Luteinizing Hormone Effect on Luteal Cells Is Dependent on the Corpus Luteum Stage in Felids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Transport and Isolation of CL
2.2. Isolation of Luteal Cells
2.3. Cell Culture Conditions and Cell Diameter Evaluation
2.4. 3β-hydroxysteroid dehydrogenase Assay
2.5. P4 Extraction and ELISA Measurements
2.6. Immunohistochemistry and Evaluation of Immunoreactivity
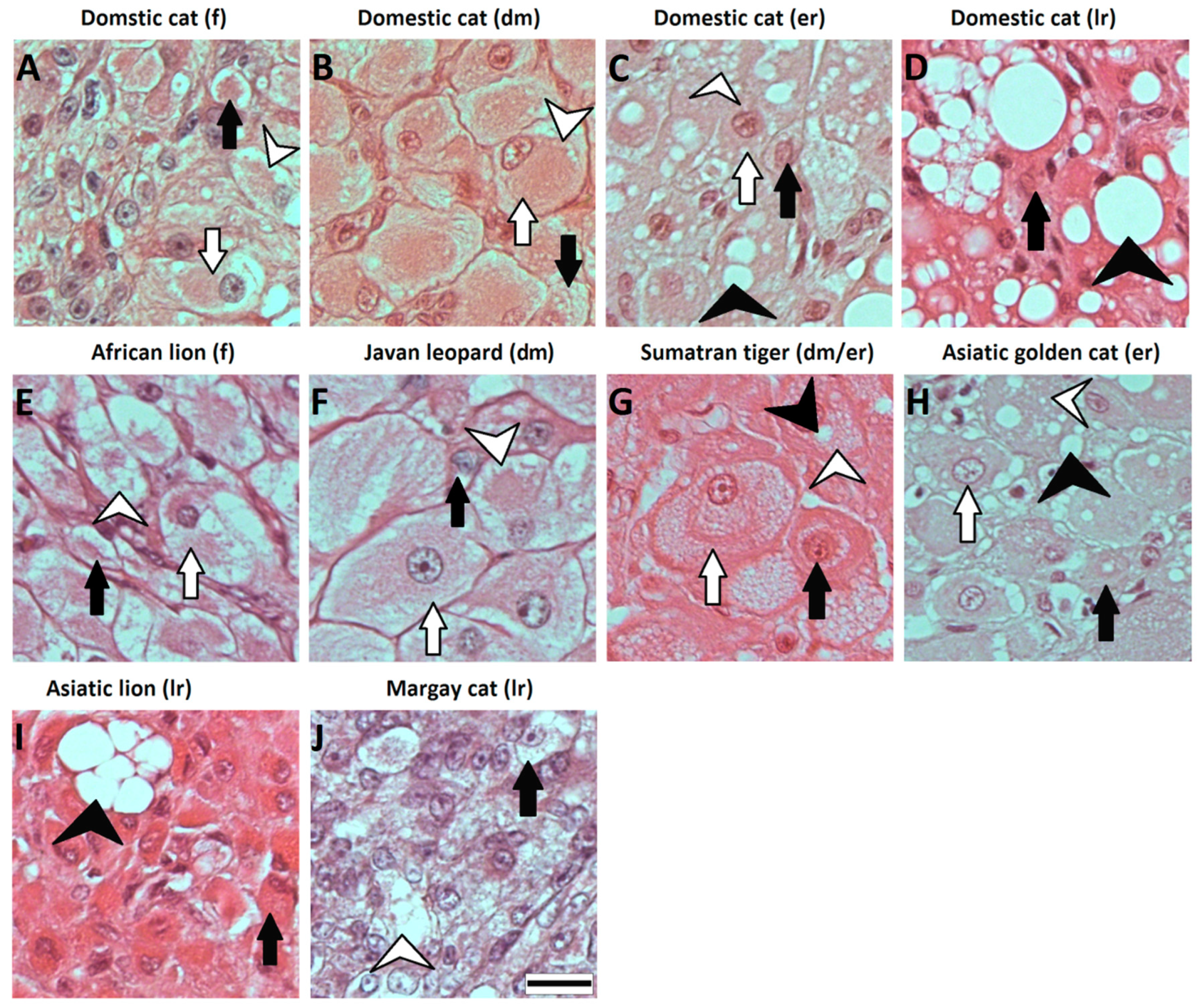
2.7. Assessment of CL Stages in Wild Felids
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Isolated and Cultured Cells
3.2. Identification of Steroidogenic Cells in Freshly Isolated and Cultured Cells
3.3. P4 Secretion in Luteal Cell Cultures
3.4. Protein Expression of HSD3B by Immunohistology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.L. Female reproductive cycles of wild female felids. Anim. Reprod. Sci. 2011, 124, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.J.; Thomas, D.G.; Yapura, J.; Potter, M.A. Reproductive biology of the 38 extant felid species: A review. Mammal Rev. 2018, 49, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L. Comparative ovarian function and reproductive monitoring of endangered mammals. Theriogenology 2018, 109, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Thongphakdee, A.; Tipkantha, W.; Punkong, C.; Chatdarong, K. Monitoring and controlling ovarian activity in wild felids. Theriogenology 2018, 109, 14–21. [Google Scholar] [CrossRef]
- Thongphakdee, A.; Sukparangsi, W.; Comizzoli, P.; Chatdarong, K. Reproductive biology and biotechnologies in wild felids. Theriogenology 2020, 150, 360–373. [Google Scholar] [CrossRef]
- Schramm, R.D.; Briggs, M.B.; Reeves, J.J. Spontaneous and induced ovulation in the lion (Panthera leo). Zoo Biol. 1994, 13, 301–307. [Google Scholar] [CrossRef]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms Controlling the Function and Life Span of the Corpus Luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Amelkina, O.; Braun, B.C.; Dehnhard, M.; Jewgenow, K. The corpus luteum of the domestic cat: Histologic classification and intraluteal hormone profile. Theriogenology 2015, 83, 711–720. [Google Scholar] [CrossRef]
