziel, B.D.; Fryxell, J.M. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol. Lett. 2008, 11, 637–650. [Google Scholar] [CrossRef]
- Kernohan, B.J.; Gitzen, R.A.; Millspaugh, J.J. Analysis of animal space use and movements. In Radio-tracking and Animal Populations; Academic Press: San Diego, CA, USA, 2001; pp. 126–166. [Google Scholar]
- Harestad, A.S.; Bunnel, F.L. Home range and body weight–A re-evaluation. Ecology 1979, 60, 389–402. [Google Scholar] [CrossRef]
- Haskell, J.P.; Ritchie, M.E.; Olff, H. Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature 2002, 418, 527–530. [Google Scholar] [CrossRef]
- Morellet, N.; Bonenfant, C.; Borger, L.; Ossi, F.; Cagnacci, F.; Heurich, M.; Kjellander, P.; Linnell, J.D.C.; Nicoloso, S.; Sustr, P.; et al. Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within Europe. Anim. Ecol. 2013, 82, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Tamburello, N.; Côté, I.M.; Dulvy, N.K. Energy and the scaling of animal space use. Am. Nat. 2015, 186, 196–211. [Google Scholar] [CrossRef]
- Johansson, Ö.; Rauset, G.R.; Samelius, G.; McCarthy, T.; Andrén, H.; Tumursukh, L.; Mishra, C. Land sharing is essential for snow leopard conservation. Biol. Conserv. 2016, 203, 1–7. [Google Scholar] [CrossRef]
- Treves, A.; Karanth, K.U. Human-carnivore conflict and perspectives on carnivore management worldwide. Conserv. Biol. 2003, 17, 1491–1499. [Google Scholar] [CrossRef]
- Sunquist, M.; Sunquist, F. Wild Cats of the World; The University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Karanth, K.U.; Nichols, J.D. Estimation of tiger densities in India using photographic captures and recaptures. Ecology 1998, 79, 2852–2862. [Google Scholar] [CrossRef]
- Carbone, C.; Christie, C.S.K.; Coulson, T.; Franklin, N.; Ginsberg, J.R.; Griffiths, M.; Holden, J.; Kawanishi, L.; Kinnaird, M.; Laidlaw, R.; et al. The use of photographic rates to estimate of tigers and other cryptic mammals. Anim. Conserv. 2001, 4, 75–79. [Google Scholar] [CrossRef]
- Wallace, R.B.; Gomez, H.; Ayala, G.; Espinoza, F. Camera trapping of jaguar (Panthera onca) in the Tuichi Valley, Bolivia. Mastozool. Neotrop. 2003, 10, 133–139. [Google Scholar]
- Maffei, L.; Cuéllar, E.; Noss, A. One thousand jaguars (Panthera onca) in Bolivia’s Chaco? Camera trapping in the Kaa-Iya National Park. J. Zool. 2004, 262, 295–304. [Google Scholar] [CrossRef]
- Silver, S.C.; Ostro, L.E.T.; Marsh, L.K.; Maffei, L.; Noss, A.J.; Kelly, M.J.; Wallace, R.B.; Gomez, H.; Ayala, G. The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis. Oryx 2004, 38, 1–7. [Google Scholar] [CrossRef]
- Soisalo, M.K.; Cavalcanti, S.M.C. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio telemetry. Biol. Conserv. 2006, 129, 487–496. [Google Scholar] [CrossRef]
- Ghoddousi, A.; Hamidi, A.H.K.; Ghadirian, T.; Ashayeri, T.D.; Moshiri, H.; Khorozyan, I. The status of the Persian leopard in Bamu National Park, Iran. Cat News 2008, 49, 10–13. [Google Scholar]
- Jackson, R.M.; Rhoe, S.D.; Wangchuk, R.; Hunter, D.D. Surveying snow leopard populations with emphasis on camera trapping. In A Handbook; The Snow Leopard Conservancy: Sonoma, CA, USA, 2005. [Google Scholar]
- Marker, L.L.; Fabiano, E.; Nghikembua, M. The use of remote camera traps to estimate density of free ranging cheetahs in North-Central Namibia. Cat News 2008, 49, 22–24. [Google Scholar]
