Application of a Handheld Near-Infrared Spectrometer to Predict Gelatinized Starch, Fiber Fractions, and Mineral Content of Ground and Intact Extruded Dry Dog Food
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Reference Analyses
2.3. Near-Infrared Spectroscopy Analysis
2.4. Chemometric Analysis
3. Results
3.1. Chemical Composition
3.2. Handheld Near-Infrared Prediction Models
4. Discussion
4.1. Chemical Composition Stated in the Label
4.2. Chemical Composition and Handheld NIRS Evaluation
4.3. Near Infrared Spectrum
4.4. Prediction Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weber, M.P.; Biourge, V.C.; Nguyen, P.G. Digestive sensitivity varies according to size of dogs: A review. J. Anim. Physiol. Anim. Nutr. (Berl). 2017, 101, 1–9. [Google Scholar] [CrossRef]
- Inal, F.; Alatas, M.S.; Kahraman, O.; Inal, S.; Uludağ, M.; Gürbüz, E.; Polat, E.S. Barley as an alternative to rice in dog food. Turkish J. Vet. Anim. Sci. 2017, 41, 770–774. [Google Scholar] [CrossRef]
- Li, E.; Dhital, S.; Hasjim, J. Effects of grain milling on starch structures and flour/starch properties. Starch/Staerke 2013, 66, 15–27. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Fortes, C.M.L.S.; Carciofi, A.C.; Sakomura, N.K.; Kawauchi, I.M.; Vasconcellos, R.S. Digestibility and metabolizable energy of some carbohydrate sources for dogs. Anim. Feed Sci. Technol. 2010, 156, 121–125. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Fahey, G.C.; Merchen, N.R.; Corbin, J.E.; Hamilton, A.K.; Serbe, K.A.; Lewis, S.M.; Hirakawa, D.A. Dietary fiber for dogs: I. Effects of graded levels of dietary beet pulp on nutrient intake, digestibility, metabolizable energy and digesta mean retention time. J. Anim. Sci. 1990, 68, 4221–4228. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dogs and Cats; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Russell, J.; Bass, P. Canine gastric emptying of fiber meals: Influence of meal viscosity and antroduodenal motility. Am. J. Physiol. 1985, 249, 662–667. [Google Scholar] [CrossRef]
- De-Oliveira, L.D.; Takakura, F.S.; Kienzle, E.; Brunetto, M.A.; Teshima, E.; Pereira, G.T.; Vasconcellos, R.S.; Carciofi, A.C. Fibre analysis and fibre digestibility in pet foods - a comparison of total dietary fibre, neutral and acid detergent fibre and crude fibre. J. Anim. Physiol. Anim. Nutr. (Berl). 2012, 96, 895–906. [Google Scholar] [CrossRef]
- Rosol, T.J.; Capen, C.C. Pathophysiology of calcium, phosphorus, and magnesium metabolism in animals. Vet. Clin. North Am.-Small Anim. Pract. 1996, 26, 1155–1184. [Google Scholar] [CrossRef]
- Cline, J. Calcium and Vitamin D Metabolism, Deficiency, and Excess. Top. Companion Anim. Med. 2012, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.; De Nadai Fernandes, E.A.; Bacchi, M.A. Neutron activation analysis for assessing chemical composition of dry dog foods. J. Radioanal. Nucl. Chem. 2012, 291, 245–250. [Google Scholar] [CrossRef]
- Pereira, A.M.; Pinto, E.; Matos, E.; Castanheira, F.; Almeida, A.A.; Baptista, C.S.; Segundo, M.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Mineral composition of dry dog foods: Impact on nutrition and potential toxicity. J. Agric. Food Chem. 2018, 66, 7822–7830. [Google Scholar] [CrossRef] [PubMed]
- Goi, A.; Manuelian, C.L.; Currò, S.; De Marchi, M. Prediction of mineral composition in commercial extruded dry dog food by near-infrared reflectance spectroscopy. Animals 2019, 9, 640. [Google Scholar] [CrossRef] [Green Version]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef] [Green Version]
