Biological Sound vs. Anthropogenic Noise: Assessment of Behavioural Changes in Scyliorhinus canicula Exposed to Boats Noise
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing
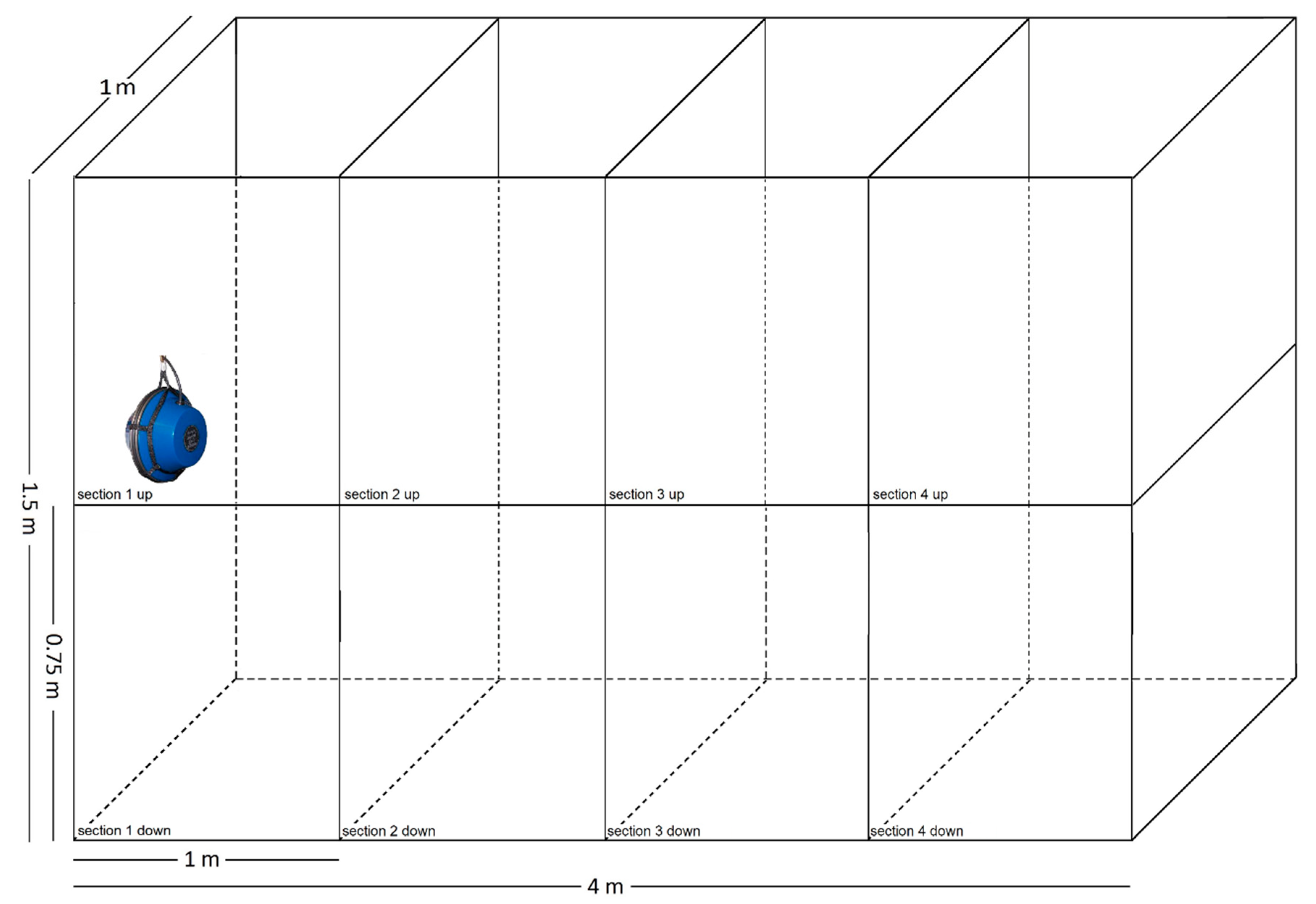
2.2. Experimental Design
- -
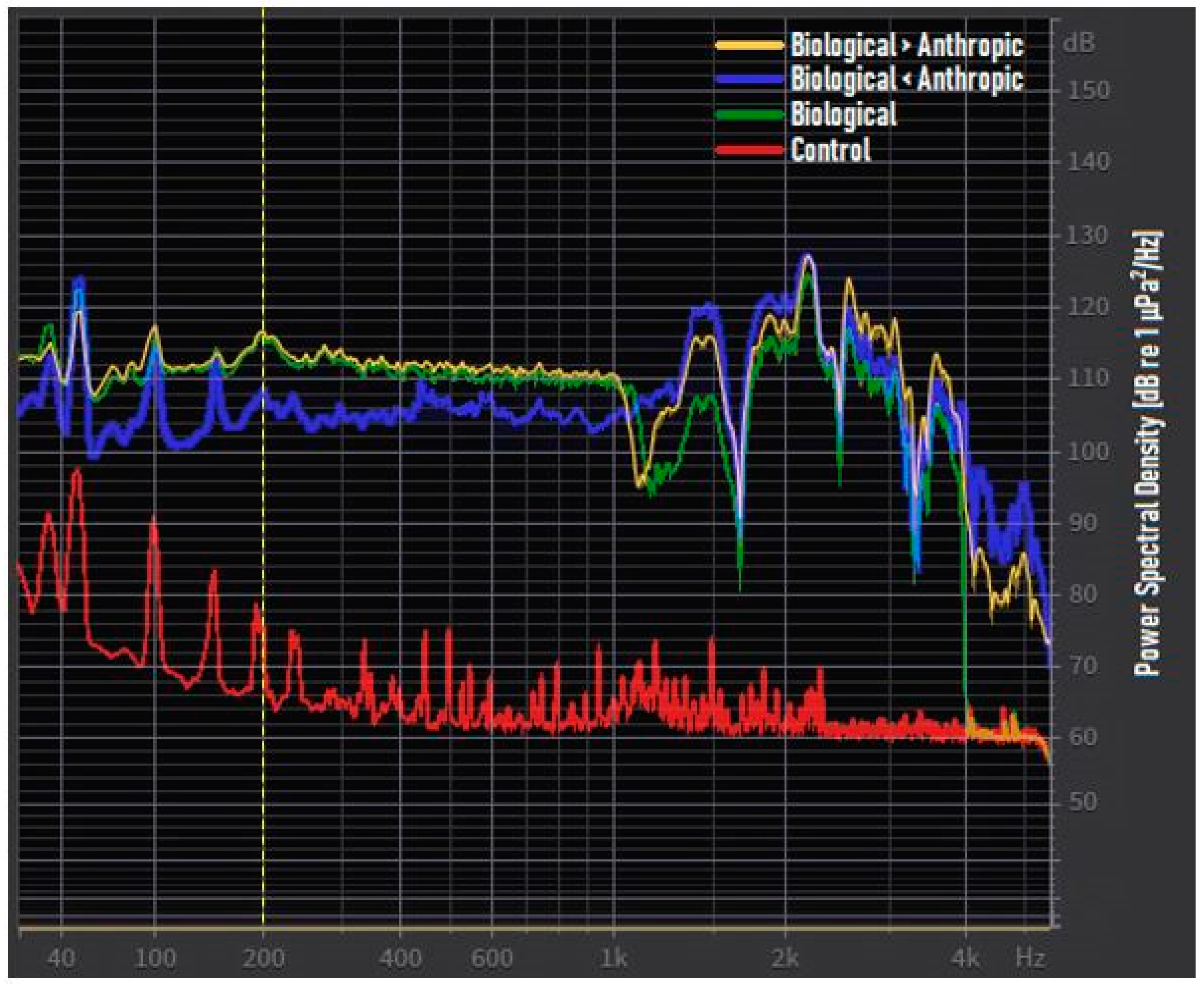
- (B) biological acoustic condition: A ten-minute audio file recreating the main acoustic components of a marine rocky soundscape, using signals from snapping shrimps, sea urchins grazing, and siniferous fish.
- -
- (B > A) biological > anthropogenic acoustic condition: A ten-minute audio file where the abovementioned track was mixed with another ten-minute audio file, resembling an intense shipping traffic marine area. Hydrofoils, recreational boats, ferries, and fishing boat noises were used to achieve this target. In this condition, the biological sounds were 6 dB higher above the anthropogenic noise.
- -
- (B < A) biological < anthropogenic acoustic condition: A ten-minute audio file similar to the abovementioned “biological > anthropogenic” track but, in this case, the biological sounds were 6 dB lower than anthropogenic noise.
- -
- (C) Control condition: Characterized only by low-level background noise of the experimental aquarium.
2.3. Acquisition, Editing and Projection of Acoustic Stimuli
2.4. Behavioural and Audio Monitoring System and Analysis
2.5. Statistical Analysis
3. Results
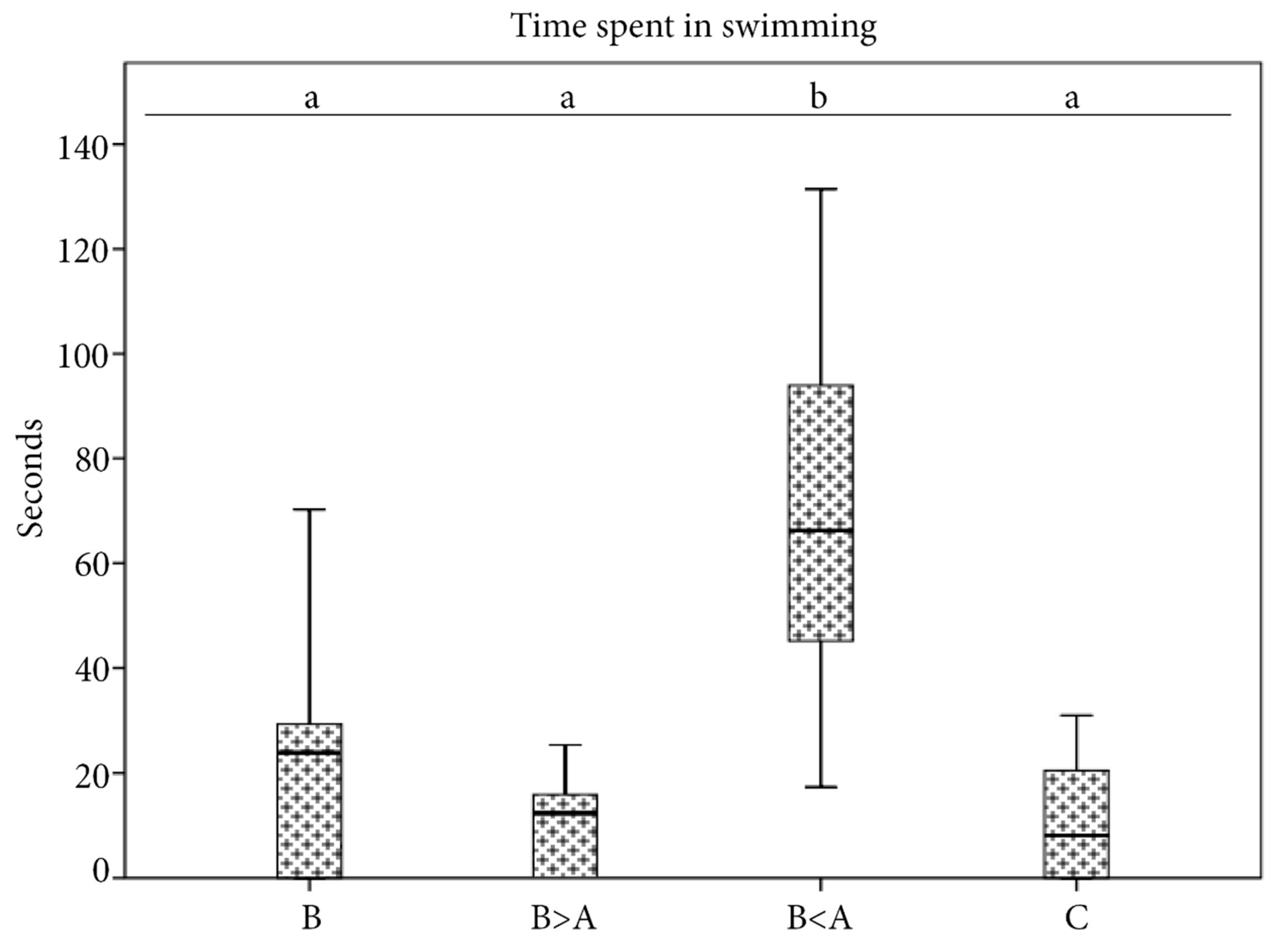
3.1. Overall Time Spent in Swimming
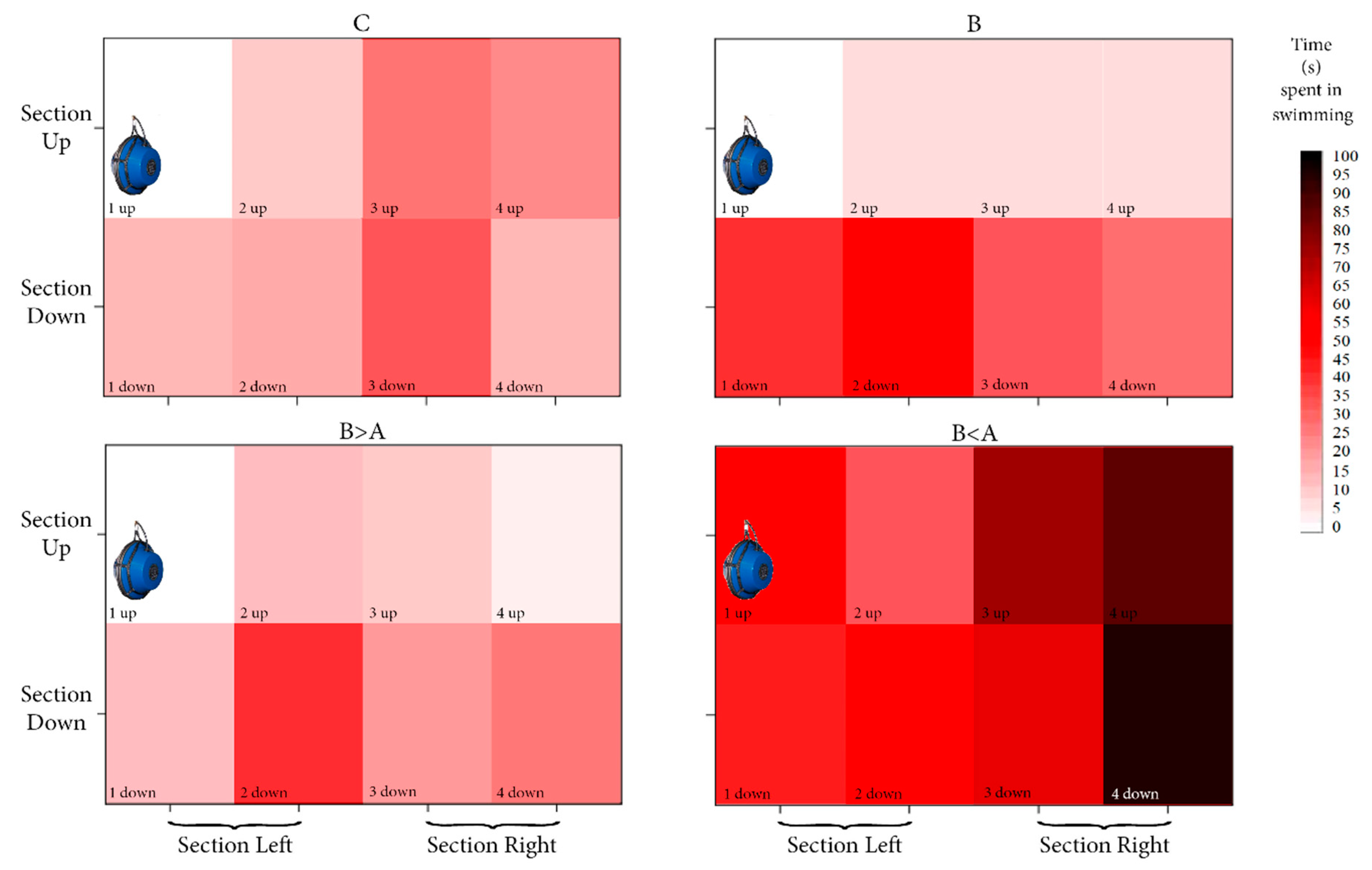
3.2. Spatial Occupancy of Aquarium
4. Discussion
4.1. Behavioural Response in the Control Condition
4.2. Behavioural Response in the Biological and Biological > Anthropogenic Conditions
4.3. Behavioural Response in the “Biological < Anthropogenic” Condition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Buscaino, G.; Gavio, A.; Galvan, D.; Filiciotto, F.; Maccarrone, V.; de Vincenzi, G.; Mazzola, S.; Orensanz, J. Acoustic signals and behaviour of Ovalipes trimaculatus in the context of reproduction. Aquat. Biol. 2015, 24, 61–73. [Google Scholar] [CrossRef]
- Coquereau, L.; Grall, J.; Clavier, J.; Jolivet, A.; Chauvaud, L. Acoustic behaviours of large crustaceans in NE Atlantic coastal habitats. Aquat. Biol. 2016, 25, 151–163. [Google Scholar] [CrossRef]
- de Vincenzi, G.; Parisi, I.; Torri, M.; Papale, E.; Mazzola, S.; Nuth, C.; Buscaino, G. Influence of environmental parameters on the use and spatiotemporal distribution of the vocalizations of bearded seals (Erignathus barbatus) in Kongsfjorden, Spitsbergen. Polar Biol. 2019, 42, 1241–1254. [Google Scholar] [CrossRef]
- de Vincenzi, G.; Filiciotto, F.; Maccarrone, V.; Mazzola, S.; Buscaino, G. Behavioural responses of the European spiny lobster, Palinurus elephas (Fabricius, 1787), to conspecific and synthetic sounds. Crustaceana 2015, 88, 523–540. [Google Scholar] [CrossRef]