- Trolle, M.; Kery, M. Estimation of ocelot density in the Pantanal using capture-recapture analysis of camera-trapping data. J. Mammal. 2003, 84, 607–614. [Google Scholar] [CrossRef]
- Gil-Sanchez, J.M.; Moral, M.; Bueno, J.; Rodrigues-Siles, J.; Lillo, S.; Perez, J.; Martin, J.M.; Valenzuela, G.; Garrote, G.; Torralba, B.; et al. The use of camera trapping for estimating Iberian lynx (Lynx pardinus) home ranges. Eur. J. Wildl. Res. 2011. [Google Scholar] [CrossRef]
- Maffei, L.; Noss, A. How small is too small? Camera trap survey areas and density estimates for ocelots in the Bolivian Chaco. Biotropica 2008, 40, 71–75. [Google Scholar] [CrossRef]
- Johansson, O.; Koehler, G.; Rauset, G.R.; Samelius, G.; Andren, H.; Mishra, C.; Lhagvasuren, P.; McCarthy, T.; Low, M. Sex-specific seasonal variation in puma and snow leopard home range utilization. Ecosphere 2018. [Google Scholar] [CrossRef]
- Goodrich, J.M.; Miquelle, D.G.; Smirnov, E.N.; Kerley, L.L.; Quigley, H.B.; Hornocker, M.G. Spatial structure of Amur (Siberian) tigers (Panthera tigris altaica) on Sikhote-Alin Biosphere Zapovednik, Russia. J. Mammal. 2010, 91, 737–748. [Google Scholar] [CrossRef]
- Lukarevsky, V.; Vereshchangin, A.P.; Lukarevsky, A.V. The spatial structure of a snow leopard population (Panthera uncia, Felidae, carnivora) in east Kyrgyzstan. Ecol. Montenegrina 2020, 33, 17–28. [Google Scholar] [CrossRef]
- Naude, V.N.; Balme, G.A.; O’Rain, J.; Hunter, L.T.B.; Fattebert, J.; Dickerson, T.; Bishop, J.M. Unsustainable anthropogenic mortality disrupts natal dispersal and promotes inbreeding in leopards. Ecol. Evol. 2020. [Google Scholar] [CrossRef]
- Sandell, M. The mating tactics and spacing patterns of solitary carnivores. In Carnivore Behavior, Ecology, and Evolution; Gittleman, J.L., Ed.; Springer: Boston, MA, USA, 1989; pp. 164–182. [Google Scholar]
- Rabinowitz, A. The density and behaviour of large cats in a dry tropical forest in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Nat. Hist. Soc. Bull. Siam Soc. 1989, 37, 235–251. [Google Scholar]
- Karanth, K.U.; Sunquist, M.E. Behavioral correlates of predation by tiger (Panthera tigris), leopard (Panthera pardus) and dhole (Cuon alpinus) in Nagarhole, India. J. Zool. 2000, 250, 255–265. [Google Scholar] [CrossRef]
- Eisenberg, J.F.; Lockhart, M. An Ecological Reconnaissance of Wilpattu National Park, Ceylon; Smithsonian University Press: Washington, DC, USA, 1972. [Google Scholar]
- Grassman, L.I., Jr. Ecology and behavior of the Indochinese leopard in Kaeng Krachan National Park, Thailand. Nat. Hist. Bull. Siam. Soc. 1999, 47, 77–93. [Google Scholar]
- Rich, L.N.; Mitchell, M.S.; Gude, J.A.; Sime, C.A. Anthropogenic mortality, intraspecific competition, and prey availability influence territory sizes of wolves in Montana. J. Mammal. 2012, 93, 722–731. [Google Scholar] [CrossRef]
- Mondal, k.; Bhattacharjee, S.; Gupta, S.; Sankar, K.; Qureshi, Q. Home range and resource selection of ‘problem’ leopards trans-located to forested habitat. Curr. Sci. 2013, 105, 338–345. [Google Scholar]
- Pilfold, N.W.; Derocher, A.E.; Richardson, E. Influence of intraspecific competition on the distribution of a wide-ranging, non-territorial carnivore. Glob. Ecol. Biogeogr. 2014, 23, 425–435. [Google Scholar] [CrossRef]