- Zontov, Y.V.; Balyklova, K.S.; Titova, A.V.; Rodionova, O.Y.; Pomerantsev, A.L. Chemometric aided NIR portable instrument for rapid assessment of medicine quality. J. Pharm. Biomed. Anal. 2016, 131, 87–93. [Google Scholar] [CrossRef]
- Kademi, H.I.; Ulusoy, B.H.; Hecer, C. Applications of miniaturized and portable near infrared spectroscopy (NIRS) for inspection and control of meat and meat products. Food Rev. Int. 2018, 35, 201–220. [Google Scholar] [CrossRef]
- Thong, Y.J.; Nguyen, T.; Zhang, Q.; Karunanithi, M.; Yu, L. Predicting food nutrition facts using pocket-size near-infrared sensor. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Seogwipo, South Korea, 11–15 July 2017; pp. 742–745. [Google Scholar]
- Li, M.; Qian, Z.; Shi, B.; Medlicott, J.; East, A. Evaluating the performance of a consumer scale SCiOTM molecular sensor to predict quality of horticultural products. Postharvest Biol. Technol. 2018, 145, 183–192. [Google Scholar] [CrossRef]
- Kartakoullis, A.; Comaposada, J.; Cruz-Carrión, A.; Serra, X.; Gou, P. Feasibility study of smartphone-based Near Infrared Spectroscopy (NIRS) for salted minced meat composition diagnostics at different temperatures. Food Chem. 2019, 278, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.B.; Babu, K.S.; Amamcharla, J.K. Prediction of total protein and intact casein in cheddar cheese using a low-cost handheld short-wave near-infrared spectrometer. Lwt 2019, 109, 319–326. [Google Scholar] [CrossRef]
- De Marchi, M.; Penasa, M.; Zidi, A.; Manuelian, C.L. Invited review: Use of infrared technologies for the assessment of dairy products—Applications and perspectives. J. Dairy Sci. 2018, 101, 10589–10604. [Google Scholar] [CrossRef] [Green Version]
- Manuelian, C.L.; Currò, S.; Penasa, M.; Cassandro, M.; De Marchi, M. Characterization of major and trace minerals, fatty acid composition, and cholesterol content of Protected Designation of Origin cheeses. J. Dairy Sci. 2017, 100, 3384–3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- İnal, F.; Alataş, M.S.; Kahraman, O.; İnal, Ş.; Uludağ, M.; Gürbüz, E.; Polat, E.S. Using of Pelleted and Extruded Foods in Dog Feeding. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 131–136. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O.; Abrams, S. Protocol for NIR calibrations: Monitoring analysis results and recalibration. In Near Infrared Spectroscopy (NIRS): Analysis of Forage Quality. USDA-ARS Agriculture Handbook, No. 643; Martens, G., Shenk, J., Barton, F., Eds.; US Government Printing Office: Washington, DC, USA, 1989; pp. 104–110. [Google Scholar]
- Williams, P. The RPD Statistic: A Tutorial Note. NIR News 2014, 25, 22–26. [Google Scholar] [CrossRef]
- Hall, M.B. Determination of dietary starch in animal feeds and pet food by an enzymatic-colorimetric method: Collaborative study. J. AOAC Int. 2015, 98, 397–409. [Google Scholar] [CrossRef]
- Tran, Q.D.; van Lin, C.G.J.M.; Hendriks, W.H.; van der Poel, A.F.B. Lysine reactivity and starch gelatinization in extruded and pelleted canine diets. Anim. Feed Sci. Technol. 2007, 138, 162–168. [Google Scholar] [CrossRef]
- Tran, Q.D.; Hendrix, W.H.; van der Poel, A.F.B. Effects of extrusion processing on nutrients in dry pet food. J. Sci. Food Agric. 2008, 1926, 1487–1493. [Google Scholar] [CrossRef]
- Sallander, M.; Hedhammar, Å.; Rundgren, M.; Lindberg, J.E. A Study on the Nutrient and Energy Content of Commercial Dog Feeds; 2001; pp. 1–17. Available online: https://pub.epsilon.slu.se/1501/6/MarSalFINAL5.pdf (accessed on 12 September 2020).