- Parisi, I.; de Vincenzi, G.; Torri, M.; Papale, E.; Mazzola, S.; Bonanno, A.; Buscaino, G. Underwater vocal complexity of Arctic seal Erignathus barbatus in Kongsfjorden (Svalbard). J. Acoust. Soc. Am. 2017, 142, 3104–3115. [Google Scholar] [CrossRef] [PubMed]
- Tyack, P.L. Acoustic Communication Under the Sea. In Animal Acoustic Communication; Springer Science and Business Media LLC: Berlin, Germany, 1998; pp. 163–220. [Google Scholar]
- Caruso, F.; Sciacca, V.; Parisi, I.; Viola, S.; de Vincenzi, G.; Bocconcelli, A.; Mooney, T.A.; Sayigh, L.S.; Li, S.; Filiciotto, F.; et al. Acoustic recordings of rough-toothed dolphin (Steno bredanensis) offshore Eastern Sicily (Mediterranean Sea). J. Acoust. Soc. Am. 2019, 146, EL286–EL292. [Google Scholar] [CrossRef]
- Tyack, P.L.; Clark, C.W. Communication and Acoustic Behavior of Dolphins and Whales. In Hearing by Whales and Dolphins; Springer Science and Business Media LLC: Berlin, Germany, 2000; Volume 12, pp. 156–224. [Google Scholar]
- Popper, A.N.; Hawkins, A. A sound approach to assessing the impact of underwater noise on marine fishes and invertebrates. In The Effects of Noise on Aquatic Life II; Springer Science and Business Media LLC: Berlin, Germany, 2016. [Google Scholar]
- Abdulla, A. Maritime Traffic Effects on Biodiversity in the Mediterranean Sea: Review of Impacts, Priority Areas and Mitigation Measures; IUCN: Gland, Switzerland, 2008; ISBN 978-2-8317-1079-2. [Google Scholar]
- Hildebrand, J.A. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 2009, 395, 5–20. [Google Scholar] [CrossRef]
- Ross, D. Ship Sources of Ambient Noise. IEEE J. Ocean. Eng. 2005, 30, 257–261. [Google Scholar] [CrossRef]
- Filiciotto, F.; Vazzana, M.; Celi, M.; Maccarrone, V.; Ceraulo, M.; Buffa, G.; Di Stefano, V.; Mazzola, S.; Buscaino, G. Behavioural and biochemical stress responses of Palinurus elephas after exposure to boat noise pollution in tank. Mar. Pollut. Bull. 2014, 84, 104–114. [Google Scholar] [CrossRef]
- Filiciotto, F.; Vazzana, M.; Celi, M.; Maccarrone, V.; Ceraulo, M.; Buffa, G.; Arizza, V.; de Vincenzi, G.; Grammauta, R.; Mazzola, S.; et al. Underwater noise from boats: Measurement of its influence on the behaviour and biochemistry of the common prawn (Palaemon serratus, Pennant 1777). J. Exp. Mar. Biol. Ecol. 2016, 478, 24–33. [Google Scholar] [CrossRef]
- Kunc, H.P.; McLaughlin, K.E.; Schmidt, R. Aquatic noise pollution: Implications for individuals, populations, and ecosystems. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160839. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, M.; Celi, M.; Maricchiolo, G.; Genovese, L.; Corrias, V.; Quinci, E.M.; de Vincenzi, G.; Maccarrone, V.; Cammilleri, G.; Mazzola, S.; et al. Are mussels able to distinguish underwater sounds? Assessment of the reactions of Mytilus galloprovincialis after exposure to lab-generated acoustic signals. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 201, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Voellmy, I.K.; Purser, J.; Flynn, D.; Kennedy, P.; Simpson, S.D.; Radford, A.N. Acoustic noise reduces foraging success in two sympatric fish species via different mechanisms. Anim. Behav. 2014, 89, 191–198. [Google Scholar] [CrossRef]
- Chan, A.A.Y.-H.; Giraldo-Perez, P.; Smith, S.; Blumstein, D.T. Anthropogenic noise affects risk assessment and attention: The distracted prey hypothesis. Biol. Lett. 2010, 6, 458–461. [Google Scholar] [CrossRef]
- Simpson, S.D.; Purser, J.; Radford, A.N. Anthropogenic noise compromises antipredator behaviour in European eels. Glob. Chang. Biol. 2015, 21, 586–593. [Google Scholar] [CrossRef]
- Hawkins, A.D. Underwater Sound and Fish Behaviour, 129–169. In The Behaviour of Telcost Fishcs; Pitcher, T.J., Ed.; Croom Helm: London, UK; Sydney, Australia, 1993. [Google Scholar]
- van Opzeeland, I.; Slabbekoorn, H. Importance of Underwater Sounds for Migration of Fish and Aquatic Mammals. In The Effects of Noise on Aquatic Life; Springer Science and Business Media LLC: Berlin, Germany, 2012; pp. 357–359. [Google Scholar]
- Myrberg, A.A., Jr.; Gordon, C.R.; Klimley, A.P. Attraction of Free Ranging Sharks by Low Frequency Sound, with Comments on Its Biological Significance. In Sound Reception in Fish; Elsevier: Amsterdam, The Netherlands, 1976; pp. 205–228. [Google Scholar]
- Casper, B.M.; Mann, D.A. Evoked Potential Audiograms of the Nurse Shark (Ginglymostoma cirratum) and the Yellow Stingray (Urobatis jamaicensis). Environ. Boil. Fishes 2006, 76, 101–108. [Google Scholar] [CrossRef]
- Chapuis, L. The Acoustic World of Sharks. Ph.D. Thesis, University of Western Australia, Crawley, Australia, 2017. [Google Scholar]
- Corwin, J.T. Peripheral auditory physiology in the lemon shark: Evidence of parallel otolithic and non-otolithic sound detection. J. Comp. Physiol. A 1981, 142, 379–390. [Google Scholar] [CrossRef]
- Fay, R.R.; Kendall, J.I.; Popper, A.N.; Tester, A.L. Vibration detection by the macula neglecta of sharks. Comp. Biochem. Physiol. Part A Physiol. 1974, 47, 1235–1240. [Google Scholar] [CrossRef]
- Gardiner, J.M.; Hueter, R.E.; Maruska, K.P.; Sisneros, J.A.; Casper, B.M.; Mann, D.A.; Demski, L.S. Sensory Physiology and Behavior of Elasmobranchs. Biol. Sharks Relat. 2012, 1, 349–401. [Google Scholar]
- Kelly, J.C.; Nelson, D.R. Hearing thresholds of the horn shark, Heterodontus francisci. J. Acoust. Soc. Am. 1975, 58, 905–909. [Google Scholar] [CrossRef]
- Tester, A.L.; Kendall, J.I.; Milisen, W.B. Morphology of the Ear of the Shark Genus Carcharhinus, with Particular Reference to the Macula Neglecta; University of Hawaii Press: Honolulu, HI, USA, 1972. [Google Scholar]