- Kumar, A.V.; Karanth, K.U.; Jathanna, D. Tigers and leopards coexist despite similarities in space use and habitat selection. Catnews 2020, 71, 20–23. [Google Scholar]
- Noor, A.; Mir, Z.R.; Veeraswami, G.G.; Habib, B. Density of leopard in a moist-temperate forest of western Himalaya, India. Trop. Ecol. 2020. [Google Scholar] [CrossRef]
- Odden, M.; Athreya, V.; Rattan, S.; Linnell, J.D.C. Adaptable Neighbours: Movement patterns of GPS-Collared Leopards in Human Dominated Landscapes in India. PLoS ONE 2014, 9, e112044. [Google Scholar] [CrossRef] [PubMed]
- Anco, C.; Kolokotronis, S.-O.; Henschel, P.; Cunningham, S.W.; Amato, G.; Hekkala, E. Historical mitochondrial diversity in African leopards (Panthera pardus) revealed by archival museum specimens. Mitochondrial DNA Part A 2018, 29, 455–473. [Google Scholar] [CrossRef]
- Fischer, J.; Abson, D.J.; Butsic, V.; Chappell, M.J.; Ekroos, J.; Hanspach, J.; Keummerle, T.; Smith, H.G.; Von Wehrden, H. Land sparing vs land sharing: Moving forward. Conserv. Lett. 2014, 7, 149–157. [Google Scholar] [CrossRef]
- Kumbhojkar, S.; Yosef, R.; Kosicki, J.; Kwiatkowska, P.; Tryjanowski, P. Coexistence with humans influences the diet of leopards (Panthera pardus fusca). Oryx 2020, in press. [Google Scholar]
- Tarugara, A.; Clegg, B.W.; Gandiwa, E.; Muposhi, V.K. Cost-benefit analysis of increasing sampling effort in a baited camera trap survey of an African leopard (Panthera pardus) population. Glob. Ecol. Conserv. 2019, 18, e00627. [Google Scholar] [CrossRef]
- Cheyne, S.M.; Stark, D.J.; Limin, S.H.; Macdonald, D.W. First estimates of population ecology and threats to Sunda clouded leopards Neofelis diardi in a peat-swamp forest, Indonesia. Endanger. Species Res. 2013, 22, 1–9. [Google Scholar] [CrossRef]
- Srivathsa, A.; Kumar, S.N.; Karanth, K.U. Home range size of the dhole from camera trap surveys. Canid Biol. Conserv. 2017, 20, 1–4. [Google Scholar]
- Simcharoen, S.; Pattanavibool, A.; Karanth, K.U.; Nichols, J.D.; Kumar, N.S. How many tigers Panthera tigris are there in Huai Kha Khaeng Wildlife Sanctuary, Thailand? An estimate using photographic capture-recapture sampling. Oryx 2007, 41, 447–453. [Google Scholar] [CrossRef]
- Athreya, V.; Odden, M.; Linnell, J.D.C.; Krishnaswamy, J.; Karanth, K.U. Big cats in our backyards: Persistence of large carnivores in a human dominated landscape in India. PLoS ONE 2013, 3, e57872. [Google Scholar] [CrossRef] [PubMed]
- Kalle, R.; Ramesh, T.; Qureshi, Q.; Sankar, K. Density of tiger and leopard in a tropical deciduous forest of Mudumalai Tiger Reserve, southern India, as estimated using photographic capture-recapture sampling. Acta Theriol. 2011, 56, 335–342. [Google Scholar] [CrossRef]
- Devens, C.; Tshabalala, T.; McManus, J.; Smuts, B. Counting the spots: Use of a spatially explicit capture-recapture technique and GPS data to estimate leopard (Panthera pardus) density in Eastern and Western Cape, South Africa. Afr. J. Ecol. 2018, 56, 850–859. [Google Scholar] [CrossRef]
- Srivathsa, A.; Parameshwaran, R.; Sharma, S.; Karanth, K.U. Estimating population sizes of leopard cats in the Western Ghats using camera surveys. J. Mammal. 2015, 96, 742–750. [Google Scholar] [CrossRef]