- Opitz, B.; Smith, P.M.; Kienzle, E.; Earle, K.E.; Maskell, I.E. Comparison of various methods of fiber analysis in pet foods. J. Nutr. 1998, 128, 2795S–2797S. [Google Scholar] [CrossRef]
- Heuberger, R.; Wakshlag, J. The relationship of feeding patterns and obesity in dogs. J. Anim. Physiol. Anim. Nutr. (Berl). 2011, 95, 98–105. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Ahlstrøm, Ø.; Skredey, A. Nutrient Digestibility of Commercial Dog Foods Using Mink as a Model. Growth (Lakeland) 2004, 134, 2141S–2144S. [Google Scholar] [CrossRef]
- Alomar, D.; Hodgkinson, S.; Abarzúa, D.; Fuchslocher, R.; Alvarado, C.; Rosales, E. Nutritional evaluation of commercial dry dog foods by near infrared reflectance spectroscopy. J. Anim. Physiol. Anim. Nutr. (Berl). 2006, 90, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Murray, I. Effect of sample presentation and animal muscle species on the analysis of meat by near infrared reflectance spectroscopy. J. Near Infrared Spectrosc. 2002, 10, 37–44. [Google Scholar] [CrossRef]
- Ozaki, Y. Near-Infrared Spectroscopy—Its Versatility in Analytical. Anal. Sci. 2012, 28, 545–562. [Google Scholar] [CrossRef] [Green Version]
- Šašić, S.; Ozaki, Y. Short-wave near-infrared spectroscopy of biological fluids. 1. Quantitative analysis of fat, protein, and lactose in raw milk by partial least-squares regression and band assignment. Anal. Chem. 2001, 73, 64–71. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 1987; ISBN 091325049X. [Google Scholar]
- Cozzolino, D.; Barlocco, N.; Vadell, A.; Ballesteros, F.; Gallieta, G. The use of visible and near-infrared reflectance spectroscopy to predict colour on both intact and homogenised pork muscle. LWT-Food Sci. Technol. 2003, 36, 195–202. [Google Scholar] [CrossRef]
- Goi, A.; Manuelian, C.L.; Righi, F.; De Marchi, M. At-line prediction of gelatinized starch and fiber fractions in extruded dry dog food using different near-infrared spectroscopy technologies. Animals 2020, 10, 862. [Google Scholar] [CrossRef]