- Casper, B.M. The Hearing Abilities of Elasmobranch Fishes. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2006. [Google Scholar]
- Myrberg, A.A. The Acoustical Biology of Elasmobranchs. In The Behavior and Sensory Biology of Elasmobranch Fishes: An Anthology in Memory of Donald Richard Nelson; Springer Science and Business Media LLC: Berlin, Germany, 2001; pp. 31–46. [Google Scholar]
- Chapuis, L.; Collin, S.P.; Yopak, K.E.; McCauley, R.D.; Kempster, R.M.; Ryan, L.A.; Schmidt, C.; Kerr, C.C.; Gennari, E.; Egeberg, C.A.; et al. The effect of underwater sounds on shark behaviour. Sci. Rep. 2019, 9, 6924. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Williams, E.K.; Hickey, C.W. Avoidance behaviour of freshwater fish and shrimp exposed to ammonia and low dissolved oxygen separately and in combination. N. Z. J. Mar. Freshw. Res. 2001, 35, 625–633. [Google Scholar] [CrossRef]
- Mauro, M.; Pérez-Arjona, I.; Perez, E.J.B.; Ceraulo, M.; Bou-Cabo, M.; Benson, T.; Espinosa, V.; Beltrame, F.; Mazzola, S.; Vazzana, M.; et al. The effect of low frequency noise on the behaviour of juvenile Sparus aurata. J. Acoust. Soc. Am. 2020, 147, 3795–3807. [Google Scholar] [CrossRef] [PubMed]
- Buscaino, G.; Filiciotto, F.; Buffa, G.; Bellante, A.; Di Stefano, V.; Assenza, A.; Fazio, F.; Caola, G.; Mazzola, S. Impact of an acoustic stimulus on the motility and blood parameters of European sea bass (Dicentrarchus labrax L.) and gilthead sea bream (Sparus aurata L.). Mar. Environ. Res. 2010, 69, 136–142. [Google Scholar] [CrossRef]
- Berthe, C.; Lecchini, D. Influence of boat noises on escape behaviour of white-spotted eagle ray Aetobatus ocellatus at Moorea Island (French Polynesia). Compt. Rendus Biol. 2016, 339, 99–103. [Google Scholar] [CrossRef]
- Sarà, G.; Dean, J.M.; D’Amato, D.; Buscaino, G.; Oliveri, A.; Genovese, S.; Ferro, S.; Buffa, G.; Martire, M.L.; Mazzola, S. Effect of boat noise on the behaviour of bluefin tuna Thunnus thynnus in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2007, 331, 243–253. [Google Scholar] [CrossRef]
- Clark, C.; Ellison, W.; Southall, B.; Hatch, L.; Van Parijs, S.; Frankel, A.; Ponirakis, D. Acoustic masking in marine ecosystems: Intuitions, analysis, and implication. Mar. Ecol. Prog. Ser. 2009, 395, 201–222. [Google Scholar] [CrossRef]
- Filiciotto, F.; Moyano, M.P.S.; de Vincenzi, G.; Hidalgo, F.; Sciacca, V.; Bazterrica, M.C.; Corrias, V.; Lorusso, M.; Mazzola, S.; Buscaino, G.; et al. Are semi-terrestrial crabs threatened by human noise? Assessment of behavioural and biochemical responses of Neohelice granulata (Brachyura, Varunidae) in tank. Mar. Pollut. Bull. 2018, 137, 24–34. [Google Scholar] [CrossRef]
- Nowacek, D.P.; Thorne, L.H.; Johnston, D.W.; Tyack, P.L. Responses of cetaceans to anthropogenic noise. Mammal Rev. 2007, 37, 81–115. [Google Scholar] [CrossRef]