- Johansson, Ö.; Samelius, G.; Wikberg, E.; Chapron, G.; Mishra, C.; Low, M. Identification errors in camera-trap studies result in systematic population overestimation. Sci. Rep. 2020, 10, 6393. [Google Scholar] [CrossRef] [PubMed]
- Pacas, C.J.; Paquet, P.C. Analysis of black bear home-range using a geographic information system. Bears Biol. Manag. 1994, 9, 419–425. [Google Scholar] [CrossRef]
- Anderson, D.J. A new non parametric estimation technique: Ecological archives. Ecology 1982, 63, 103–112. [Google Scholar] [CrossRef]
- Kang, T.H.; Kim, D.H.; Lee, H.; Cho, H.J.; Hur, W.H.; Han, S.H.; Kim, Y.J.; Paek, W.K.; Jin, S.D.; Paik, I.H. Analysis of home range of Eurasian eagle owl (Bubo bubo) by WT-100. J. Asia Pac. Biodivers. 2013, 6, 369–373. [Google Scholar] [CrossRef]
- Boyle, S.A.; Lourenco, W.C.; Silva, L.R.D.; Smith, A.T. Home range estimates vary with sample size and methods. Folia Primatol. 2008, 80, 33–42. [Google Scholar] [CrossRef]
- Downs, J.A.; Horner, M.W. A characteristic-hull based method for home range estimation. Trans. GIS J. 2009, 13, 527–537. [Google Scholar] [CrossRef]
- Sankar, K.; Pabla, H.S.; Patil, C.K.; Nigam, P.; Qureshi, Q.; Navneethan, B.; Manjreakar, M.; Virkar, P.S.; Mondal, K. Home range, habitat use of re-introduced gaur (Bos gauras gauras) in Bandhavgarh Tiger Reserve, Central India. Trop. Conserv. Sci. 2013, 6, 50–69. [Google Scholar] [CrossRef]
- Stein, A.B.; Fuller, T.K.; Destefano, S.; Marker, L.L. Leopard population and home range estimates in north-central Namibia. Afr. J. Ecol. 2011, 49, 383–387. [Google Scholar] [CrossRef]
- Bailey, T.N. The African Leopard; Columbia University Press: New York, NY, USA, 1993; p. 429. [Google Scholar]
- Iliany, G. Leopard Panthera Pardus in Israel. Cat News 1990, 12, 4–5. [Google Scholar]
- Jackson, C.R.; Groom, R.J.; Jordan, N.R.; McNutt, J.W. The effect of relatedness and pack size in territory overlap in African wild dogs. Mov. Ecol. 2017, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.B.W.; Trillmich, F. Kin in space: Social viscosity in a spatially and genetically substructured network. R. Soc. Lond. B Biol. Sci. 2008, 22, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Fattebert, J.; Robinson, H.S.; Balme, G.; Slotow, R.; Hunter, L. Structural habitat predicts functional dispersal habitat of a large carnivore: How leopards change spots. Ecol. Appl. 2015, 25, 1911–1921. [Google Scholar] [CrossRef]
- Pulliam, H.R. Sources and sinks: Empirical evidence and population consequences. In Population Dynamics in Ecological Space and Time; Rhodes, O.E., Chesser, R.K., Smith, M.H., Eds.; University of Chicago Press: Chicago, IL, USA, 1996; pp. 45–69. [Google Scholar]
- Sangay, T.; Rajaratnam, R.; Vernes, K. Wildlife Camera trapping in the Himalayan Kingdom of Bhutan with recommendations for the future. Wildl. Manag. Res. 2014, 87–98. [Google Scholar]
- Blomqvist, L.; Sten, I. Reproductive biology of the snow leopard, Panthera uncia. Int. Ped. Book Snow Leopards 1982, 2, 71–79. [Google Scholar]
- Smith, J.L.D.; McDougal, C. The contribution of variance in lifetime reproduction to effective population size in tigers. Conserv. Biol. 1991, 5, 484–490. [Google Scholar] [CrossRef]
- Jackson, R. Home Range, Movement and Habitat Use of Snow Leopard (Uncia uncia) in Nepal. Ph.D. Thesis, University of London, London, UK, 1996. [Google Scholar]