- Pérez-Marín, D.; Paz, P.; Guerrero, J.E.; Garrido-Varo, A.; Sánchez, M.T. Miniature handheld NIR sensor for the on-site non-destructive assessment of post-harvest quality and refrigerated storage behavior in plums. J. Food Eng. 2010, 99, 294–302. [Google Scholar] [CrossRef]
- Kong, X.; Xie, J.; Wu, X.; Huang, Y.; Bao, J. Rapid prediction of acid detergent fiber, neutral detergent fiber, and acid detergent lignin of rice materials by near-infrared spectroscopy. J. Agric. Food Chem. 2005, 53, 2843–2848. [Google Scholar] [CrossRef]
- García, J.; Cozzolino, D. Use of near infrared reflectance (NIR) spectroscopy to predict chemical composition of forages in broad-based calibration models of forages in broad-based calibration models. Agric. Técnica 2006, 66, 41–47. [Google Scholar] [CrossRef]
- Redshaw, E.S.; Weisenburger, R.D.; Mathison, G.W.; Milligan, L.P. Near Infrared Reflectance Spectroscopy for Predicting Forage Composition and Voluntary Consumption and Digestibility in Cattle and Sheep. Can. J. Anim. Sci. 1986, 66, 103–115. [Google Scholar] [CrossRef]
- Karoui, R.; Mouazen, A.M.; Dufour, E.; Pillonel, L.; Picque, D.; Bosset, J.O.; De Baerdemaeker, J. Mid-infrared spectrometry: A tool for the determination of chemical parameters in Emmental cheeses produced during winter. Lait 2006, 86, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. J. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Schmitt, S.; Garrigues, S.; de la Guardia, M. Determination of the mineral composition of foods by infrared spectroscopy: A review of a green alternative. Crit. Rev. Anal. Chem. 2014, 44, 186–197. [Google Scholar] [CrossRef]
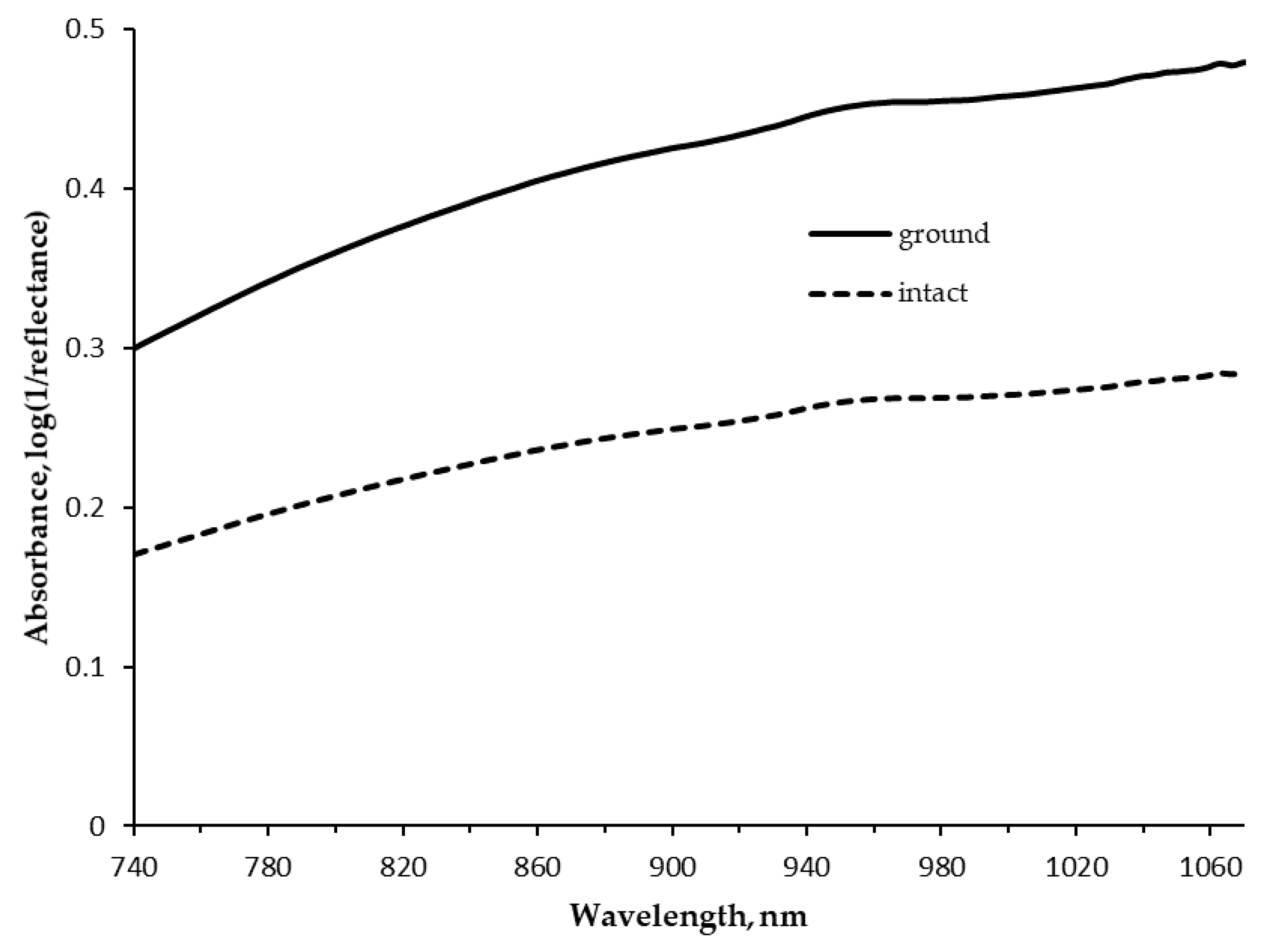
| Item | Mean | SD | Minimum | Maximum | CV |
|---|---|---|---|---|---|
| DM | 92.00 | 0.00 | 92.00 | 92.00 | 0.0 |
| Crude protein | 30.27 | 4.95 | 23.91 | 41.30 | 16.4 |
| Ether extract | 16.87 | 3.60 | 10.33 | 21.74 | 21.3 |
| Crude ash | 7.24 | 1.26 | 2.50 | 9.78 | 17.5 |
