Feed Restriction Induced Changes in Behavior, Corticosterone, and Microbial Programming in Slow- and Fast-Growing Chicken Breeds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Data Collection
2.2.1. Body Weight
2.2.2. Behavior in the Home Cage
2.2.3. Corticosterone
2.2.4. Cecum Microbiota by 16S rRNA
2.3. Statistical Analysis
3. Results
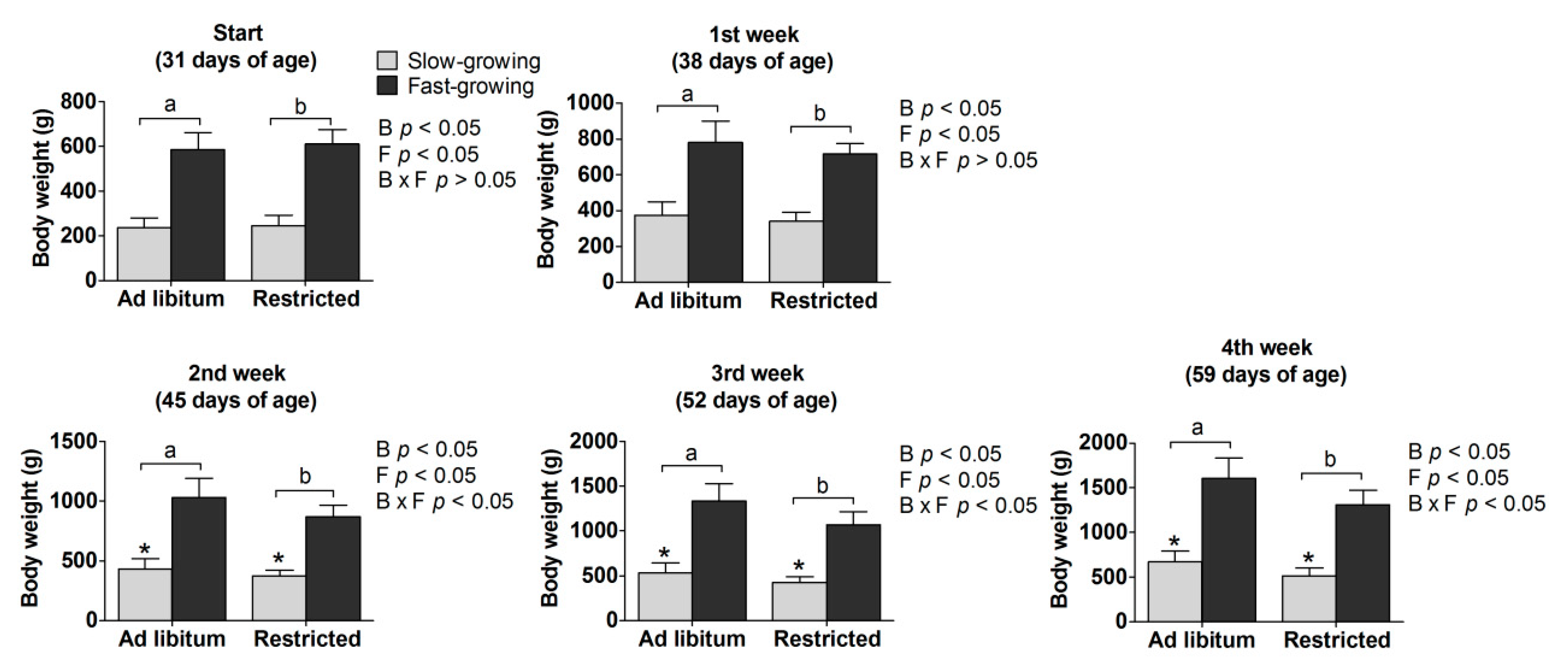
3.1. Body Weight
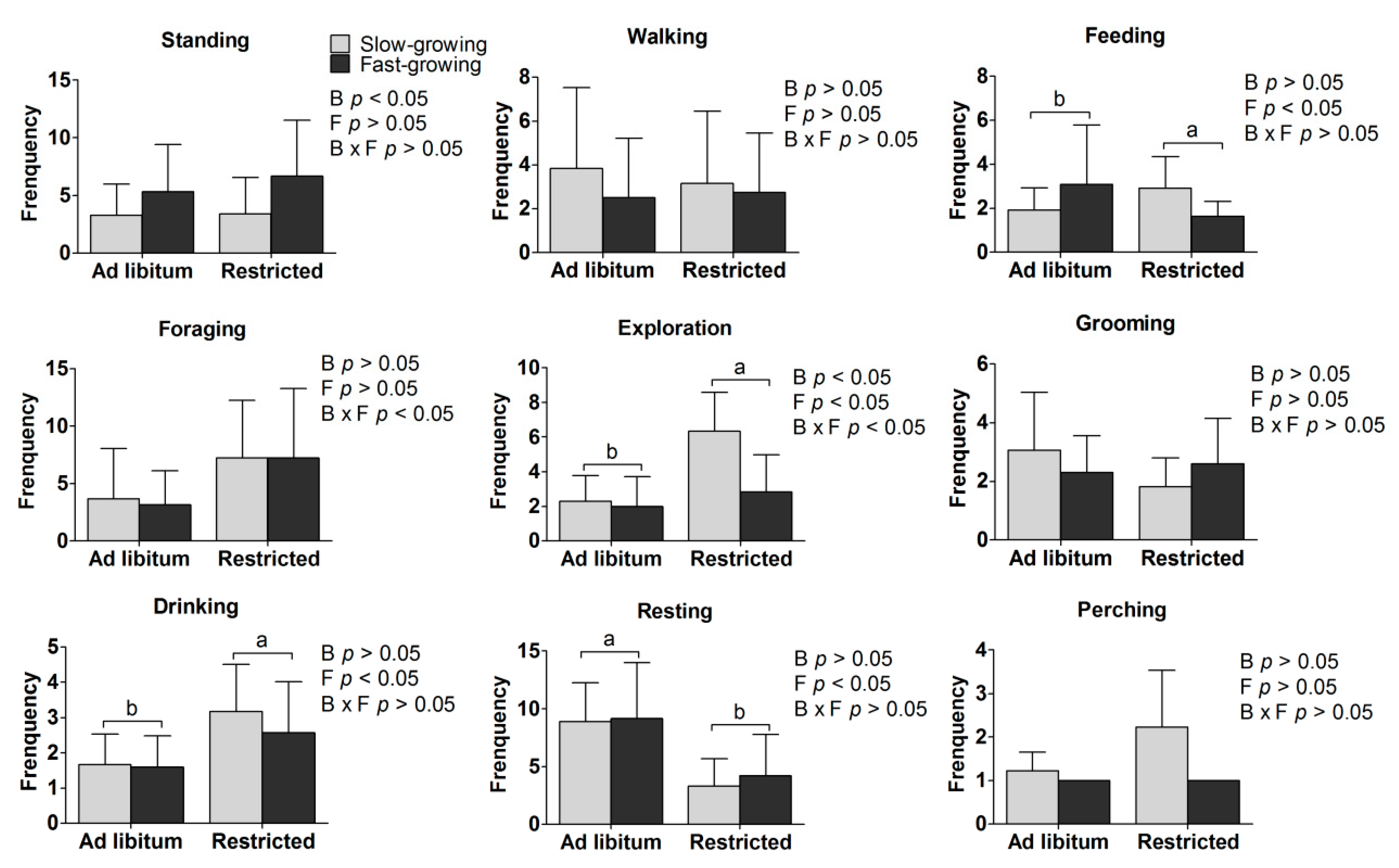
3.2. In Situ Behavior
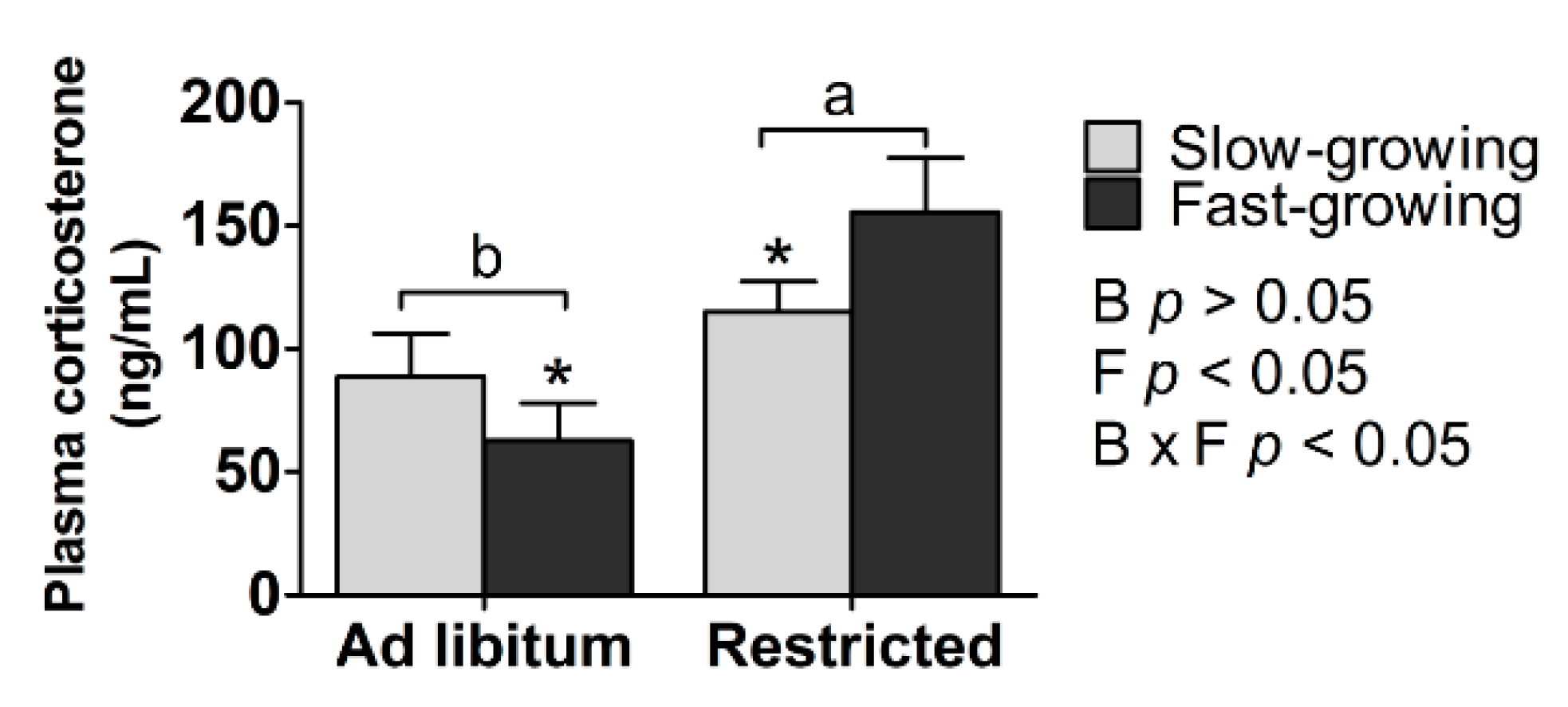
3.3. Corticosterone Concentration
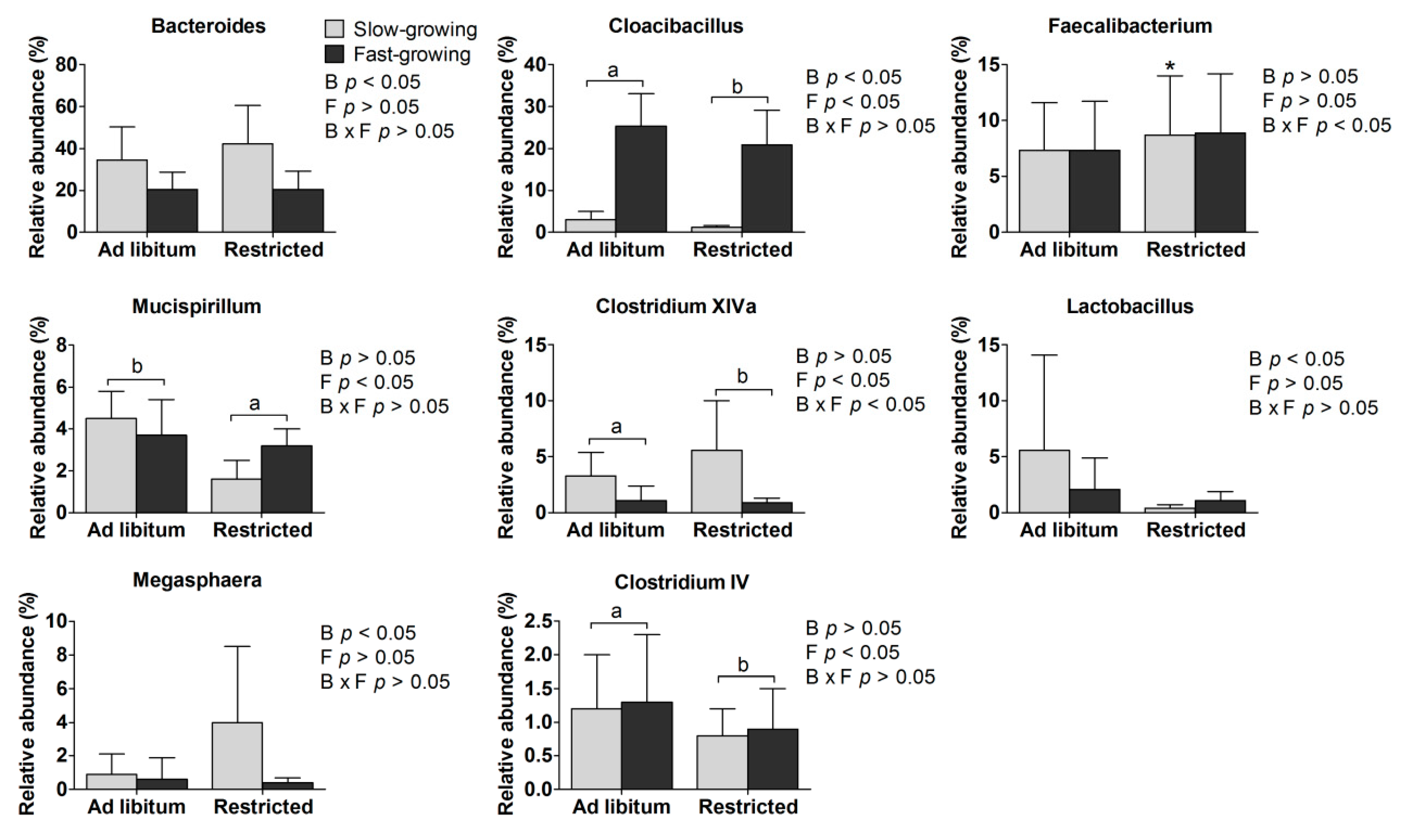
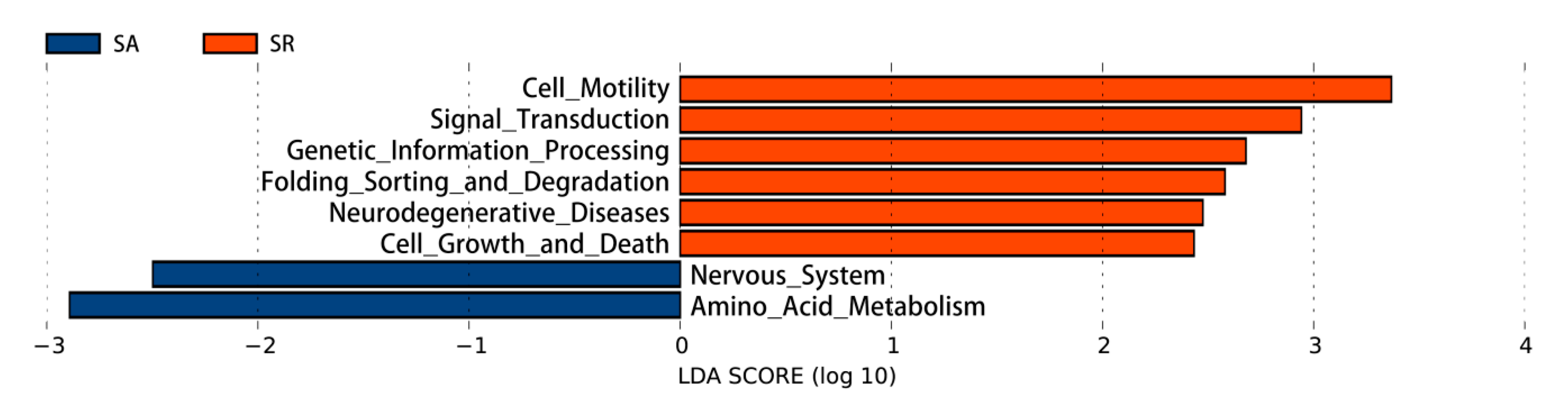
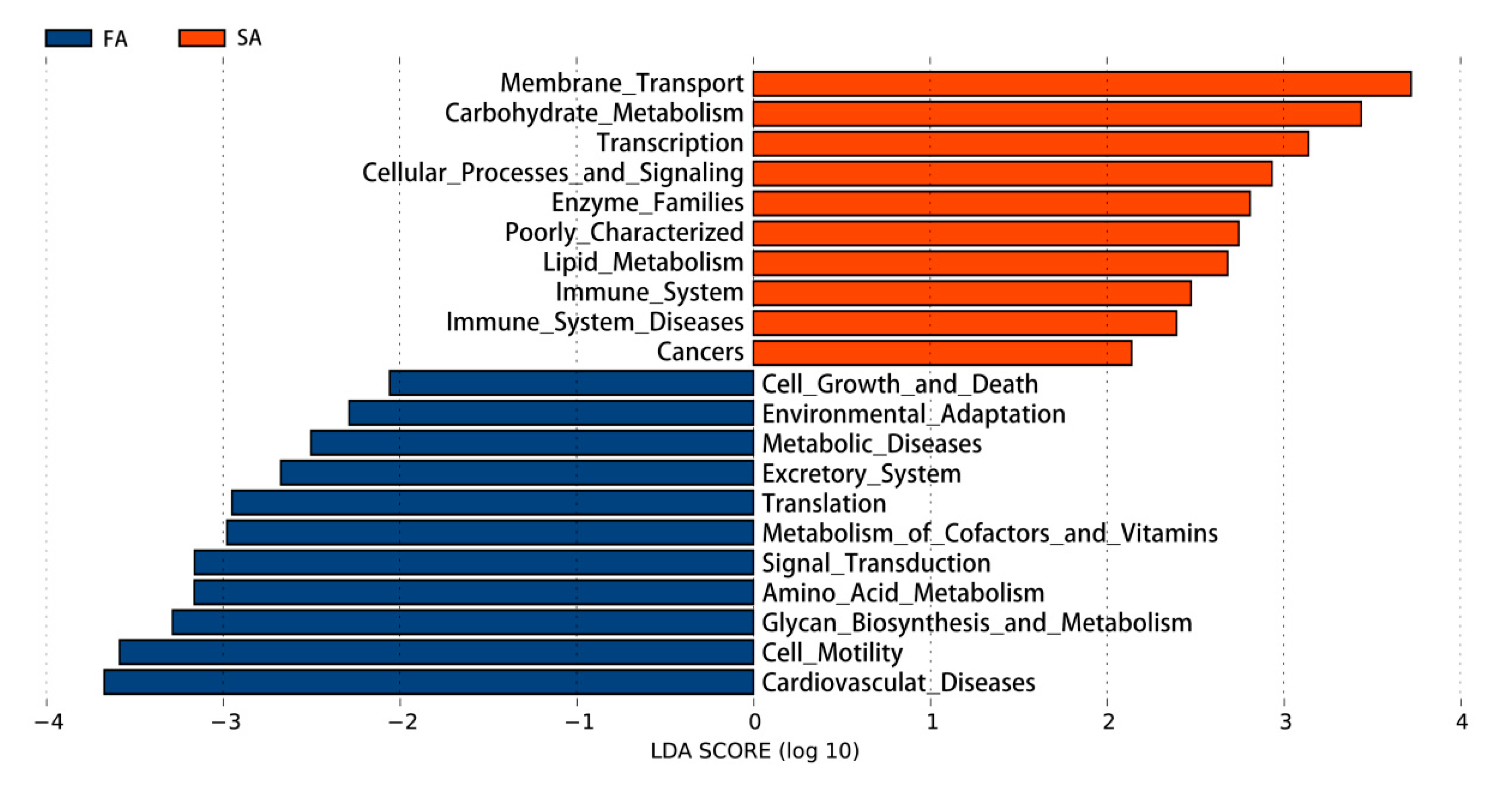
3.4. Gut Microbiota
3.4.1. Gut Microbiome at the Genus Level
3.4.2. Functions of the Gut Microbiota
4. Discussion
4.1. Body Weight
4.2. In Situ Behaviors
4.3. Corticosterone
4.4. Gut Microbiota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Nasser, A.; Al-Khalaifa, H.; Al-Saffar, A.; Khalil, F.; Albahouh, M.; Ragheb, G.; Al-Haddad, A.; Mashaly, M. Overview of chicken taxonomy and domestication. World Poult. Sci. J. 2007, 63, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.H.; Wang, K.H.; Wang, J.Y.; Ding, C.; Yang, N.; Dai, G.J.; Li, B.C.; Zhang, X.Y.; Liu, H.L.; Li, G.Z.; et al. Poultry Genetic Resources in China, 1st ed.; Shanghai Scientific and Technological Press: Shanghai, China, 2004. [Google Scholar]
- Wu, P.; Dai, G.; Chen, F.; Chen, L.; Zhang, T.; Xie, K.; Wang, J.; Zhang, G.; Du, S.J. Transcriptome profile analysis of leg muscle tissues between slow- and fast-growing chickens. PLoS ONE 2018, 13, e0206131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Xu, H.; Li, Z.; Zheng, X.; Jia, X.; Nie, Q.; Zhang, X.; Nicoletta, L. Comparison of the genome-wide DNA methylation profiles between fast-growing and slow-growing broilers. PLoS ONE 2013, 8, e56411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanatico, A.C.; Pillai, P.B.; Hester, P.Y.; Falcone, C.; Mench, J.A.; Owens, C.M.; Emmert, J.L. Performance, livability, and carcass yield of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor cccess. Poult. Sci. 2008, 87, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Fanatico, A.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007, 86, 2245–2255. [Google Scholar] [CrossRef]
