The Effects of Red Light on Mammalian Sperm Rely upon the Color of the Straw and the Medium Used
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Suppliers
2.2. Animals and Ejaculates
2.3. Experimental Design
2.4. Analysis of Sperm Motility
2.5. Flow Cytometry
2.5.1. Analysis of Plasma Membrane Integrity
2.5.2. Analysis of Acrosome Integrity
2.5.3. Analysis of Mitochondrial Membrane Potential
2.5.4. Analysis of Intracellular ROS Levels: H2O2 and O2−
2.5.5. Intracellular Calcium Levels
2.6. Statistical Analyses
3. Results
3.1. Plasma Membrane Integrity
3.2. Acrosomal Integrity
3.3. Sperm Motility
3.4. Mitochondrial Membrane Potential
3.5. Intracellular Peroxide and Superoxide Levels
3.6. Intracellular Calcium Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalczyk, A.; Czerniawska-Piatkowska, E.; Kuczaj, M. Factors influencing the popularity of artificial insemination of mares in Europe. Animals 2019, 9, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loomis, P.R. Advanced Methods for Handling and Preparation of Stallion Semen. Vet. Clin. N. Am. Equine Pract. 2006, 22, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Papas, M.; Gacem, S.; Noto, F.; Delgado-Bermúdez, A.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Effects of red-light irradiation on the function and survival of fresh and liquid-stored donkey semen. Theriogenology 2020, 149. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Paventi, G.; Pizzuto, R.; Di Iorio, M.; Bailey, J.L.; Manchisi, A.; Passarella, S. Helium-neon laser irradiation of cryopreserved ram sperm enhances cytochrome c oxidase activity and ATP levels improving semen quality. Theriogenology 2016, 86, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Zan-Bar, T.; Bartoov, B.; Segal, R.; Yehuda, R.; Lavi, R.; Lubart, R.; Avtalion, R.R. Influence of Visible Light and Ultraviolet Irradiation on Motility and Fertility of Mammalian and Fish Sperm. Photomed. Laser Surg. 2005, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, N.; Meluzzi, A.; Manchisi, A.; Passarella, S. Improvement of stored turkey semen quality as a result of He-Ne laser irradiation. Anim. Reprod. Sci. 2005, 85, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Landthaler, M.; Haina, D.; Schill, W.B. The effects of laser light on sperm motility and velocity in vitro. Andrologia 2009, 16, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Claroni, F.; Gandini, L.; Lombardo, F.; Barbieri, C.; Lino, A.; Dondero, F. Laser radiation and motility patterns of human sperm. Syst. Biol. Reprod. Med. 1989, 23, 229–234. [Google Scholar] [CrossRef]
