Anti-Coccidial Effect of Rumex Nervosus Leaf Powder on Broiler Chickens Infected with Eimeria Tenella Oocyst
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Housing, Infection, and Experimental Design with Broiler Chickens
2.3. RN Leaf Powder Preparation and Its Nutritional Composition Analysis
2.4. Anti-Coccidial Evaluation
2.5. Performance Indices
2.6. Statistical Analysis
3. Results
3.1. Nutrient Analysis and Phytochemical Composition of RN Leaves
3.2. Anti-Coccidial Activity
3.3. Performance and Production Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peek, H.; Landman, W. Coccidiosis in poultry: Anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Coop, R.; Wall, R. Veterinary Parasitology, 3rd ed.; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Michels, M.; Bertolini, L.; Esteves, A.; Moreira, P.; Franca, S. Anticoccidial effects of coumestans from Eclipta alba for sustainable control of Eimeria tenella parasitosis in poultry production. Vet. Parasitol. 2011, 177, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Baki, A.-A.S.; Al-Quraishy, S. Prevalence of Coccidia (Eimeria spp.) Infection in Domestic Rabbits, Oryctolagus cuniculus, in Riyadh, Saudi Arabia. Pak. J. Zool. 2013, 45, 1329–1333. [Google Scholar]
- Al-Quraishy, S.; Abdel-Baki, A.; Dkhil, M. Eimeria tenella infection among broiler chicks Gallus domesticus in Riyadh city, Saudi Arabia. J. King Saud Univ. Sci. 2009, 21, 191–193. [Google Scholar] [CrossRef]
- Dubey, J.P. Coccidiosis in Livestock, Poultry, Companion Animals and Humans, 1st ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2019; p. 394. [Google Scholar]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Jang, S.I.; Lillehoj, E.P.; Bravo, D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 2013, 92, 2635–2643. [Google Scholar] [CrossRef]
- Amerah, A.; Ravindran, V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poult. Sci. 2015, 94, 673–680. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, W.; Shi, P.; Zhang, Y.; Huang, Z. Protective effect of berberine on the intestinal caecum in chicks with Eimeria tenella. Avian Biol. Res. 2016, 9, 235–239. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Cho, S.H.; Chung, T.K.; Kim, I.H. Anti-coccidial effect of essential oil blends and vitamin D on broiler chickens vaccinated with purified mixture of coccidian oocyst from Eimeria tenella and Eimeria maxima. Poult. Sci. 2019, 98, 2919–2926. [Google Scholar] [CrossRef]
- Nouroozikoh, T.; Shirali, S.; Rahbari, S. Salinomycin Impact on Eimeria Species Oocyst Excretion in Poultry by Litter Monitoring. Vet. Res. Biol. Prod. 2018, 32, 11–18. [Google Scholar]
- Abdelrahman, W.; Mohnl, M.; Teichmann, K.; Doupovec, B.; Schatzmayr, G.; Lumpkins, B.; Mathis, G. Comparative evaluation of probiotic and salinomycin effects on performance and coccidiosis control in broiler chickens. Poult. Sci. 2014, 93, 3002–3008. [Google Scholar] [CrossRef]