- Porter, T.E. Differences in embryonic growth hormone secretion between slow and fast growing chicken strains. Growth Horm. IGF Res. 1998, 8, 133–139. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Ruggeri, S.; Martino, M.; Moscati, L.; Pesca, C.; Castellini, C. Adaptive response to exercise of fast-growing and slow-growing chicken strains: Blood oxidative status and non-enzymatic antioxidant defense. Poult. Sci. 2017, 96, 4096–4102. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Persia, M.E.; Feierstein, E.; Kingham, B.; Saylor, W.W. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult. Sci. 2009, 88, 2610–2619. [Google Scholar] [CrossRef]
- McDevitt, R.M.; McEntee, G.M.; Rance, K.A. Bone breaking strength and apparent metabolisability of calcium and phosphorus in selected and unselected broiler chicken genotypes. Br. Poult. Sci. 2006, 47, 613–621. [Google Scholar] [CrossRef]
- Savory, C.J.; Maros, K. Influence of degree of food restriction, age and time of day on behaviour of broiler breeder chickens. Behav. Process. 1993, 29, 179. [Google Scholar] [CrossRef]
- Jezová, D.; Skultétyová, I.; Makatsori, A.; Moncek, F.; Duncko, R. Hypothalamo-pituitary-adrenocortical axis function and hedonic behavior in adult male and female rats prenatally stressed by maternal food restriction. Stress 2002, 5, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.W.; Robinson, F.E. The application of short-term feed restriction to broiler chicken production: A review. J. Appl. Poult. Res. 1992, 1, 147–153. [Google Scholar] [CrossRef]
- Li, L.; Zhao, G.; Ren, Z.; Duan, L.; Zheng, H.; Wang, J.; He, Y. Effects of early feed restriction programs on production performance and hormone level in plasma of broiler chickens. Front. Agric. China 2011, 5, 94–101. [Google Scholar] [CrossRef]