- Firestone, R.S.; Esfandiari, N.; Moskovtsev, S.I.; Burstein, E.; Videna, G.T.; Librach, C.; Bentov, Y.; Casper, R.F. The Effects of Low-Level Laser Light Exposure on Sperm Motion Characteristics and DNA Damage. J. Androl. 2012, 33, 469–473. [Google Scholar] [CrossRef]
- Salman Yazdi, R.; Bakhshi, S.; Jannat Alipoor, F.; Akhoond, M.R.; Borhani, S.; Farrahi, F.; Lotfi Panah, M.; Sadighi Gilani, M.A. Effect of 830-nm diode laser irradiation on human sperm motility. Lasers Med. Sci. 2014, 29, 97–104. [Google Scholar] [CrossRef]
- Ban Frangez, H.; Frangez, I.; Verdenik, I.; Jansa, V.; Virant Klun, I. Photobiomodulation with light-emitting diodes improves sperm motility in men with asthenozoospermia. Lasers Med. Sci. 2015, 30, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.; El-Sawy, M. Light-emitting diode exposure enhances sperm motility in men with and without asthenospermia: Preliminary results. Arch. Ital. Urol. Androl. 2015, 87, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeste, M.; Codony, F.; Estrada, E.; Lleonart, M.; Balasch, S.; Peña, A.; Bonet, S.; Rodríguez-Gil, J.E. Specific LED-based red light photo-stimulation procedures improve overall sperm function and reproductive performance of boar ejaculates. Sci. Rep. 2016, 6, 22569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezo, F.; Zambrano, F.; Uribe, P.; Ramírez-Reveco, A.; Romero, F.; Sanchéz, R. LED-based red light photostimulation improves short-term response of cooled boar semen exposed to thermal stress at 37 °C. Andrologia 2019, 51, e13237. [Google Scholar] [CrossRef]
- Blanco Prieto, O.; Catalán, J.; Lleonart, M.; Bonet, S.; Yeste, M.; Rodríguez-Gil, J.E. Red-light stimulation of boar semen prior to artificial insemination improves field fertility in farms: A worldwide survey. Reprod. Domest. Anim. 2019, 54, rda.13470. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Rosato, M.P.; Paventi, G.; Pizzuto, R.; Gambacorta, M.; Manchisi, A.; Passarella, S. The irradiation of rabbit sperm cells with He–Ne laser prevents their in vitro liquid storage dependent damage. Anim. Reprod. Sci. 2010, 119, 123–129. [Google Scholar] [CrossRef]