| Crude fibers | 3.58 | 2.29 | 2.28 | 15.22 | 63.8 |
| Nitrogen-free extract | 41.75 | 8.06 | 25.54 | 54.89 | 19.3 |
| Item | n | Mean | SD | Minimum | Maximum | CV |
|---|---|---|---|---|---|---|
| Starch, % | ||||||
| Total | 81 | 32.26 | 7.15 | 11.50 | 43.23 | 22.2 |
| Gelatinized | 81 | 21.69 | 5.86 | 9.15 | 35.00 | 27.0 |
| Insoluble fiber, % | ||||||
| NDF | 99 | 16.01 | 6.07 | 7.74 | 36.55 | 37.9 |
| ADF | 99 | 4.27 | 2.36 | 1.98 | 16.84 | 55.4 |
| ADL | 99 | 1.66 | 0.76 | 0.41 | 4.34 | 45.5 |
| Cellulose | 99 | 2.61 | 1.79 | 1.06 | 12.54 | 68.6 |
| Hemicellulose | 99 | 11.74 | 4.80 | 5.19 | 28.19 | 40.9 |
| Macrominerals, % | ||||||
| Ca | 99 | 1.37 | 0.47 | 0.44 | 3.58 | 34.4 |
| P | 99 | 0.99 | 0.29 | 0.35 | 1.84 | 29.5 |
| K | 99 | 0.70 | 0.31 | 0.28 | 1.52 | 43.9 |
| Na | 99 | 0.51 | 0.17 | 0.12 | 0.91 | 33.4 |
| S | 99 | 0.38 | 0.13 | 0.20 | 0.79 | 33.6 |
| Mg | 99 | 0.11 | 0.02 | 0.08 | 1.50 | 15.3 |
| Trace minerals, mg/kg | ||||||
| Fe | 99 | 344.31 | 86.94 | 121.68 | 658.52 | 25.2 |
| Zn | 99 | 179.44 | 53.14 | 35.11 | 307.39 | 29.6 |
| Al | 99 | 152.88 | 51.38 | 51.73 | 288.23 | 33.6 |
| Mn | 99 | 66.66 | 23.00 | 14.15 | 230.75 | 34.5 |
| Cu | 99 | 22.67 | 5.47 | 9.34 | 43.80 | 24.1 |
| Sr | 99 | 17.83 | 9.62 | 5.68 | 64.23 | 53.9 |
| Ba | 99 | 5.41 | 2.17 | 1.39 | 13.64 | 40.1 |
| B | 86 | 4.52 | 1.95 | 0.99 | 9.77 | 43.2 |
| Cr | 99 | 1.99 | 1.06 | 0.59 | 8.38 | 53.2 |
| Ni | 99 | 1.21 | 0.34 | 0.52 | 2.45 | 27.8 |
| Mo | 99 | 0.67 | 0.20 | 0.23 | 1.29 | 29.8 |
| V | 88 | 0.38 | 0.24 | 0.13 | 1.43 | 64.4 |
| Li | 99 | 0.19 | 0.09 | 0.08 | 0.59 | 46.7 |
| Item | n | LF | Mean | SD | R2C | SEC | R2CrV | SECrV | RPD |
|---|---|---|---|---|---|---|---|---|---|
| Ground kibbles | |||||||||
| Total starch | 75 | 6 | 32.89 | 6.54 | 0.91 | 1.91 | 0.81 | 2.80 | 2.33 |
| Gelatinized starch | 76 | 7 | 22.00 | 5.78 | 0.87 | 2.08 | 0.84 | 2.27 | 2.54 |
| NDF | 87 | 6 | 15.60 | 5.15 | 0.71 | 2.78 | 0.56 | 3.39 | 1.52 |