- Jackson, R.; Ahlborn, G. Snow leopards (Panthera uncia) in Nepal- Home range and movements. Nat. Geo. Res. 1989, 5, 161–175. [Google Scholar]
- Chundawat, R.S. Habitat selection by a snow leopard in Hemis National Park, India. Int. Ped. Book Snow Leopards 1990, 6, 85–92. [Google Scholar]
- Kshettry, A.; Vaidyanathan, S.; Athreya, A. Leopard in a tea-cup: A stusy of leopard habitat-use and human-leopard interaction in north-eastern India. PLoS ONE 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Sunquist, M.; Sunquist, F. Handbook of the Mammals of the World. 1. Carnivores; Lynx Edicions: Barcelona, Spain, 2009; pp. 133–134. [Google Scholar]
- Ostro, L.E.; Young, T.P.; Silver, S.C.; Koontz, F.W. A geographic information system method for estimating home range size. J. Wildl. Manag. 1999, 63, 748–755. [Google Scholar] [CrossRef]
- Oli, M.K. Winter home ranges of snow leopard in Nepal. Mammalia 1997, 61, 355–360. [Google Scholar]
- Odden, M.; Wegge, P. Spacing and activity patterns of leopards Panthera pardus in Royal Bardia National Park, Nepal. Wildlife Biol. 2004, 11, 145–152. [Google Scholar] [CrossRef]
- Marker, L.L.; Dickman, A.J. Factors affecting leopard (Panthera pardus) spatial ecology, with particular reference to Namibian farmlands. S. Afr. J. Wildl. Res. 2005, 35, 105–115. [Google Scholar]
- Jenny, D. Spatial organisation of leopards Panthera pardus in Tai National Park, Ivory Coast: Is Rainforest habitat a ‘tropical haven’? J. Zool. 1996, 240, 427–440. [Google Scholar] [CrossRef]
- Simcharoen, S.; Barlow, A.C.; Simcharoen, A.; Smith, J.L. Home range size and daytime habitat selection of leopards in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Biol. Conserv. 2008, 141, 2242–2250. [Google Scholar] [CrossRef]
- Steyn, V.; Funston, P.J. Land-use and socio-spatial organization of female leopards in a semi-arid wooded savanna, Botswana. S. Afr. J. Wildl. Res. 2009, 39, 126–132. [Google Scholar] [CrossRef]
- Khan, U.; Ferretti, F.; Shah, S.A.; Lovari, S. A large carnivore among people and livestock: The common leopard. In Problematic Wildlife II; Springer: Cham, Switzerland, 2020; pp. 93–110. [Google Scholar]
- Karanth, K.U.; Sunquist, M.E. Prey selection by tiger, leopard and dhole in tropical forests. J. Anim. Ecol. 1995, 64, 439–450. [Google Scholar] [CrossRef]
- Breitenmoser, U.; Angst, C.; Landry, J.M.; Breitenmoser-Wursten, C.; Linnell, J.D.C.; Weber, J. Non-lethal techniques for reducing depredation. In People and Wildlife. Conflict or Co-Existence; Woodroffe, R., Thirgood, S., Rabinowitz, A., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 49–71. [Google Scholar]
- Kandel, S.R.; Lamichhane, B.R.; Subedi, N. Leopard (Pantera pardus) density and diet in a forest corridor of Terai: Implications for conservation and conflict management. Wild. Res. 2020. [Google Scholar] [CrossRef]
- Thapa, T.B. Habitat Suitability Evaluation for Leopard (Panthera pardus) Using Remote Sensing and GIS in and around Chitwan National Park, Nepal. Ph.D. Thesis, Saurashtra University, Rajkot, India, 2011. [Google Scholar]
- Lamichhane, B.R.; Leirs, H.; Persoon, G.A.; Subedi, N.; Dhakal, M.; Oli, B.N.; Malla, S. Factors associated with co-occurrence of large carnivores in a human-dominated landscape. Biol. Cons. 2019, 28, 1473–1491. [Google Scholar] [CrossRef]