- Corral-Baqués, M.I.; Rigau, T.; Rivera, M.; Rodríguez, J.E.; Rigau, J. Effect of 655-nm diode laser on dog sperm motility. Lasers Med. Sci. 2005, 20, 28–34. [Google Scholar] [CrossRef]
- Corral-Baqués, M.I.; Rivera, M.M.; Rigau, T.; Rodríguez-Gil, J.E.; Rigau, J. The effect of low-level laser irradiation on dog spermatozoa motility is dependent on laser output power. Lasers Med. Sci. 2009, 24, 703–713. [Google Scholar] [CrossRef]
- Abdel-Salam, Z.; Dessouki, S.H.M.; Abdel-Salam, S.A.M.; Ibrahim, M.A.M.; Harith, M.A. Green laser irradiation effects on buffalo semen. Theriogenology 2011, 75, 988–994. [Google Scholar] [CrossRef]
- Catalán, J.; Llavanera, M.; Bonilla-Correal, S.; Papas, M.; Gacem, S.; Rodríguez-Gil, J.E.; Yeste, M.; Miró, J. Irradiating frozen-thawed stallion sperm with red-light increases their resilience to withstand post-thaw incubation at 38 °C. Theriogenology 2020, 157, 85–95. [Google Scholar] [CrossRef]
- Catalán, J.; Papas, M.; Gacem, S.; Mateo-Otero, Y.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Red-Light Irradiation of Horse Spermatozoa Increases Mitochondrial Activity and Motility through Changes in the Motile Sperm Subpopulation Structure. Biology 2020, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Papas, M.; Trujillo-Rojas, L.; Blanco-Prieto, O.; Bonilla-Correal, S.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Red LED Light Acts on the Mitochondrial Electron Chain of Donkey Sperm and Its Effects Depend on the Time of Exposure to Light. Front. Cell Dev. Biol. 2020, 8, 588621. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Castillo-Martín, M.; Bonet, S.; Rodríguez-Gil, J.E. Impact of light irradiation on preservation and function of mammalian spermatozoa. Anim. Reprod. Sci. 2018, 194, 19–32. [Google Scholar] [CrossRef]