- Tonda, R.; Rubach, J.; Lumpkins, B.; Mathis, G.; Poss, M. Effects of tannic acid extract on performance and intestinal health of broiler chickens following coccidiosis vaccination and/or a mixed-species Eimeria challenge. Poult. Sci. 2018, 97, 3031–3042. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Becker, S.; Xiao, Y.; Lyu, W.; Yang, Q.; Zhu, H.; Yang, H.; Zhao, J.; Zhang, G. Differential impact of subtherapeutic antibiotics and ionophores on intestinal microbiota of broilers. Microorganisms 2019, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Iqbal, Z.; Blake, D.; Khan, M.; Saleemi, M. Anticoccidial drug resistance in fowl coccidia: The state of play revisited. Worlds Poult. Sci. J. 2011, 67, 337–350. [Google Scholar] [CrossRef]
- Wunderlich, F.; Al-Quraishy, S.; Steinbrenner, H.; Sies, H.; Dkhil, M.A. Towards identifying novel anti-Eimeria agents: Trace elements, vitamins, and plant-based natural products. Parasitol. Res. 2014, 113, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Barbour, E.; Ayyash, D.; Iyer, A.; Harakeh, S.; Kumosani, T. A review of approaches targeting the replacement of coccidiostat application in poultry production. Rev. Bras. Cienc. 2015, 17, 405–418. [Google Scholar] [CrossRef]
- Wiedosari, E.; Wardhana, A.H. Anticoccidial activity of Artemisinin and Extract of Artemesia annua leaves in chicken infected by Eimeria tenella. Ind. J. Anim. Vet. Sci. 2018, 22, 196–204. [Google Scholar] [CrossRef]
- Abbas, A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.K.; Khan, J.A.; Mahmood, M.S.; Saleemi, M.K. In vivo anticoccidial effects of Beta vulgaris (sugar beet) in broiler chickens. Microb. Pathog. 2017, 111, 139–144. [Google Scholar] [CrossRef]
- Allen, P.C. Dietary supplementation with Echinacea and development of immunity to challenge infection with coccidia. Parasitol. Res. 2003, 91, 74–78. [Google Scholar] [CrossRef]
- Yim, D.; Kang, S.S.; Kim, D.W.; Kim, S.H.; Lillehoj, H.S.; Min, W. Protective effects of Aloe vera-based diets in Eimeria maxima-infected broiler chickens. Exp. Parasitol. 2011, 127, 322–325. [Google Scholar] [CrossRef]
- Toulah, F.; Ismeel, H.; Khan, S. Effect of treatment with Neem (Azadirachta indica) compared with Baycox drug on the caecum of chicken experimentally infected with Eimeria tenella. J. Egypt. Soc. Parasitol. 2010, 40, 93–106. [Google Scholar]
- Ullah, M.I.; Akhtar, M.; Awais, M.M.; Anwar, M.I.; Khaliq, K. Evaluation of immunostimulatory and immunotherapeutic effects of tropical mushroom (Lentinus edodes) against eimeriasis in chicken. Trop. Anim. Health Prod. 2018, 50, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Colwell, D.; Gilleard, J. Botanicals: An alternative approach for the control of avian coccidiosis. Worlds Poult. Sci. J. 2012, 68, 203–215. [Google Scholar] [CrossRef]