- Parks, S.; Clark, C.W.; Tyack, P.L. Short- and long-term changes in right whale calling behavior: The potential effects of noise on acoustic communication. J. Acoust. Soc. Am. 2007, 122, 3725–3731. [Google Scholar] [CrossRef]
- Wintrebert, P. L’embryon de Scylliorhinus canicula L. Gill Considéré Comme Animal de Laboratoire. Bull. Soc. Zool. Fr. 1920, 45, 311–341. [Google Scholar]
- Delarbre, C.; Barriel, V.; Tillier, S.; Janvier, P.; Gachelin, G. The main features of the craniate mitochondrial DNA between the ND1 and the COI genes were established in the common ancestor with the lancelet. Mol. Biol. Evol. 1997, 14, 807–813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rasch, L.J.; Martin, K.J.; Cooper, R.L.; Metscher, B.D.; Underwood, C.J.; Fraser, G.J. An ancient dental gene set governs development and continuous regeneration of teeth in sharks. Dev. Biol. 2016, 415, 347–370. [Google Scholar] [CrossRef]
- Molist, P.; Barja, P.; Anadón, R.; Rodríguez-Moldes, I.; López, J.M.; Quintela, I.; Cerviño, M.C.; González, A. Distribution of choline acetyltransferase immunoreactivity in the brain of an elasmobranch, the lesser spotted dogfish (Scyliorhinus canicula). J. Comp. Neurol. 2000, 420, 139–170. [Google Scholar] [CrossRef]
- Coolen, M.; Menuet, A.; Chassoux, D.; Compagnucci, C.; Henry, S.; Lévèque, L.; Da Silva, C.; Gavory, F.; Samain, S.; Wincker, P. The Dogfish Scyliorhinus canicula: A Reference in Jawed Vertebrates. Cold Spring Harb. Protoc. 2008, 2008, pdb.emo111. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, M.; Vallarino, M.; Lovejoy, D.A.; Vandesande, F.; King, J.A.; Pierantoni, R.; Peter, R.E. Nature and Distribution of Gonadotropin-Releasing Hormone (GnRH) in the Brain, and GnRH and GnRH Binding Activity in Serum of the Spotted Dogfish Scyliorhinus canicula. Gen. Comp. Endocrinol. 1995, 98, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Loppion, G.; Crespel, A.; Martinez, A.-S.; Auvray, P.; Sourdaine, P. Study of the potential spermatogonial stem cell compartment in dogfish testis, Scyliorhinus canicula L. Cell Tissue Res. 2008, 332, 533–542. [Google Scholar] [CrossRef]
- Micarelli, P.; De Lucia, L. Analisi preliminari della risposta allo stress, indotto da variazioni di salinitá, verificato tramite il consumo di ossigeno da parte di Scyliorhinus canicula. Biol. Mar. Mediterr. 2012, 19, 261–262. [Google Scholar]
- Evangelista, C.; Mills, M.; Siebeck, U.E.; Collin, S.P. A comparison of the external morphology of the membranous inner ear in elasmobranchs. J. Morphol. 2010, 271, 483–495. [Google Scholar] [CrossRef]
- Casper, B.M.; Mann, D. Dipole hearing measurements in elasmobranch fishes. J. Exp. Biol. 2007, 210, 75–81. [Google Scholar] [CrossRef]