- Thapa, K.; Shrestha, R.; Karki, J.; Thapa, G.J.; Subedi, N.; Pradhan, N.M.B.; Kelly, M.J. Leopard Panthera pardus fusca,density in the seasonally dry, subtropical forest in the Bhabhar of Terai Arc, Nepal. Adv. Ecol. 2014, 286949. [Google Scholar] [CrossRef]













| Sr. No. | Leopard | Sex | No. of Photo-Captures/Individual | No. of Photo-Captures Discarded/Individual Due to Repetition | Home Range (MCP) km2 | Core Range (KDE) km2 |
|---|---|---|---|---|---|---|
| 1 | Sultan | M | 11 | 3 | 1.28 | 0.062 |
| 2 | Baghira | M | 61 | 23 | 3.06 | 0.355 |
| 3 | Bahadur | M | 33 | 11 | 3.49 | 0.772 |
| 4 | Rambo | M | 116 | 46 | 4.18 | 0.712 |
| 5 | Unidentified male_1 | M | 10 | 2 | 0.25 | 0.111 |
| 6 | Nathwali | F | 18 | 6 | 0.05 | 0.048 |
| 7 | Zara | F | 11 | 1 | 0.29 | 0.185 |
| 8 | Sharmili | F | 46 | 18 | 1.51 | 0.109 |
| 9 | Leela | F | 43 | 13 | 1.58 | 0.341 |
| 10 | Flora | F | 51 | 19 | 1.82 | 0.106 |
| 11 | Mrs Khan | F | 103 | 64 | 3.06 | 0.146 |
| 12 | Tim tim | F | 35 | 22 | 3.36 | 3.36 |
| 13 | Jalebi | F | 51 | 26 | 3.39 | 3.39 |
| 14 | Juliet | F | 8 | 0 | 2.24 | 0.995 |
| 15 | Cleopatra | F | 11 | 0 | 1.57 | 1.097 |
| 16 | LK Female | F | 4 | 0 | NI | NI |
| 17 | Basanti | F | 3 | 0 | NI | NI |
| 18 | Unidentified F1 | F | 1 | 0 | NI | NI |
| 19 | Unidentified F2 | F | 3 | 0 | NI | NI |
| 20 | Laxmi | F | 7 | 0 | NI | NI |
| 21 | Budi Ma | F | 3 | 0 | NI | NI |
| 22 | Tim tim cub | F | 15 | 0 | 3.36 | 0.268 |
| 23 | Unidentified M2 | M | 1 | 0 | NI | NI |
| 24 | Unidentified M3 | M | 5 | 0 | NI | NI |
| 25 | Flora cub | M | 11 | 0 | NI | NI |
| N | Sultan | Baghira | Bahadur | Rambo | UM | Nathwali | Zara | Sharmili | Leela | Flora | Mrs Khan | Tim Tim | Jalebi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | 8 | 5 | 5 | |||||||||
| 2 | |||||||||||||
| 3 | 2 | 8 | 8 | 3 | |||||||||
| 4 | |||||||||||||
| 5 | 64 | 13 | 50 | 72 | 22 | 26 | |||||||
| 6 | 7 | 3 | 8 | 3 | 53 | 2 | 25 | ||||||
| 7 | 3 | ||||||||||||
| 8 | 1 | 38 | |||||||||||
| 9 | 7 | 26 | 16 | 3 | 3 | 48 | 7 | ||||||
| 10 | 4 | 2 | 8 | 2 | 7 | 1 | 20 | ||||||
| 11 | 2 | 4 | |||||||||||
| 12 | 3 | 1 | 4 | 5 | |||||||||
| 13 | 7 | 4 | 19 | 2 | 6 | ||||||||
| 14 | 7 | 3 | 2 | 20 | 25 | 4 | 10 | 6 | 3 | ||||
| 15 | 7 | 2 | 3 | 20 | 7 | 3 | 4 | ||||||
| 16 | |||||||||||||
| 17 | 4 | ||||||||||||
| 18 | 42 | 2 | 49 | 75 | 17 | 42 | 5 | ||||||
| 19 | 33 | 7 | 54 | ||||||||||
| 20 | 12 | 50 | 9 | 38 | |||||||||
| 21 | 28 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumbhojkar, S.; Yosef, R.; Mehta, A.; Rakholia, S. A Camera-Trap Home-Range Analysis of the Indian Leopard (Panthera pardus fusca) in Jaipur, India. Animals 2020, 10, 1600. https://doi.org/10.3390/ani10091600
Kumbhojkar S, Yosef R, Mehta A, Rakholia S. A Camera-Trap Home-Range Analysis of the Indian Leopard (Panthera pardus fusca) in Jaipur, India. Animals. 2020; 10(9):1600. https://doi.org/10.3390/ani10091600
Chicago/Turabian StyleKumbhojkar, Swapnil, Reuven Yosef, Abhinav Mehta, and Shrey Rakholia. 2020. "A Camera-Trap Home-Range Analysis of the Indian Leopard (Panthera pardus fusca) in Jaipur, India" Animals 10, no. 9: 1600. https://doi.org/10.3390/ani10091600
APA StyleKumbhojkar, S., Yosef, R., Mehta, A., & Rakholia, S. (2020). A Camera-Trap Home-Range Analysis of the Indian Leopard (Panthera pardus fusca) in Jaipur, India. Animals, 10(9), 1600. https://doi.org/10.3390/ani10091600