- Quiroz-Castañeda, R.E.; Dantán-González, E. Control of avian coccidiosis: Future and present natural alternatives. Biomed. Res. Int. 2015, 2015, 430610. [Google Scholar] [CrossRef] [PubMed]
- Al-Naqeb, G. Antioxidant and antibacterial activities of some Yemeni medicinal plants. Int. J. Herb. Med. 2015, 3, 6–11. [Google Scholar]
- Alhotan, R.A.; Abudabos, A. Anticoccidial and antioxidant effects of plants derived polyphenol in broilers exposed to induced coccidiosis. Environ. Sci. Pollut. Res. 2019, 26, 14194–14199. [Google Scholar] [CrossRef]
- Goodla, L.; Manubolu, M.; Pathakoti, K.; Jayakumar, T.; Sheu, J.-R.; Fraker, M.; Tchounwou, P.B.; Poondamalli, P.R. Protective effects of ammannia baccifera against CCl4-induced oxidative stress in rats. Int. J. Environ. Res. 2019, 16, 1440. [Google Scholar] [CrossRef]
- Al-Asmari, A.R.K.; Siddiqui, Y.M.; Athar, M.T.; Al-Buraidi, A.; Al-Eid, A.; Horaib, G.B. Antimicrobial activity of aqueous and organic extracts of a Saudi medicinal plant: Rumex nervosus. J. Pharm. Bioallied Sci. 2015, 7, 300–303. [Google Scholar] [CrossRef]
- Al-Sunafi, S.M.Y. Pharmacognostical Study of Rumex nervosus Vahl. Family (Polygonaceae) growing in Yemen. Master’s Thesis, Cairo University, Giza, Egypt, 2016. [Google Scholar]
- Shankar, P.R.; Balasubramanium, R. Antimicrobial resistance: Global report on surveillance 2014. Australas. Med. J. 2014, 7, 237–238. [Google Scholar]
- Desta, K.T.; Lee, W.S.; Lee, S.J.; Kim, Y.H.; Kim, G.S.; Lee, S.J.; Kim, S.T.; Abd El-Aty, A.; Warda, M.; Shin, H.C. Antioxidant activities and liquid chromatography with electrospray ionization tandem mass spectrometry characterization and quantification of the polyphenolic contents of Rumex nervosus Vahl leaves and stems. J. Sep. Sci. 2016, 39, 1433–1441. [Google Scholar] [CrossRef]
- Quradha, M.M.; Khan, R.; Rehman, M.U.; Abohajeb, A. Chemical composition and in vitro anticancer, antimicrobial and antioxidant activities of essential oil and methanol extract from Rumex nervosus. Nat. Prod. Res. 2019, 33, 2554–2559. [Google Scholar] [CrossRef]
- Qasem, M.A.; Dkhil, M.A.; Al-Shaebi, E.M.; Murshed, M.; Mares, M.; Al-Quraishy, S. Rumex nervosus leaf extracts enhance the regulation of goblet cells and the inflammatory response during infection of chickens with Eimeria tenella. J. King Saud Univ. Sci. 2020, 32, 1818–1823. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Qasem, M.A.; Al-Shaebi, E.M.; Murshed, M.; Mares, M.M.; Dkhil, M.A. Rumex nervosus changed the oxidative status of chicken caecum infected with Eimeria tenella. J. King Saud Univ. Sci. 2020, 32, 2207–2211. [Google Scholar] [CrossRef]
- Ali, A.; Nasser, A.; Al-Sokari, S.S.; Mothana, R.; Hamed, M.; Waigh, M.; Cos, P.; Maes, L. In vitro antiprotozoal activity of five plant extracts from Albaha region. World J. Pharm. Res. 2016, 5, 338–346. [Google Scholar]