- Picciulin, M.; Calcagno, G.; Sebastianutto, L.; Bonacito, C.; Codarin, A.; Costantini, M.; Ferrero, E.A. Diagnostics of nocturnal calls of Sciaena umbra (L., fam. Sciaenidae) in a nearshore Mediterranean marine reserve. Bioacoustics 2013, 22, 109–120. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.W.; Davies, S.J.; Bone, Q. On the Diel Rhythms in Metabolism and Activity of Post-Hatching Lesser Spotted Dogfish, Scyliorhinus canicula. J. Fish Boil. 1993, 43, 749–754. [Google Scholar] [CrossRef]
- Lyle, J.M. Food and feeding habits of the lesser spotted dogfish, Scyliorhinus canicula (L.), in Isle of Man waters. J. Fish Biol. 1983, 23, 725–737. [Google Scholar] [CrossRef]
- Olaso, I.; Rodríguez-Marín, E. Decapod Crustaceans in the Diets of Demersal Fish in the Cantabrian Sea. In Proceedings of the ICES Marine Science Symposia, Copenhagen, Denmark, 2–4 October 1989; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1991; Volume 199, pp. 209–221. [Google Scholar]
- Olaso, I.; Velasco, F.; Sanchez, F.; Serrano, A.; Rodríguez-Cabello, C.; Cendrero, O. Trophic Relations of Lesser-Spotted Catshark (Scyliorhinus canicula) and Blackmouth Catshark (Galeus melastomus) in the Cantabrian Sea. J. Northwest Atl. Fish. Sci. 2005, 35, 481–494. [Google Scholar] [CrossRef]
- Mnasri, N.; El Kamel, O.; Boumaiza, M.; Reynaud, C.; Capape, C. Food and Feeding Habits of the Small-Spotted Catshark, Scyliorhinus canicula (Chondrichthyes: Scyliorhinidae) from the Northern Coast of Tunisia (Central Mediterraneean). Cah. Boil. Mar. 2012, 53, 139–150. [Google Scholar]
- Pijanowski, B.C.; Farina, A.; Gage, S.H.; Dumyahn, S.L.; Krause, B.L. What is soundscape ecology? An introduction and overview of an emerging new science. Landsc. Ecol. 2011, 26, 1213–1232. [Google Scholar] [CrossRef]
- Ceraulo, M.; Papale, E.; Caruso, F.; Filiciotto, F.; Grammauta, R.; Parisi, I.; Mazzola, S.; Farina, A.; Buscaino, G. Acoustic comparison of a patchy Mediterranean shallow water seascape: Posidonia oceanica meadow and sandy bottom habitats. Ecol. Indic. 2018, 85, 1030–1043. [Google Scholar] [CrossRef]
- Boussard, A. The Reactions of Roach (Rutilus rutilus) and Rudd (Scardinius erythrophthalmus) to Noises Produced by High Speed Boating. In Proceedings of the 2nd British Freshwater Fisheries Conference, Liverpool, UK, 13–15 April 1981; University of Liverpool: Liverpool, UK, 1981; pp. 188–200. [Google Scholar]
- Pearson, W.H.; Skalski, J.R.; Malme, C.I. Effects of Sounds from a Geophysical Survey Device on Behavior of Captive Rockfish (Sebastes spp.). Can. J. Fish. Aquat. Sci. 1992, 49, 1343–1356. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Bouton, N.; Van Opzeeland, I.; Coers, A.; Cate, C.T.; Popper, A.N. A noisy spring: The impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 2010, 25, 419–427. [Google Scholar] [CrossRef]