- Arrazola, A.; Mosco, E.; Widowski, T.M.; Guerin, M.T.; Kiarie, E.G.; Torrey, S. The effect of alternative feeding strategies for broiler breeder pullets: 1. Welfare and performance during rearing. Poult. Sci. 2019, 98, 3377–3390. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Tolkamp, B.J.; Kyriazakis, I.; Lawrence, A.B. ‘Freedom from hunger’ and preventing obesity: The animal welfare implications of reducing food quantity or quality. Anim. Behav. 2009, 77, 275–288. [Google Scholar] [CrossRef]
- Lippens, M. Early and temporary quantitative food restriction of broiler chickens. 1. Effects on performance characteristics, mortality and meat quality. Br. Poult. Sci. 2000, 41, 343–354. [Google Scholar] [CrossRef]
- Zhan, X.A.; Wang, M.; Ren, H.; Zhao, R.Q.; Li, J.X.; Tan, Z.L. Effect of early feed restriction on metabolic programming and compensatory growth in broiler chickens. Poult. Sci. 2007, 86, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, M.J.; Constantinidou, C.; Cogan, T.A.; Bedford, M.R.; Penn, C.W.; Pallen, M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 2014, 9, e91941. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. The neglected organ: Bacterial flora has a crucial immunostimulatory role. Perspect. Biol. Med. 1992, 35, 251–260. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Yeoman, C.J.; Chia, N.; Jeraldo, P.; Sipos, M.; Goldenfeld, N.D.; White, B.A. The microbiome of the chicken gastrointestinal tract. Anim. Health Res. Rev. 2012, 13, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaway, T.R.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; Mcreynolds, J.L.; Edrington, T.S.; Byrd, J.A.; Anderson, R.C.; Krueger, N.; Nisbet, D.J. Evaluation of the bacterial diversity in cecal contents of laying hens fed various molting diets by using bacterial tag-encoded FLX amplicon pyrosequencing. Poult. Sci. 2009, 88, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Seidavi, A.; Asadpour, L.; Corazzin, M. Effects of physical form of diet and intensity and duration of feed restriction on the growth performance, blood variables, microbial flora, immunity, and carcass and organ characteristics of broiler chickens. Livest. Sci. 2015, 180, 150–157. [Google Scholar] [CrossRef]