- De Blas, G.A.; Darszon, A.; Ocampo, A.Y.; Serrano, C.J.; Castellano, L.E.; Hernández-González, E.O.; Chirinos, M.; Larrea, F.; Beltrán, C.; Treviño, C.L. TRPM8, a Versatile Channel in Human Sperm. PLoS ONE 2009, 4, e6095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahat, A.; Eisenbach, M. Human Sperm Thermotaxis Is Mediated by Phospholipase C and Inositol Trisphosphate Receptor Ca2+ Channel1. Biol. Reprod. 2010, 82, 606–616. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, G.M.; Orta, G.; Reddy, T.; Koppers, A.J.; Martínez-López, P.; De La Vega-Beltràn, J.L.; Lo, J.C.Y.; Veldhuis, N.; Jamsai, D.; McIntyre, P.; et al. Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc. Natl. Acad. Sci. USA 2011, 108, 7034–7039. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the Mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Boryshpolets, S.; Afanzar, O.; Brandis, A.; Nevo, R.; Kiss, V.; Eisenbach, M. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Prieto, O.; Catalán, J.; Trujillo-Rojas, L.; Peña, A.; Rivera del Álamo, M.M.; Llavanera, M.; Bonet, S.; Fernández-Novell, J.M.; Yeste, M.; Rodríguez-Gil, J.E. Red LED Light Acts on the Mitochondrial Electron Chain of Mammalian Sperm via Light-Time Exposure-Dependent Mechanisms. Cells 2020, 9, 2546. [Google Scholar] [CrossRef]
- Lubart, R.; Friedmann, H.; Levinshal, T.; Lavie, R.; Breitbart, H. Effect of light on calcium transport in bull sperm cells. J. Photochem. Photobiol. B Biol. 1992, 15, 337–341. [Google Scholar] [CrossRef]
- Karu, T. Photobiology of low-power laser effects. Health Phys. 1989, 56, 691–704. [Google Scholar] [CrossRef]
- Gabel, C.P.; Carroll, J.; Harrison, K. Sperm motility is enhanced by Low Level Laser and Light Emitting Diode photobiomodulation with a dose-dependent response and differential effects in fresh and frozen samples. Laser Ther. 2018, 27, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Tuner, J.; Hode, L. Laser Therapy: Clinical Practice and Scientific; Prima Books AB: Grängesberg, Sweden, 2002; pp. 12–22. [Google Scholar]
- Preece, D.; Chow, K.W.; Gomez-Godinez, V.; Gustafson, K.; Esener, S.; Ravida, N.; Durrant, B.; Berns, M.W. Red light improves spermatozoa motility and does not induce oxidative DNA damage. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kenney, M.R. Minimal contamination techniques for breeding mares: Techniques and priliminary findings. Proc. Am. Assoc. Equine Pract. 1975, 327–336. [Google Scholar]