- Limenih, Y.; Umer, S.; Wolde-Mariam, M. Ethnobotanical study on traditional medicinal plants in Dega Damot woreda, Amhara Region, North Ethiopia. Int. J. Res. Pharm. Chem. 2015, 5, 258–273. [Google Scholar]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef] [PubMed]
- El-Ashram, S.; Suo, X. Electrical cream separator coupled with vacuum filtration for the purification of eimerian oocysts and trichostrongylid eggs. Sci. Rep. 2017, 7, 43346. [Google Scholar]
- Lee, H.-A.; Hong, S.; Chung, Y.-H.; Song, K.-D.; Kim, O. Anticoccidial effects of Galla rhois extract on Eimeria tenella-infected chicken. Lab. Anim. Res. 2012, 28, 193–197. [Google Scholar] [CrossRef]
- Melesse, A.; Masebo, M.; Abebe, A. The Substitution Effect of Noug Seed (Guizotia Abyssinica) Cake with Cassava Leaf (Manihot Escutulata C.) Meal on Feed Intake, Growth Performance, and Carcass Traits in Broiler Chickens. J. Anim. Hus. Dairy Sci. 2018, 2, 1–9. [Google Scholar]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. 2018, 98, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Ma, C.; Pan, L.; Li, G.; Yang, J.; Hong, J.; Cai, H.; Ren, X. Vaccination of chickens with DNA vaccine encoding Eimeria acervulina 3-1E and chicken IL-15 offers protection against homologous challenge. Exp. Parasitol. 2011, 127, 208–214. [Google Scholar] [CrossRef]
- Lan, L.; Zuo, B.; Ding, H.; Huang, Y.; Chen, X.; Du, A. Anticoccidial evaluation of a traditional chinese medicine—Brucea javanica—in broilers. Poult. Sci. 2016, 95, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Singh, V.; Thakur, V. Effect of Calotropis procera (madar) and amprolium supplementation on parasitological parameters of broilers during mixed Eimeria species infection. Vet. World. 2017, 10, 864–868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holdsworth, P.; Conway, D.; McKenzie, M.; Dayton, A.; Chapman, H.; Mathis, G.; Skinner, J.; Mundt, H.-C.; Williams, R. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet. Parasitol. 2004, 121, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Raju, M.; Rao, S.; Lavanya, G.; Reddy, E.; Sunder, G.S. Replacement of normal maize with quality protein maize on performance, immune response and carcass characteristics of broiler chickens. Asian Australas. J. Anim. Sci. 2010, 23, 1626–1631. [Google Scholar] [CrossRef]
- Behnamifar, A.; Rahimi, S.; Kiaei, M.; Fayazi, H. Comparison of the effect of probiotic, prebiotic, salinomycin and vaccine in control of coccidiosis in broiler chickens. Iran. J. Vet. Res. 2019, 20, 51–54. [Google Scholar]
- SAS. SAS Institute/OR 9.3 User’s Guide: Mathematical Programming Examples; SAS Institute: Hong Kong, China, 2012. [Google Scholar]
- Desta, K.T.; Kim, G.S.; Hong, G.E.; Kim, Y.H.; Lee, W.S.; Lee, S.J.; Jin, J.S.; Abd El-Aty, A.; Shin, H.C.; Shim, J.H. Dietary-flavonoid-rich flowers of Rumex nervosus Vahl: Liquid chromatography with electrospray ionization tandem mass spectrometry profiling and in vitro anti-inflammatory effects. J. Sep. Sci. 2015, 38, 3345–3353. [Google Scholar] [CrossRef]