- Jahanpour, H.; Seidavi, A.; Qotbi, A.A.A.; Delgado, F.; Gamboa, S. Effect of intensity and duration of quantitative feed restriction on broiler caecum microbiota. Indian J. Anim. Sci. 2014, 84, 554–558. [Google Scholar] [CrossRef]
- Chen, S.; Yan, C.; Xiang, H.; Xiao, J.; Liu, J.; Zhang, H.; Wang, J.; Liu, H.; Zhang, X.; Ou, M. Transcriptome changes underlie alterations in behavioral traits in different types of chicken. J. Anim. Sci. 2020, 98, 6. [Google Scholar] [CrossRef] [PubMed]
- Pourabedin, M.; Zhao, X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015, 362, fnv122. [Google Scholar] [CrossRef] [Green Version]
- Sofie, T.; Michiel, O.D.B.; Bram, B.; Sascha, T.; Vincent, S.; Van, H.J.D.; Nele, W.; Jaco, V. Comparative evaluation of four bacteria-specific primer pairs for 16S rRNA gene surveys. Front. Microbiol. 2017, 8, 494. [Google Scholar] [CrossRef]
- Qiong, W.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261. [Google Scholar] [CrossRef] [Green Version]
- Masri, S.A.; Alshamy, Z.; Hünigen, H.; Rhe, I.; Plendl, J. Comparison of dual-purpose chicken and broilers at market body weight: A morphometric pilot study revealing anatomical differences. In Proceedings of the 31st Conference of the European Association of Veterinary Anatomists, Vienna, Austria, 27–30 July 2016. [Google Scholar]
- Robinson, F.E.; Classen, H.L.; Hanson, J.A.; Onderka, D.K. Growth performance, feed efficiency and the incidence of skeletal and metabolic disease in full-fed and feed restricted broiler and roaster chickens. J. Appl. Poult. Res. 1992, 1, 33–41. [Google Scholar] [CrossRef]
- Rahimi, S.; Seidavi, A.; Sahraei, M.; Blanco, F.P.; Schiavone, A.; Martínez Marín, A.L. Effects of feed restriction and diet nutrient density during re-alimentation on growth performance, carcass traits, organ weight, blood parameters and the immune response of broilers. Italian J. Anim. Sci. 2015, 14, 4037. [Google Scholar] [CrossRef]
- Schütz, K.E.; Forkman, B.R.; Jensen, P. Domestication effects on foraging strategy, social behaviour and different fear responses: A comparison between the red junglefowl (Gallus gallus) and a modern layer strain. Appl. Anim. Behav. Sci. 2001, 74, 1–14. [Google Scholar] [CrossRef]