- Prieto-Martínez, N.; Vilagran, I.; Morató, R.; Rodríguez-Gil, J.E.; Yeste, M.; Bonet, S. Aquaporins 7 and 11 in boar spermatozoa: Detection, localisation and relationship with sperm quality. Reprod. Fertil. Dev. 2016, 28, 663–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.A.; Spidlen, J.; Boyce, K.; Cai, J.; Crosbie, N.; Dalphin, M.; Furlong, J.; Gasparetto, M.; Goldberg, M.; Goralczyk, E.M.; et al. MIFlowCyt: The minimum information about a flow cytometry experiment. Cytom. Part A 2008, 73A, 926–930. [Google Scholar] [CrossRef] [Green Version]
- Petrunkina, A.M.; Waberski, D.; Bollwein, H.; Sieme, H. Identifying non-sperm particles during flow cytometric physiological assessment: A simple approach. Theriogenology 2010, 73, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- Rathi, R.; Colenbrander, B.; Bevers, M.M.; Gadella, B.M. Evaluation of in vitro capacitation of stallion spermatozoa. Biol. Reprod. 2001, 65, 462–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murillo, M.M.; Carmona-Cuenca, I.; Del Castillo, G.; Ortiz, C.; Roncero, C.; Sánchez, A.; Fernández, M.; Fabregat, I. Activation of NADPH oxidase by transforming growth factor-β in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-κB-dependent mechanism. Biochem. J. 2007, 405, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, H.D.; Welch, G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Kalivendi, S.; Zhang, H.; Joseph, J.; Nithipatikom, K.; Vásquez-Vivar, J.; Kalyanaraman, B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: Potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003, 34, 1359–1368. [Google Scholar] [CrossRef]
- Yeste, M.; Fernández-Novell, J.M.; Ramió-Lluch, L.; Estrada, E.; Rocha, L.G.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Concha, I.I.; Ramírez, A.; Rodríguez-Gil, J.E. Intracellular calcium movements of boar spermatozoa during ‘in vitro’ capacitation and subsequent acrosome exocytosis follow a multiple-storage place, extracellular calcium-dependent model. Andrology 2015, 3, 729–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadirvel, G.; Kumar, S.; Kumaresan, A.; Kathiravan, P. Capacitation status of fresh and frozen-thawed buffalo spermatozoa in relation to cholesterol level, membrane fluidity and intracellular calcium. Anim. Reprod. Sci. 2009, 116, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Yeste, M.; Rivera Del Alamo, M.M.; Domingo, J.; Casao, A.; Rodriguez-Gil, J.E.; Pérez-Pé, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Effect of seminal plasma proteins on the motile sperm subpopulations in ram ejaculates. Reprod. Fertil. Dev. 2017, 29, 394–405. [Google Scholar] [CrossRef]