- Chapman, C.J. Some Observations on the Reactions of Fish to Sound. In Proceedings of the Sound Reception in Fish: Proceedings of a Symposium, Utrecht, The Netherlands, 16–18 April 1975; p. 241. [Google Scholar]
- Hawkins, A.D.; Chapman, C.J. Masked auditory thresholds in the cod, Gadus morhua L. J. Comp. Physiol. A 1975, 103, 209–226. [Google Scholar] [CrossRef]
- Knudsen, F.R.; Enger, P.S.; Sand, O. Awareness reactions and avoidance responses to sound in juvenile Atlantic salmon, Salmo salar L. J. Fish Biol. 1992, 40, 523–534. [Google Scholar] [CrossRef]
- Bracciali, C.; Campobello, D.; Giacoma, C.; Sarà, G. Effects of Nautical Traffic and Noise on Foraging Patterns of Mediterranean Damselfish (Chromis chromis). PLoS ONE 2012, 7, e40582. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.; Killen, S.S.; Armstrong, J.D.; Metcalfe, N.B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B Biol. Sci. 2011, 278, 3465–3473. [Google Scholar] [CrossRef] [PubMed]
- Sabet, S.S.; Neo, Y.; Slabbekoorn, H. Impact of Anthropogenic Noise on Aquatic Animals: From Single Species to Community-Level Effects. In The Effects of Noise on Aquatic Life II; Springer Science and Business Media LLC: Berlin, Germany, 2016; Volume 875, pp. 957–961. [Google Scholar]
- Ellison, W.T.; Southall, B.; Clark, C.; Frankel, A.S. A New Context-Based Approach to Assess Marine Mammal Behavioral Responses to Anthropogenic Sounds. Conserv. Biol. 2011, 26, 21–28. [Google Scholar] [CrossRef]
- Boisclair, D.; Sirois, P. Testing Assumptions of Fish Bioenergetics Models by Direct Estimation of Growth, Consumption, and Activity Rates. Trans. Am. Fish. Soc. 1993, 122, 784–796. [Google Scholar] [CrossRef]
- Koch, F.; Wieser, W. Partitioning of Energy in Fish: Can Reduction of Swimming Activity Compensate for the Cost of Production? J. Exp. Biol. 1983, 107, 141–146. [Google Scholar]
- Banner, A.; Hyatt, M. Effects of Noise on Eggs and Larvae of Two Estuarine Fishes. Trans. Am. Fish. Soc. 1973, 102, 134–136. [Google Scholar] [CrossRef]
- Lagardère, J.P. Effects of noise on growth and reproduction of Crangon crangon in rearing tanks. Mar. Biol. 1982, 71, 177–185. [Google Scholar] [CrossRef]
- Wysocki, L.E.; Davidson, J.W.; Smith, M.E.; Frankel, A.S.; Ellison, W.T.; Mazik, P.M.; Popper, A.N.; Bebak, J. Effects of aquaculture production noise on hearing, growth, and disease resistance of rainbow trout Oncorhynchus mykiss. Aquaculture 2007, 272, 687–697. [Google Scholar] [CrossRef]
- Maccarrone, V.; Filiciotto, F.; de Vincenzi, G.; Mazzola, S.; Buscaino, G. An Italian proposal on the monitoring of underwater noise: Relationship between the EU Marine Strategy Framework Directive (MSFD) and marine spatial planning directive (MSP). Ocean Coast. Manag. 2015, 118, 215–224. [Google Scholar] [CrossRef]