- Siegel, P.B. Evolution of the modern broiler and feed efficiency. Annu. Rev. Anim. Biosci. 2014, 2, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Price, E.O. Behavioral Genetics and the Process of Animal Domestication; Academic Press: San Diego, CA, USA, 1998; pp. 31–65. [Google Scholar]
- Favati, A.; Zidar, J.; Thorpe, H.; Jensen, P.; Løvlie, H. The ontogeny of personality traits in the red junglefowl, Gallus gallus. Behav. Ecol. 2015, 27, 484–493. [Google Scholar] [CrossRef]
- Beilharz, R.G.; Nitter, G. The missing E: The role of the environment in evolution and animal breeding. J. Anim. Breed. Genet. 1998, 115, 439–453. [Google Scholar] [CrossRef]
- Karin, E.; Schütz, J.P. Effects of resource allocation on behavioural strategies: A comparison of Red Junglefowl (Gallus gallus) and two domesticated breeds of poultry. Ethology 2001, 107, 753–765. [Google Scholar] [CrossRef]
- Fondevila, G.; Archs, J.L.; Cámara, L.; de Juan, A.F.; Mateos, G.G. The length of the feed restriction period affects eating behavior, growth performance, and the development of the proximal part of the gastrointestinal tract of young broilers. Poult. Sci. 2020, 99, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Aranibar, C.D.; Chen, C.; Davis, A.J.; Daley, W.I.; Dunkley, C.; Kim, W.K.; Usher, C.; Webster, A.B.; Wilson, J.L. Impact of an alternate feeding program on broiler breeder pullet behavior, performance, and plasma corticosterone. Poult. Sci. 2020, 99, 829–838. [Google Scholar] [CrossRef]
- de Jong, I.C.; van Voorst, A.S.; Blokhuis, H.J. Parameters for quantification of hunger in broiler breeders. Physiol. Behav. 2003, 78, 773–783. [Google Scholar] [CrossRef]
- Rajman, M.; Jurani, M.; Lamosova, D.; Macajova, M.; Sedlackova, M.; Kost’al, L.; Jezova, D.; Vyboh, P. The effects of feed restriction on plasma biochemistry in growing meat type chickens (Gallus gallus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 145, 363–371. [Google Scholar] [CrossRef]
- Kitaysky, A.S.; Kitaiskaia, E.V.; Wingfield, J.C.; Piatt, J.F. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J. Comp. Physiol. B 2001, 171, 701–709. [Google Scholar] [CrossRef]