- Siqueira, A.F.P.; Maria, F.S.; Mendes, C.M.; Hamilton, T.R.S.; Dalmazzo, A.; Dreyer, T.R.; da Silva, H.M.; Nichi, M.; Milazzotto, M.P.; Visintin, J.A.; et al. Effects of photobiomodulation therapy (PBMT) on bovine sperm function. Lasers Med. Sci. 2016, 31, 1245–1250. [Google Scholar] [CrossRef]
- Quintero-Moreno, A.; Miró, J.; Teresa Rigau, A.; Rodríguez-Gil, J.E. Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates. Theriogenology 2003, 59, 1973–1990. [Google Scholar] [CrossRef]
- Begum, R.; Powner, M.B.; Hudson, N.; Hogg, C.; Jeffery, G. Treatment with 670 nm Light Up Regulates Cytochrome C Oxidase Expression and Reduces Inflammation in an Age-Related Macular Degeneration Model. PLoS ONE 2013, 8, e57828. [Google Scholar] [CrossRef] [Green Version]
- Cohen, N.; Lubart, R.; Rubinstein, S.; Breitbart, H. Light Irradiation of Mouse Spermatozoa: Stimulation of In Vitro Fertilization and Calcium Signals. Photochem. Photobiol. 1998, 68, 407–413. [Google Scholar] [CrossRef]
- De Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Jones, D.P. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta Bioenerg. 1998, 1366, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Ferrusola, C.; Macías García, B.; Gallardo-Bolaños, J.M.; González-Fernández, L.; Rodríguez-Martinez, H.; Tapia, J.A.; Peña, F.J. Apoptotic markers can be used to forecast the freezeability of stallion spermatozoa. Anim. Reprod. Sci. 2009, 114, 393–403. [Google Scholar] [CrossRef]
- Yeste, M. Boar spermatozoa within the oviductal environment (II): Sperm capacitation. In Boar Reproduction: Fundamentals and New Biotechnological Trends; Bonet, S., Casas, I., Holt, W.V., Yeste, M., Eds.; Springer: Berlin, Germany, 2013; pp. 281–342. [Google Scholar]