- Ariza-Nieto, C.J. Evaluation of Oregano (Origanum Vulgare) Essential Oils in Swine Production System; University of Minnesota: Minneapolis, MN, USA, 2006. [Google Scholar]
- Castelo, A.; Del Menezzi, C.; Resck, I. Seasonal variation in the yield and the chemical composition of essential oils from two Brazilian native arbustive species. J. Appl. Sci. 2012, 12, 753–760. [Google Scholar] [CrossRef]
- Muthamilselvan, T.; Kuo, T.-F.; Wu, Y.-C.; Yang, W.-C. Herbal remedies for coccidiosis control: A review of plants, compounds, and anticoccidial actions. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Asad, M.; Getachew, A.; Ahmad, M. Antidiarrheal activity of methanolic extract of Rumex nervosus. J. Pharm. Res. 2004, 3, 67–69. [Google Scholar] [CrossRef][Green Version]
- Geetha, M.; Palanivel, K. A Review on Poultry Coccidiosis. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3345–3349. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Alyemni, A.H.; Swilam, E.O.; Al-Ghadi, M. Comparative Anticoccidial Effect of some Natural Products against Eimeria spp. Infection on Performance Traits, Intestinal Lesion and Occyte Number in Broiler. Pak. J. Zool. 2017, 49, 1989–1995. [Google Scholar] [CrossRef]
- Tanweer, A.J.; Saddique, U.; Bailey, C.; Khan, R. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol. Res. 2014, 113, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Novaes, J.; Rangel, L.T.L.; Ferro, M.; Abe, R.Y.; Manha, A.P.; de Mello, J.C.; Varuzza, L.; Durham, A.M.; Madeira, A.M.B.; Gruber, A. A comparative transcriptome analysis reveals expression profiles conserved across three Eimeria spp. of domestic fowl and associated with multiple developmental stages. Int. J. Parasitol. 2012, 42, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Abdisa, T.; Hasen, R.; Tagesu, T.; Regea, G.; Tadese, G. Poultry Coccidiosis and its Prevention. Control. J. Vet. Anim. Res. 2019, 2, 103. [Google Scholar]
- Mitsch, P.; Zitterl-Eglseer, K.; Köhler, B.; Gabler, C.; Losa, R.; Zimpernik, I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult. Sci. 2004, 83, 669–675. [Google Scholar] [CrossRef]
- Akanbi, O.B.; Taiwo, V.O. The effect of a Local isolate and Houghton strain of Eimeria tenella on clinical and growth parameters following challenge in chickens vaccinated with IMMUCOX® and LIVACOX® vaccines. J. Parasit. Dis. 2020, 44, 395–402. [Google Scholar] [CrossRef]
- Adarsh, A.; Chettiyar, B.; Kanthesh, B.; Raghu, N. Phytochemical Screening and Antimicrobial Activity of “Cinnamon zeylanicum”. Int. J. Pharm. Res. Innov. 2020, 13, 22–33. [Google Scholar]
- El-Hack, M.E.A.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef]