| ADF | 93 | 6 | 3.84 | 1.23 | 0.57 | 0.80 | 0.45 | 0.91 | 1.35 |
| ADL | 93 | 5 | 1.57 | 0.62 | 0.76 | 0.30 | 0.64 | 0.37 | 1.68 |
| Cellulose | 86 | 7 | 2.23 | 0.78 | 0.56 | 0.52 | 0.39 | 0.61 | 1.28 |
| Hemicellulose | 94 | 9 | 11.72 | 4.67 | 0.73 | 2.41 | 0.58 | 3.00 | 1.56 |
| K | 94 | 7 | 0.67 | 0.29 | 0.60 | 0.18 | 0.56 | 0.19 | 1.51 |
| Na | 93 | 8 | 0.51 | 0.16 | 0.67 | 0.09 | 0.56 | 0.11 | 1.53 |
| S | 92 | 10 | 0.37 | 0.12 | 0.85 | 0.04 | 0.72 | 0.06 | 1.92 |
| Mg | 94 | 6 | 0.11 | 0.02 | 0.68 | 0.01 | 0.55 | 0.01 | 1.45 |
| Ni, mg/kg | 89 | 4 | 1.16 | 0.29 | 0.60 | 0.18 | 0.41 | 0.22 | 1.32 |
| V, mg/kg | 83 | 5 | 0.35 | 0.20 | 0.69 | 0.11 | 0.44 | 0.15 | 1.33 |
| Li, mg/kg | 91 | 5 | 0.18 | 0.06 | 0.60 | 0.04 | 0.51 | 0.04 | 1.50 |
| Intact kibbles | |||||||||
| Total starch | 72 | 7 | 32.27 | 6.43 | 0.89 | 2.13 | 0.77 | 3.09 | 2.08 |
| Gelatinized starch | 74 | 5 | 21.55 | 5.69 | 0.89 | 1.89 | 0.83 | 2.32 | 2.45 |
| NDF | 94 | 8 | 15.42 | 5.26 | 0.78 | 2.49 | 0.61 | 3.27 | 1.61 |
| ADF | 91 | 7 | 3.77 | 1.15 | 0.69 | 0.64 | 0.56 | 0.76 | 1.51 |
| ADL | 91 | 6 | 1.56 | 0.61 | 0.78 | 0.29 | 0.71 | 0.33 | 1.86 |
| Cellulose | 90 | 8 | 2.22 | 0.74 | 0.59 | 0.47 | 0.44 | 0.54 | 1.37 |
| Hemicellulose | 95 | 8 | 11.64 | 4.73 | 0.83 | 1.98 | 0.63 | 2.86 | 1.65 |
| K | 92 | 5 | 0.67 | 0.29 | 0.80 | 0.13 | 0.74 | 0.15 | 1.98 |
| Na | 95 | 4 | 0.52 | 0.17 | 0.65 | 0.10 | 0.47 | 0.12 | 1.38 |
| S | 92 | 5 | 0.37 | 0.12 | 0.69 | 0.07 | 0.62 | 0.07 | 1.61 |
| Mg | 95 | 6 | 0.11 | 0.02 | 0.64 | 0.01 | 0.55 | 0.01 | 1.55 |
| Sr, mg/kg | 95 | 5 | 16.52 | 6.99 | 0.53 | 4.81 | 0.40 | 5.40 | 1.29 |
| Cr, mg/kg | 93 | 5 | 1.85 | 0.70 | 0.61 | 0.44 | 0.48 | 0.51 | 1.37 |
| Ni, mg/kg | 94 | 7 | 1.17 | 0.29 | 0.65 | 0.17 | 0.43 | 0.22 | 1.32 |
| Li, mg/kg | 95 | 3 | 0.18 | 0.06 | 0.56 | 0.04 | 0.47 | 0.05 | 1.20 |
| Item | Calibration set 1 | Validation set 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | SECrV | R2CrV | Bias | Slope | SEP | R2ExV | RPDExV | |