- Correia, J.; Michelangeli, F.; Publicover, S. Regulation and roles of Ca2+ stores in human sperm. Reproduction 2015, 150, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Breitbart, H.; Levinshal, T.; Cohen, N.; Friedmann, H.; Lubart, R. Changes in calcium transport in mammalian sperm mitochondria and plasma membrane irradiated at 633 nm (HeNe laser). J. Photochem. Photobiol. B Biol. 1996, 34, 117–121. [Google Scholar] [CrossRef]
- Blanco-Prieto, O.; Catalán, J.; Rojas, L.; Delgado-Bermúdez, A.; Llavanera, M.; Rigau, T.; Bonet, S.; Yeste, M.; Rivera del Álamo, M.; Rodríguez-Gil, J. Medium-term effects of the diluted pig semen irradiation with red LED light on the integrity of nucleoprotein structure and resilience to withstand thermal stress. Theriogenology 2020. [Google Scholar] [CrossRef]

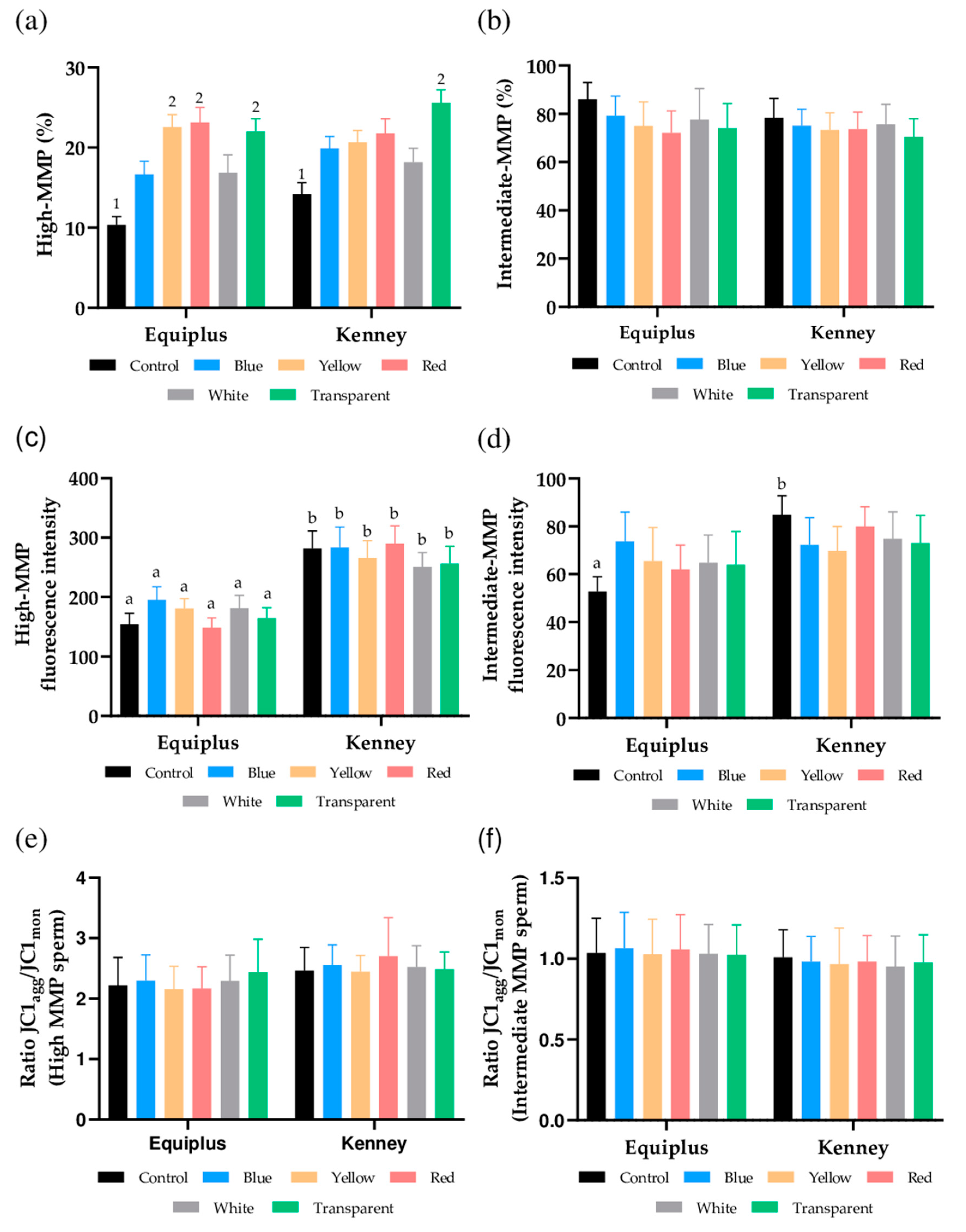
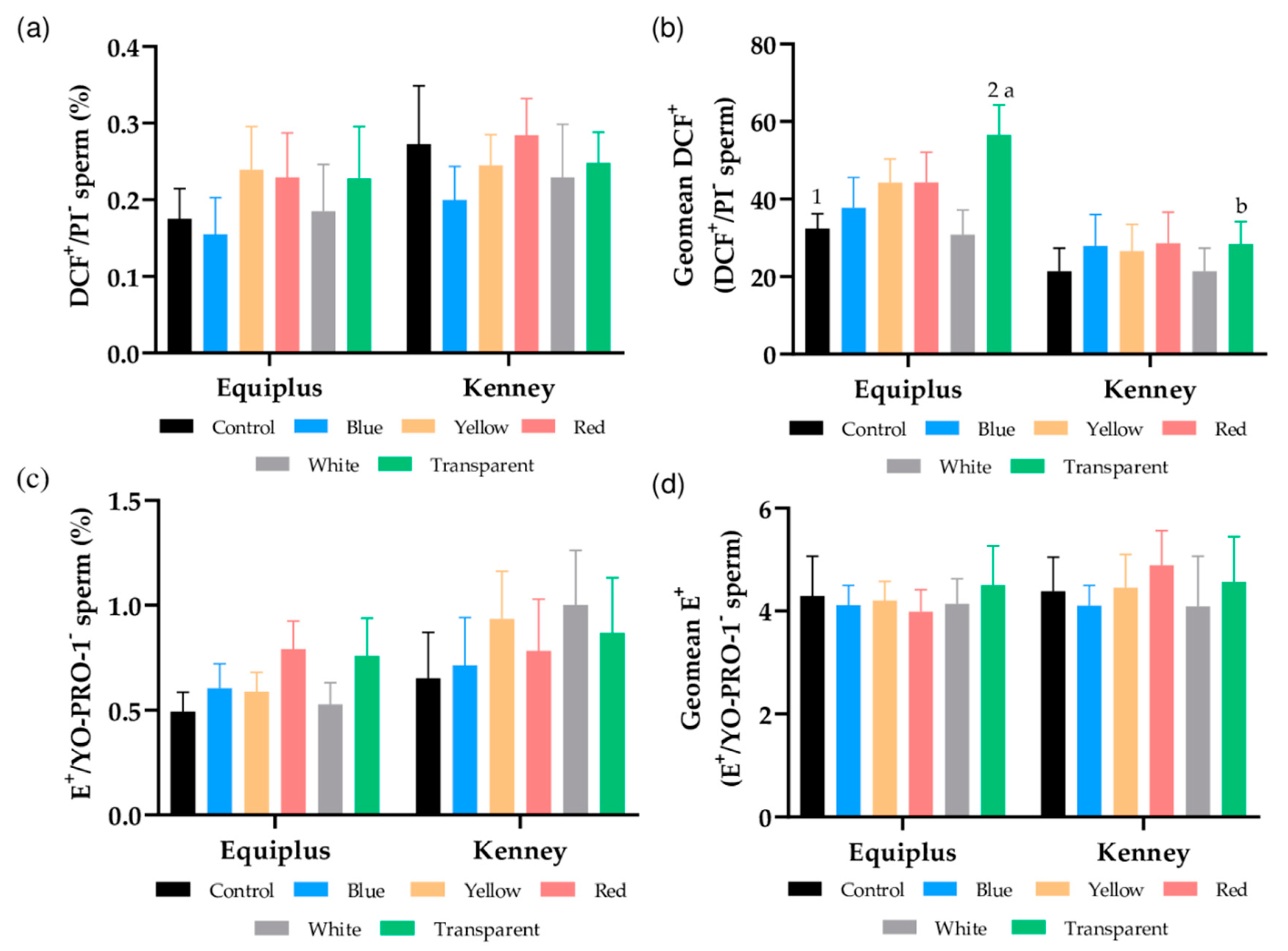
| Extender | Treatment (Straw Color) | Kinetic Variables (Mean ± SEM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | WOB (%) | ALH (µm) | BCF (Hz) | ||
| Equiplus | Control | 91.2 ± 3.1 ¹ | 54.0 ± 2.9 | 74.2 ± 3.2 ¹ | 59.3 ± 1.4 | 73.5 ± 1.9 | 80.8 ± 1.1 | 3.0 ± 0.2 | 8.9 ± 0.2 |
| Blue | 98.5 ± 4.4 | 60.4 ± 3.6 | 82.6 ± 4.3 | 61.2 ± 214 | 72.9 ± 1.3 ᵃ | 83.0 ± 1.4 | 3.0 ± 0.2 | 8.9 ± 0.4 | |
| Yellow | 102.0 ± 4.7 | 62.1 ± 4.1 | 82.8 ± 3.4 | 61.0 ± 1.7 | 75.6 ± 1.8 | 80.7 ± 1.4 | 3.3 ± 0.2 | 9.2 ± 0.1 | |
| Red | 106.5 ± 3.2 2 | 68.8 ± 2.6 ² | 89.3 ± 3.0 ² | 64.7 ± 2.1 | 77.2 ± 2.9 | 83.8 ± 0.9 | 3.3 ± 0.2 | 8.8 ± 0.2 | |
| White | 96.7 ± 4.0 | 61.3 ± 3.9 | 81.2 ± 4.3 | 63.1 ± 1.4 | 75.5 ± 1.6 | 83.5 ± 0.8 | 2.9 ± 0.2 | 8.7 ± 0.2 | |
| Transparent | 102.9 ± 4.9 | 62.9 ± 3.1 | 83.6 ± 4.2 | 62.8 ± 1.8 | 77.8 ± 2.1 | 82.7 ± 0.6 | 3.2 ± 0.2 | 8.9 ± 0.2 | |
| Kenney | Control | 93.8 ± 2.8 ¹ | 56.7 ± 3.6 | 72.3 ± 3.1 ¹ | 59.9 ± 2.6 | 78.7 ± 2.2 | 76.1 ± 2.7 | 2.9 ± 0.1 | 10.1 ± 0.4 |
| Blue | 97.5 ± 4.3 | 63.3 ± 2.8 | 77.7 ± 4.5 | 63.8 ± 2.2 | 82.4 ± 1.6 ᵇ | 81.0 ± 2.2 | 2.8 ± 0.1 | 9.2 ± 0.4 | |
| Yellow | 100.9 ± 4.1 | 63.4 ± 2.5 | 80.4 ± 3.9 | 63.3 ± 2.0 | 81.2 ± 2.5 | 78.6 ± 2.3 | 3.0 ± 0.1 | 9.9 ± 0.4 | |
| Red | 96.1 ± 3.6 | 62.6 ± 2.5 | 77.7 ± 4.3 | 63.7 ± 1.8 | 81.8 ± 1.8 | 78.6 ± 2.3 | 3.0 ± 0.1 | 9.8 ± 0.5 | |
| White | 92.1 ± 3.2 | 56.0 ± 2.5 | 70.8 ± 3.7 | 60.7 ± 2.1 | 80.1 ± 2.0 | 76.2 ± 2.5 | 3.0 ± 0.1 | 10.4 ± 0.4 | |
| Transparent | 107.4 ± 3.0 2 | 66.3 ± 2.8 | 86.0 ± 3.0 2 | 62.0 ± 2.2 | 78.8 ± 2.4 | 79.1 ± 2.5 | 3.1 ± 0.1 | 9.8 ± 0.5 | |