- de Jong, I.C.; van Voorst, S.; Ehlhardt, D.A.; Blokhuis, H.J. Effects of restricted feeding on physiological stress parameters in growing broiler breeders. Br. Poult. Sci. 2002, 43, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Trocino, A.; White, P.; Bordignon, F.; Ferrante, V.; Bertotto, D.; Birolo, M.; Pillan, G.; Xiccato, G. Effect of feed restriction on the behaviour and welfare of broiler chickens. Animals 2020, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Tahamtani, F.M.; Moradi, H.; Riber, A.B. Effect of qualitative feed restriction in broiler breeder pullets on stress and clinical welfare indicators. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Hocking, P.M.; Maxwell, M.H.; Mitchell, M.A. Relationships between the degree of food restriction and welfare indices in broiler breeder females. Br. Poult. Sci. 1996, 37, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Jong, I.C.D.; Fillerup, M.; Blokhuis, H.J. Effect of scattered feeding and feeding twice a day during rearing on indicators of hunger and frustration in broiler breeders. Appl. Anim. Behav. Sci. 2005, 92, 61–76. [Google Scholar] [CrossRef]
- Hocking, P.M.; Maxwell, M.H.; Mitchell, M.A. Welfare assessment of broiler breeder and layer females subjected to food restriction and limited access to water during rearing. Br. Poult. Sci. 1993, 34, 443–458. [Google Scholar] [CrossRef]
- Savory, C.J.; Mann, J.S. Is there a role for corticosterone in expression of abnormal behaviour in restricted-fed fowls? Physiol. Behav. 1997, 62, 7–13. [Google Scholar] [CrossRef]
- Astheimer, L.B.; Wingfield, B.J.C. Interactions of Corticosterone with Feeding, Activity and Metabolism in Passerine Birds. Ornis. Scand. 1992, 23, 355–365. [Google Scholar] [CrossRef]
- Ericsson, M.; Fallahsharoudi, A.; Bergquist, J.; Kushnir, M.M.; Jensen, P. Domestication effects on behavioural and hormonal responses to acute stress in chickens. Physiol. Behav. 2014, 133, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, G.; Siegel, P.; He, C.; Meng, H. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci. Rep. 2013, 3, 1163. [Google Scholar] [CrossRef]