- Abbas, R.; Iqbal, Z.; Mansoor, M. Role of natural antioxidants for the control of coccidiosis in poultry. Pak. Vet. J. 2013, 33, 401–407. [Google Scholar]
- Thangavel, G.; Mukkalil, R.; Chirakkal, H. Plant Parts and Extracts Having Anticoccidial Activity. U.S. Patent 10,568,923, 25 February 2020. [Google Scholar]
- Abudabos, A.M.; Alyemni, A.H.; Hussein, E.O.; Al-Ghadi, M.A.Q. Anticoccidial effect of some natural products in experimentally induced Eimeria spp. infection on carcass quality, intestinal lesion and ileal histology in broilers. J. Anim. Plant Sci. 2018, 28, 73–79. [Google Scholar]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]





| Ingredient | Period | |
|---|---|---|
| Starter | Finisher | |
| Yellow corn | 53.218 | 58.09 |
| Soybean meal | 37.85 | 32.15 |
| Wheat bran | 2.00 | 2.2 |
| Corn gluten meal | 1.4 | 0 |
| Choline chloride CL 60 | 0.05 | 0.05 |
| Corn oil | 1.5 | 4.2 |
| Dicalcuim phosphate DCP | 1.98 | 1.615 |
| Ground limestone | 0.9 | 0.79 |
| Salt | 0.400 | 0.30 |
| DL-methionine | 0.292 | 0.25 |
| Lysine-HCL | 0.21 | 0.105 |
| Vitamin–mineral premix 1 | 0.200 | 0.200 |
| Total | 100 | 100 |
| Metabolic energy (ME), kcal/kg | 3000 | 3200 |
| Crude protein, % | 23.0 | 20.0 |
| Non phytate p, % | 0.48 | 0.405 |
| Calcium, % | 0.96 | 0.81 |
| d-lysine, % | 1.28 | 1.06 |
| Sulfur amino acids, % | 0.95 | 0.83 |
| Threonine, % | 0.86 | 0.71 |
| Parameter | As-fed basis (%) |
|---|---|
| Moisture content | 5.67 |
| Dry matter | 94.33 |
| Crude protein | 13.63 |
| Ether extract | 1.54 |
| NFE | 58.58 |
| Total carbohydrate | 52.91 |
| Crude ash (Inorganic matter) | 18.01 |
| Total crude fiber | 8.24 |
| Total fiber fractions | |
| A. Acid detergent fiber | 15.48 |
| B. Neutral detergent fiber | 20.21 |
| Organic matter | 81.99 |
| Nutritive value (GE) (kcal/100 g) | 327.33 |
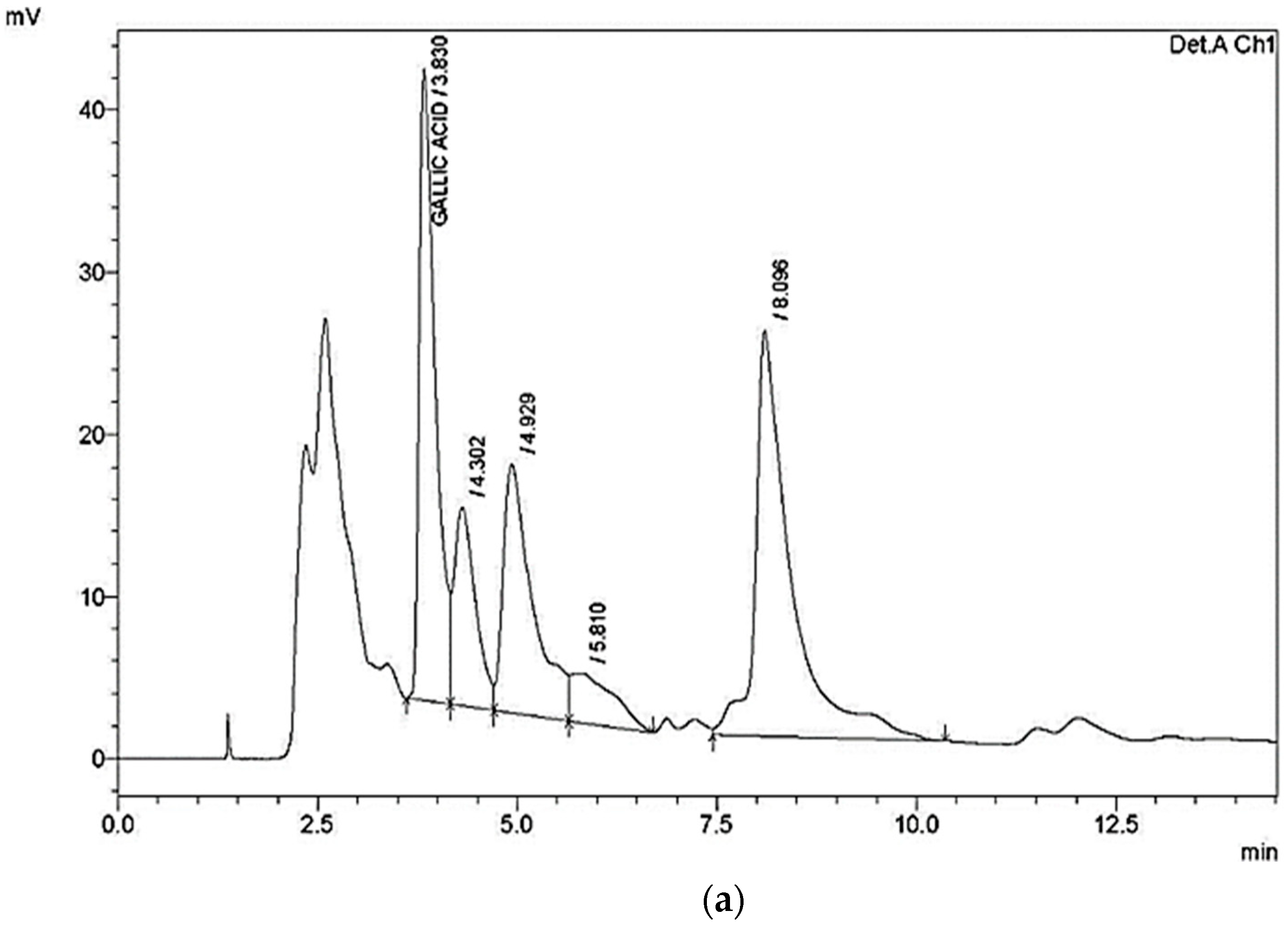
| Compound | Retention Time (RT) (min) | Area | Height | Concentration (µg/g) |
|---|---|---|---|---|
| Gallic acid | 3.830 | 554,308 | 38,945 | 700 |
| Catechin | - | - | - | - |
| Chlorogenic acid | - | - | - | - |
| Caffeine | - | - | - | - |
| Retention Time (RT) (min) | Bioactive Chemical Constituents | Quality | Molecular Weight (amu) | Molecular Formula |
|---|---|---|---|---|
| 4.211 | Oxime-, methoxy-phenyl-_ | 74 | 151.063 | C8H9NO2 |
| 7.026 | Cyclotrisiloxane, hexamethyl- | 72 | 222.056 | C6H18O3Si3 |
| 7.146 | 5-Methyl-2-phenylindolizine | 59 | 207.105 | C15H13N |
| 7.146 | 1,2-Bis(trimethylsilyl)benzene | 50 | 222.126 | C12H22Si2 |
| 25.41 | Hexadecanoic acid, methyl ester | 98 | 270.256 | C15H30O2 |
| 25.41 | Pentadecanoic acid, 14-methyl-, methyl ester | 97 | 270.256 | C17H34O2 |
| 25.41 | Tridecanoic acid, methyl ester | 86 | 228.209 | C14H28O2 |
| 25.41 | Nonadecanoic acid, methyl ester | 53 | 312.303 | C20H40O2 |
| 28.054 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 99 | 294.256 | C19H34O2 |
| 28.054 | 9,17-Octadecadienal, (Z)- | 90 | 264.245 | C18H32O |