| SP1 | SP2 | SP3 | SP4 | |||||
|---|---|---|---|---|---|---|---|---|
| N | 10,647 | 12,622 | 14,674 | 8748 | ||||
| Parameter | Mean ± SEM | Range | Mean ± SEM | Range | Mean ± SEM | Range | Mean ± SEM | Range |
| VCL (µm/s) | 146.7 ± 0.4 | 108.2–247.3 | 61.9 ± 0.2 | 10.0–126.6 | 94.5 ± 0.1 | 54.9–149.2 | 126.3 ± 0.3 | 71.9–248.3 |
| VSL (µm/s) | 108.5 ± 0.2 | 63.4–198.3 | 33.4 ± 0.1 | 4.0–54.3 | 71.4 ± 0.1 | 42.6–123.6 | 40.0 ± 0.2 | 4.2–112.6 |
| VAP (µm/s) | 126.6 ± 0.2 | 10.2–121.0 | 45.4 ± 0.1 | 7.6–80.4 | 82.5 ± 0.1 | 48.9–120.0 | 97.4 ± 0.3 | 25.1–201.5 |
| LIN (%) | 75.0 ± 0.1 | 10.0–99.5 | 56.4 ± 0.2 | 8.3–96.6 | 76.6 ± 0.1 | 42.0–99.3 | 32.0 ± 0.2 | 2.7–59.8 |
| STR (%) | 85.9 ± 0.1 | 5.3–96.9 | 74.6 ± 0.2 | 4.0–95.2 | 87.1 ± 0.08 | 52.1–99.7 | 42.4 ± 0.2 | 1.5–97.0 |
| WOB (%) | 87.2 ± 0.1 | 37.8–100.0 | 75.1 ± 0.2 | 23.6–98.6 | 88.0 ± 0.09 | 45.9–100.0 | 78.0 ± 0.2 | 19.3–99.2 |
| ALH (µm) | 3.9 ± 0.02 | 0.5–11.6 | 2.2 ± 0.01 | 0.2–6.8 | 2.6 ± 0.01 | 0.3–7.4 | 3.9 ± 0.02 | 1.0–10.3 |
| BCF (Hz) | 8.2 ± 0.03 | 0.0–21.0 | 8.7 ± 0.03 | 0.0–22.0 | 9.1 ± 0.03 | 0.0–22.0 | 7.7 ± 0.05 | 0.0–21.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalán, J.; Yánez-Ortiz, I.; Gacem, S.; Papas, M.; Bonet, S.; Rodríguez-Gil, J.E.; Yeste, M.; Miró, J. The Effects of Red Light on Mammalian Sperm Rely upon the Color of the Straw and the Medium Used. Animals 2021, 11, 122. https://doi.org/10.3390/ani11010122
Catalán J, Yánez-Ortiz I, Gacem S, Papas M, Bonet S, Rodríguez-Gil JE, Yeste M, Miró J. The Effects of Red Light on Mammalian Sperm Rely upon the Color of the Straw and the Medium Used. Animals. 2021; 11(1):122. https://doi.org/10.3390/ani11010122
Chicago/Turabian StyleCatalán, Jaime, Iván Yánez-Ortiz, Sabrina Gacem, Marion Papas, Sergi Bonet, Joan E. Rodríguez-Gil, Marc Yeste, and Jordi Miró. 2021. "The Effects of Red Light on Mammalian Sperm Rely upon the Color of the Straw and the Medium Used" Animals 11, no. 1: 122. https://doi.org/10.3390/ani11010122
APA StyleCatalán, J., Yánez-Ortiz, I., Gacem, S., Papas, M., Bonet, S., Rodríguez-Gil, J. E., Yeste, M., & Miró, J. (2021). The Effects of Red Light on Mammalian Sperm Rely upon the Color of the Straw and the Medium Used. Animals, 11(1), 122. https://doi.org/10.3390/ani11010122








