Anti-Biofilm Effect of Tea Saponin on a Streptococcus agalactiae Strain Isolated from Bovine Mastitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Condition
2.2. Inhibitory Effect Assays of TS on GBS2
2.3. Biofilm Formation Assays
2.4. Biofilm Observation by Scanning Electron Microscopy
2.5. Isolation and Purification of Total RNA and RT-qPCR Processing
2.6. Statistical Analysis
3. Results
3.1. Antibacterial Effect of TS on GBS2
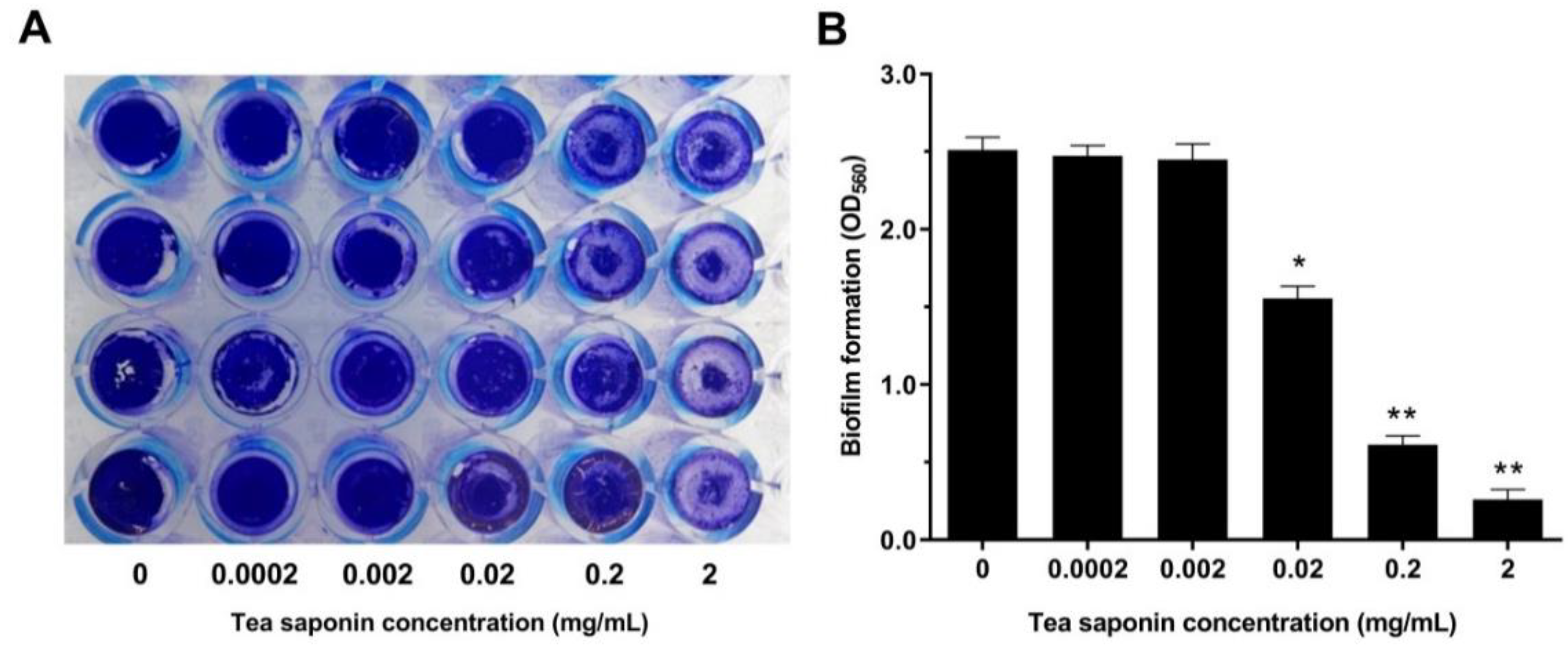
3.2. Effects of TS on Biofilm of GBS2
3.3. Effect of TS on Transcriptions of Biofilm-Associated Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Melchior, M.B.; Vaarkamp, H.; Fink-Gremmels, J. Biofilms: A role in recurrent mastitis infections? Vet. J. 2006, 171, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A. Bovine mastitis: An evolving disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Huijps, K.; Osteras, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- Tian, X.Y.; Zheng, N.; Han, R.W.; Ho, H.; Wang, J.; Wang, Y.T.; Wang, S.Q.; Li, H.G.; Liu, H.W.; Yu, Z.N. Antimicrobial resistance and virulence genes of Streptococcus isolated from dairy cows with mastitis in China. Microb. Pathog. 2019, 131, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Smulski, S.; Malinowski, E.; Kaczmarowski, M.; Lassa, H. Occurrence, forms and etiologic agents of mastitis in Poland depending on size of farm. Med. Weter. 2011, 67, 190–193. [Google Scholar]
- Martin, P.; Barkema, H.W.; Brito, L.F.; Narayana, S.G.; Miglior, F. Symposium review: Novel strategies to genetically improve mastitis resistance in dairy cattle. J. Dairy Sci. 2018, 101, 2724–2736. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Sutherland, I. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Rosini, R.; Margarit, I. Biofilm formation by Streptococcus agalactiae: Influence of environmental conditions and implicated virulence factors. Front. Cell. Infect. Microbiol. 2015, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Kaczorek, E.; Malaczewska, J.; Wojcik, R.; Siwicki, A.K. Biofilm production and other virulence factors in Streptococcus spp. isolated from clinical cases of bovine mastitis in Poland. BMC Vet. Res. 2017, 13, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaudo, C.D.; Rosini, R.; Galeotti, C.L.; Berti, F.; Necchi, F.; Reguzzi, V.; Ghezzo, C.; Telford, J.L.; Grandi, G.; Maione, D. Specific involvement of pilus type 2a in biofilm formation in group B Streptococcus. PLoS ONE 2010, 5, e9216. [Google Scholar] [CrossRef] [PubMed]
- Beyene, T.; Hayishe, H.; Gizaw, F.; Beyi, A.F.; Abunna, F.; Mammo, B.; Ayana, D.; Waktole, H.; Abdi, R.D. Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in Addis Ababa, Ethiopia. BMC Res. Notes 2017, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Kalmus, P.; Aasmae, B.; Karssin, A.; Orro, T.; Kask, K. Udder pathogens and their resistance to antimicrobial agents in dairy cows in Estonia. Acta Vet. Scand 2011, 53, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Guha, C.; Biswas, U.; Jana, P.S.; Chatterjee, A.; Samanta, I. Detection of emerging antibiotic resistance in bacteria isolated from subclinical mastitis in cattle in West Bengal. Vet. World 2017, 10, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Velez, J.R.; Cameron, M.; Rodriguez-Lecompte, J.C.; Xia, F.; Heider, L.C.; Saab, M.; McClure, J.T.; Sanchez, J. Whole-Genome Sequence Analysis of Antimicrobial Resistance Genes in Streptococcus uberis and Streptococcus dysgalactiae Isolates from Canadian Dairy Herds. Front. Vet. Sci. 2017, 4, 63. [Google Scholar] [CrossRef]
- Kromker, V.; Leimbach, S. Mastitis treatment-Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. 2017, 5, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Ma, K.; Nie, M.; Dong, Y.; Liu, Y. Role of luxS in immune evasion and pathogenicity of piscine Streptococcus agalactiae is not dependent on autoinducer-2. Fish Shellfish Immunol. 2020, 99. [Google Scholar] [CrossRef]
- Mushtaq, S.; Shah, A.M.; Shah, A.; Lone, S.A.; Hussain, A.; Hassan, Q.P.; Ali, M.N. Bovine mastitis: An appraisal of its alternative herbal cure. Microb. Pathog. 2018, 114, 357–361. [Google Scholar] [CrossRef]
- Sagesaka, Y.M.; Uemura, T.; Suzuki, Y.; Sugiura, T.; Yoshida, M.; Yamaguchi, K.; Kyuki, K. Antimicrobial and anti-inflammatory actions of tea-leaf saponin. Yakugaku Zasshi 1996, 116, 238–243. [Google Scholar] [CrossRef]
- Sur, P.; Chaudhuri, T.; Vedasiromoni, J.R.; Gomes, A.; Ganguly, D.K. Antiinflammatory and antioxidant property of saponins of tea (Camellia sinensis (L) O. Kuntze) root extract. Phytother. Res. PTR 2001, 15, 174–176. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahhmed, A.; Shin, J.H.; Baek, J.S.; Kim, M.Y.; Kim, J.D. Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram Positive and Gram Negative Bacteria, a Comprehensive Study In Vitro and In Vivo. Evid. Based Complement. Alternat. Med. 2018, 2018, 3486106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tendolkar, P.M.; Baghdayan, A.S.; Gilmore, M.S.; Shankar, N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004, 72, 6032–6039. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhang, W.; Mou, Z.; Chen, Y.; Guo, F.; Yang, E.; Wang, W. Transcriptome Analysis Reveals Silver Nanoparticle-Decorated Quercetin Antibacterial Molecular Mechanism. ACS Appl. Mater. Interfaces 2017, 9, 10047–10060. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Jiang, S.; Wessels, M.R. CsrRS and environmental pH regulate group B streptococcus adherence to human epithelial cells and extracellular matrix. Infect. Immun. 2012, 80, 3975–3984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.L. Genomic Insights Into the Distribution and Evolution of Group B Streptococcus. Front. Microbiol. 2019, 10, 1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostensson, K.; Lam, V.; Sjogren, N.; Wredle, E. Prevalence of subclinical mastitis and isolated udder pathogens in dairy cows in Southern Vietnam. Trop. Anim. Health Prod. 2013, 45, 979–986. [Google Scholar] [CrossRef]