| 28.123 | 10,13-Octadecadienoic acid, methyl ester | 99 | 294.256 | C19H34O2 |
| 28.123 | 7-Pentadecyne | 96 | 208.219 | C15H28 |
| 28.5 | Octadecanoic acid, methyl ester | 98 | 298.287 | C19H38O2 |
| Group | Treatment 1 | Bloody Diarrhea | Lesion Scores | OPG (Mean × 106) | Oocyst Value | Inhibition Rate | Cecal Length (%) |
|---|---|---|---|---|---|---|---|
| 1 | RN1g | 1.0 ab | 1.8 ab | 6.42 b | 51.15 b | 48.85 b | 15.56 ab |
| 2 | RN3g | 0.8 ab | 1.6 b | 4.77 bc | 38.01 bc | 61.99 b | 16.83 a |
| 3 | RN5g | 0.2 b | 1.4 b | 3.00 bc | 23.93 bc | 76.07 b | 16.44 a |
| 4 | Sacox | 0.0 b | 0.8 bc | 3.13 bc | 24.94 bc | 75.06 b | 15.88 ab |
| 5 | PC | 2.0 a | 2.6 a | 12.54 a | 100.00 a | 0.00 c | 11.99 b |
| 6 | NC | 0.0 b | 0.0 c | 0.00 c | 0.00c | 100.00 a | 14.79 ab |
| SEM 2 Probability | 0.205 | 0.176 | 0.8796 | 7.012 | 7.454 | 0.459 | |
| 0.0196 | <0.0001 | 0.0010 | <0.0001 | 0.0010 | 0.0180 | ||
| Group | Treatment 1 | Performance | |||||
|---|---|---|---|---|---|---|---|
| BW (kg) | BWG (g) | FI (g) | FCR (g:g) | PI | PEF | ||
| 1 | R1g | 1.128 b | 58.35 b | 96.87 b | 1.67 ab | 35.36 b | 251.90 b |
| 2 | R3g | 1.136 b | 54.37 b | 96.45 b | 1.77 a | 30.71 b | 237.49 b |
| 3 | R5g | 1.200 ab | 57.18 b | 98.80 b | 1.74 a | 33.29 b | 257.61 b |
| 4 | Sacox | 1.238 ab | 57.75 b | 104.35 ab | 1.81 a | 32.03 b | 254.19 b |
| 5 | PC | 1.120 b | 56.45 b | 99.35 b | 1.81 a | 33.14 b | 230.64 b |
| 6 | NC | 1.273 a | 83.51 a | 117.19 a | 1.41 b | 59.76 a | 336.18 a |
| SEM 2 Probability | 18.082 | 2.272 | 1.950 | 0.040 | 2.313 | 9.068 | |
| 0.043 | <0.0001 | 0.007 | 0.017 | <.0001 | 0.003 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qaid, M.M.; Al-Mufarrej, S.I.; Azzam, M.M.; Al-Garadi, M.A.; Albaadani, H.H.; Alhidary, I.A.; Aljumaah, R.S. Anti-Coccidial Effect of Rumex Nervosus Leaf Powder on Broiler Chickens Infected with Eimeria Tenella Oocyst. Animals 2021, 11, 167. https://doi.org/10.3390/ani11010167
Qaid MM, Al-Mufarrej SI, Azzam MM, Al-Garadi MA, Albaadani HH, Alhidary IA, Aljumaah RS. Anti-Coccidial Effect of Rumex Nervosus Leaf Powder on Broiler Chickens Infected with Eimeria Tenella Oocyst. Animals. 2021; 11(1):167. https://doi.org/10.3390/ani11010167
Chicago/Turabian StyleQaid, Mohammed M., Saud I. Al-Mufarrej, Mahmoud M. Azzam, Maged A. Al-Garadi, Hani H. Albaadani, Ibrahim A. Alhidary, and Riyadh S. Aljumaah. 2021. "Anti-Coccidial Effect of Rumex Nervosus Leaf Powder on Broiler Chickens Infected with Eimeria Tenella Oocyst" Animals 11, no. 1: 167. https://doi.org/10.3390/ani11010167
APA StyleQaid, M. M., Al-Mufarrej, S. I., Azzam, M. M., Al-Garadi, M. A., Albaadani, H. H., Alhidary, I. A., & Aljumaah, R. S. (2021). Anti-Coccidial Effect of Rumex Nervosus Leaf Powder on Broiler Chickens Infected with Eimeria Tenella Oocyst. Animals, 11(1), 167. https://doi.org/10.3390/ani11010167