- Lumpkins, B.S.; Batal, A.B.; Lee, M.D. Evaluation of the bacterial community and intestinal development of different genetic lines of chickens. Poult. Sci. 2010, 89, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Kwok, L.Y.; Zheng, Y.; Wang, L.; Guo, Z.; Zhang, J.; Huang, W.; Wang, Y.; Leng, L.; Li, H. Differential fecal microbiota are retained in broiler chicken lines divergently selected for fatness traits. Sci. Rep. 2016, 6, 37376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Lee, S.H.; Lillehoj, H.S.; Li, G.X.; Jang, S.I.; Babu, U.S.; Park, M.S.; Kim, D.K.; Lillehoj, E.P.; Neumann, A.P.; et al. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 2010, 89, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Vaughan, E.E. Probiotic and gut lactobacilli and bifidobacteria: Molecular approaches to study diversity and activity. Annu. Rev. Microbiol. 2009, 63, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Yan, W.; Sun, C.; Ji, C.; Zhou, Q.; Zhang, D.; Zheng, J.; Yang, N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019, 13, 1422–1436. [Google Scholar] [CrossRef]
- Eeckhaut, V.; Van Immerseel, F.; Croubels, S.; De Baere, S.; Haesebrouck, F.; Ducatelle, R.; Louis, P.; Vandamme, P. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb. Biotechnol. 2011, 4, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- van Krimpen, M.M.; de Jong, I.C. Impact of nutrition on welfare aspects of broiler breeder flocks. World Poult. Sci. J. 2014, 70, 139–150. [Google Scholar] [CrossRef]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [Green Version]
- Simon, K.; De, V.R.G.; Kemp, B.; Lammers, A. Development of ileal cytokine and immunoglobulin expression levels in response to early feeding in broilers and layers. Poult. Sci. 2014, 93, 3017–3027. [Google Scholar] [CrossRef]
- Olkowski, A.A.; Wojnarowicz, C.; Nain, S.; Ling, B.; Alcorn, J.M.; Laarveld, B. A study on pathogenesis of sudden death syndrome in broiler chickens. Res. Vet. Sci. 2008, 85, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Olkowski, A.A.; Classen, H.L. High incidence of cardiac arrhythmias in broiler chickens. J. Vet. Med. A 1998. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, A.; de Leeuw, M.; Penaud-Frezet, S.; Dimova, D.; Murphy, R.A. Phylogenetic and functional alterations in bacterial community compositions in broiler ceca as a result of mannan oligosaccharide supplementation. Appl. Environ. Microb. 2015, 81, 3460–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Behavior Classification | Behavior Definition |
|---|---|
| Standing | Standing with legs upright without locomotion. |
| Walking | Locomotion at a normal speed. |
| Exploration | Pecking around the cage wall, the bottom of the cage or scratching the ground before feeding; looking around in a non-stationary state. |
| Food seeking and feeding | Searching for food from the feeder before feed was provided and ingestion of food after its provision. |
| Foraging | Pecking around the cage wall, the bottom of the cage or the area around the feeder after feeding but without ingesting food. |
| Grooming | Preening feathers with beak or claws, combing the feathers, or stretching the wings and legs. |
| Drinking | Tapping the nipple drinking fountain. |
| Resting | Lying down whilst performing none of the other defined behaviors. |
| Perching | Standing or squatting motionless on the perch. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Xiao, J.; Chen, D.; Turner, S.P.; Li, Z.; Liu, H.; Liu, W.; Liu, J.; Chen, S.; Zhao, X. Feed Restriction Induced Changes in Behavior, Corticosterone, and Microbial Programming in Slow- and Fast-Growing Chicken Breeds. Animals 2021, 11, 141. https://doi.org/10.3390/ani11010141
Yan C, Xiao J, Chen D, Turner SP, Li Z, Liu H, Liu W, Liu J, Chen S, Zhao X. Feed Restriction Induced Changes in Behavior, Corticosterone, and Microbial Programming in Slow- and Fast-Growing Chicken Breeds. Animals. 2021; 11(1):141. https://doi.org/10.3390/ani11010141
Chicago/Turabian StyleYan, Chao, Jinlong Xiao, Di Chen, Simon P. Turner, Zhiwei Li, Hao Liu, Wen Liu, Jian Liu, Siyu Chen, and Xingbo Zhao. 2021. "Feed Restriction Induced Changes in Behavior, Corticosterone, and Microbial Programming in Slow- and Fast-Growing Chicken Breeds" Animals 11, no. 1: 141. https://doi.org/10.3390/ani11010141