- Chen, X.; Shang, F.; Meng, Y.; Li, L.; Cui, Y.; Zhang, M.; Qi, K.; Xue, T. Ethanol extract of Sanguisorba officinalis L. inhibits biofilm formation of methicillin-resistant Staphylococcus aureus in an ica-dependent manner. J. Dairy Sci. 2015, 98, 8486–8491. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Moatamedi, A.; Lotfalian, S.; Mirshokraei, P. Biofilm formation, hemolysin production and antimicrobial susceptibilities of Streptococcus agalactiae isolated from the mastitis milk of dairy cows in Shahrekord district, Iran. Vet. Res. Forum Int. Q. J. 2013, 4, 269–272. [Google Scholar]
- Wang, J.K.; Ye, J.A.; Liu, J.X. Effects of tea saponins on rumen microbiota, rumen fermentation, methane production and growth performance--a review. Trop. Anim. Health Prod. 2012, 44, 697–706. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Liu, J.X.; Lu, Y.; Zhu, W.Y.; Denman, S.E.; McSweeney, C.S. Effect of tea saponin on methanogenesis, microbial community structure and expression of mcrA gene, in cultures of rumen micro-organisms. Lett. Appl. Microbiol. 2008, 47, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, T.; Chen, D.; Zhang, N.; Si, B.; Deng, K.; Tu, Y.; Diao, Q. Effects of Tea Saponin Supplementation on Nutrient Digestibility, Methanogenesis, and Ruminal Microbial Flora in Dorper Crossbred Ewe. Animals 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Tu, Y.; Zhao, S.P.; Hao, Y.H.; Liu, J.X.; Liu, F.H.; Xiong, B.H.; Jiang, L.S. Effect of tea saponins on milk performance, milk fatty acids, and immune function in dairy cow. J. Dairy Sci. 2017, 100, 8043–8052. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Primer Name a | Oligonucleotide (5′-3′) |
|---|---|
| RT-16s-F | GTAAATGGCGAAGCA |
| RT-16s-R | TTTGGAAGCGATGAG |
| RT-cpsE-F | CTTTTACAACGACACGA |
| RT-cpsE-R | ATCCAAGATACAGACAGC |
| RT-luxS-F | TCCGCCTTATTCAGC |
| RT-luxS-R | GACCCCACCAGCAA |
| RT-neuA-F | ATAAAGGAAGCAATGGA |
| RT-neuA-R | AGGTGACCGATGACG |
| RT-csrR-F | CGCTTCGTCTCGTTA |
| RT-csrR-R | TTCTTTTGTCTTCGTTTC |
| RT-fbsC-F | TACTCCAAAACCAGTACCACC |
| RT-fbsC-R | CCTAACATAATCGCTAACCCT |
| RT-srtA-F | GTGCAGGAACGATGAAGGAA |
| RT-srtA-R | GGCTCTTGCCAGGTGTATCA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, F.; Wang, H.; Xue, T. Anti-Biofilm Effect of Tea Saponin on a Streptococcus agalactiae Strain Isolated from Bovine Mastitis. Animals 2020, 10, 1713. https://doi.org/10.3390/ani10091713
Shang F, Wang H, Xue T. Anti-Biofilm Effect of Tea Saponin on a Streptococcus agalactiae Strain Isolated from Bovine Mastitis. Animals. 2020; 10(9):1713. https://doi.org/10.3390/ani10091713
Chicago/Turabian StyleShang, Fei, Hui Wang, and Ting Xue. 2020. "Anti-Biofilm Effect of Tea Saponin on a Streptococcus agalactiae Strain Isolated from Bovine Mastitis" Animals 10, no. 9: 1713. https://doi.org/10.3390/ani10091713
APA StyleShang, F., Wang, H., & Xue, T. (2020). Anti-Biofilm Effect of Tea Saponin on a Streptococcus agalactiae Strain Isolated from Bovine Mastitis. Animals, 10(9), 1713. https://doi.org/10.3390/ani10091713