| Ground kibbles | ||||||||
| Total starch | 55 | 3.05 | 0.76 | 0.07 | 0.87 | 3.17 | 0.69 | 1.75 |
| Gelatinized starch | 54 | 2.33 | 0.81 | −0.59 | 0.82 | 2.49 | 0.89 | 2.55 |
| NDF | 67 | 3.20 | 0.53 | 0.90 | 0.79 | 3.13 | 0.56 | 1.45 |
| ADF | 67 | 0.87 | 0.43 | 0.03 | 1.27 | 0.74 | 0.61 | 1.55 |
| ADL | 61 | 0.37 | 0.65 | −0.01 | 0.74 | 0.43 | 0.62 | 1.48 |
| Intact kibbles | ||||||||
| Total starch | 56 | 3.19 | 0.77 | −0.31 | 0.95 | 3.19 | 0.72 | 1.89 |
| Gelatinized starch | 54 | 2.28 | 0.81 | −0.14 | 0.84 | 3.14 | 0.78 | 2.03 |
| NDF | 69 | 3.49 | 0.49 | 0.30 | 1.00 | 3.58 | 0.62 | 1.61 |
| ADF | 68 | 0.78 | 0.58 | 0.02 | 0.96 | 0.86 | 0.52 | 1.44 |
| ADL | 69 | 0.36 | 0.66 | −0.09 | 0.79 | 0.34 | 0.70 | 1.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goi, A.; Simoni, M.; Righi, F.; Visentin, G.; De Marchi, M. Application of a Handheld Near-Infrared Spectrometer to Predict Gelatinized Starch, Fiber Fractions, and Mineral Content of Ground and Intact Extruded Dry Dog Food. Animals 2020, 10, 1660. https://doi.org/10.3390/ani10091660
Goi A, Simoni M, Righi F, Visentin G, De Marchi M. Application of a Handheld Near-Infrared Spectrometer to Predict Gelatinized Starch, Fiber Fractions, and Mineral Content of Ground and Intact Extruded Dry Dog Food. Animals. 2020; 10(9):1660. https://doi.org/10.3390/ani10091660
Chicago/Turabian StyleGoi, Arianna, Marica Simoni, Federico Righi, Giulio Visentin, and Massimo De Marchi. 2020. "Application of a Handheld Near-Infrared Spectrometer to Predict Gelatinized Starch, Fiber Fractions, and Mineral Content of Ground and Intact Extruded Dry Dog Food" Animals 10, no. 9: 1660. https://doi.org/10.3390/ani10091660
APA StyleGoi, A., Simoni, M., Righi, F., Visentin, G., & De Marchi, M. (2020). Application of a Handheld Near-Infrared Spectrometer to Predict Gelatinized Starch, Fiber Fractions, and Mineral Content of Ground and Intact Extruded Dry Dog Food. Animals, 10(9), 1660. https://doi.org/10.3390/ani10091660