| Experimental Condition | Acoustic Features | N° Specimens Per Trial | N° Replicates | TOT Specimens Involved | TOT Trials |
|---|---|---|---|---|---|
| Control (C) | Low-level background noise of the experimental aquaria | 1 | 3 | 3 | 3 |
| Biological (B) | Acoustic file representing the main acoustic components of marine rocky soundscape | 1 | 3 | 3 | 3 |
| Biological minor of anthropogenic (B < A) | Acoustic file representing the main acoustic components of marine rocky soundscape mixed with the noise produced by the shipping traffic. The biological sounds were 6 dB less intense than the anthropogenic shipping traffic noise. | 1 | 3 | 3 | 3 |
| Biological major of anthropogenic (B > A) | Acoustic file representing the main acoustic components of marine rocky soundscape mixed with the noise produced by the shipping traffic. The biological sounds were 6 dB more intense than the anthropogenic shipping traffic noise. | 1 | 3 | 3 | 3 |
| Experimental Acoustic Condition | SPL dB re 1 μPa Band 15 Hz–5.5 kHz | |
|---|---|---|
| Control | Mean | 74.0 |
| Maximum | 98.1 | |
| Minimum | 56.4 | |
| SD | ±11.6 | |
| Biological | Mean | 107.2 |
| Maximum | 129.1 | |
| Minimum | 71.0 | |
| SD | ±13.6 | |
| Biological > anthropogenic | Mean | 107.0 |
| Maximum | 129.0 | |
| Minimum | 74.3 | |
| SD | ±13.8 | |
| Biological < anthropogenic | Mean | 101.0 |
| Maximum | 126.0 | |
| Minimum | 56.3 | |
| SD | ±19.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vincenzi, G.; Micarelli, P.; Viola, S.; Buffa, G.; Sciacca, V.; Maccarrone, V.; Corrias, V.; Reinero, F.R.; Giacoma, C.; Filiciotto, F. Biological Sound vs. Anthropogenic Noise: Assessment of Behavioural Changes in Scyliorhinus canicula Exposed to Boats Noise. Animals 2021, 11, 174. https://doi.org/10.3390/ani11010174
de Vincenzi G, Micarelli P, Viola S, Buffa G, Sciacca V, Maccarrone V, Corrias V, Reinero FR, Giacoma C, Filiciotto F. Biological Sound vs. Anthropogenic Noise: Assessment of Behavioural Changes in Scyliorhinus canicula Exposed to Boats Noise. Animals. 2021; 11(1):174. https://doi.org/10.3390/ani11010174
Chicago/Turabian Stylede Vincenzi, Giovanni, Primo Micarelli, Salvatore Viola, Gaspare Buffa, Virginia Sciacca, Vincenzo Maccarrone, Valentina Corrias, Francesca Romana Reinero, Cristina Giacoma, and Francesco Filiciotto. 2021. "Biological Sound vs. Anthropogenic Noise: Assessment of Behavioural Changes in Scyliorhinus canicula Exposed to Boats Noise" Animals 11, no. 1: 174. https://doi.org/10.3390/ani11010174
APA Stylede Vincenzi, G., Micarelli, P., Viola, S., Buffa, G., Sciacca, V., Maccarrone, V., Corrias, V., Reinero, F. R., Giacoma, C., & Filiciotto, F. (2021). Biological Sound vs. Anthropogenic Noise: Assessment of Behavioural Changes in Scyliorhinus canicula Exposed to Boats Noise. Animals, 11(1), 174. https://doi.org/10.3390/ani11010174