- Paape, S.R.; Shille, V.M.; Seto, H.; Stabenfeldt, G.H. Luteal Activity in the Pseudopregnant Cat. Biol. Reprod. 1975, 13, 470–474. [Google Scholar] [CrossRef]
- Tsutsui, T.; Stabenfeldt, G.H. Biology of ovarian cycles, pregnancy and pseudopregnancy in the domestic cat. J. Reprod. Fertil. Suppl. 1993, 47, 29–35. [Google Scholar]
- Dawson, A.B. The postpartum history of the corpus luteum of the cat. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1946, 95, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Painer, J.; Jewgenow, K.; Dehnhard, M.; Arnemo, J.M.; Linnell, J.D.C.; Odden, J.; Hildebrandt, T.B.; Goeritz, F. Physiologically Persistent Corpora lutea in Eurasian Lynx (Lynx lynx)—Longitudinal Ultrasound and Endocrine Examinations Intra-Vitam. PLoS ONE 2014, 9, e90469. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.F.; Roth, T.L.; Brown, J.L.; Wildt, D.E. Relationship of Circulating Steroid Hormones, Luteal Luteinizing Hormone Receptor and Progesterone Concentration, and Embryonic Mortality during Early Embryogenesis in the Domestic Cat. Biol. Reprod. 1995, 53, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Alila, H.W.; Hansel, W. Origin of Different Cell Types in the Bovine Corpus Luteum as Characterized by Specific Monoclonal Antibodies. Biol. Reprod. 1984, 31, 1015–1025. [Google Scholar] [CrossRef]
- Meidan, R. The Life Cycle of the Corpus Luteum; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Schmidt, P.M.; Chakraborty, P.K.; Wildt, D.E. Ovarian activity, circulating hormones and sexual behavior in the cat. II. Relationships during pregnancy, parturition, lactation and the postpartum estrus. Biol. Reprod. 1983, 28, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Wildt, D.E.; Chan, S.Y.; Seager, S.W.; Chakraborty, P.K. Ovarian Activity, Circulating Hormones, and Sexual Behavior in the Cat. I. Relationships during the Coitus-Induced Luteal Phase and the Estrous Period without Mating. Biol. Reprod. 1981, 25, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Banks, D.H.; Stabenfeldt, G. Luteinizing Hormone Release in the Cat in Response to Coitus on Consecutive Days of Estrus. Biol. Reprod. 1982, 26, 603–611. [Google Scholar] [CrossRef] [Green Version]
- Painer, J.; Goeritz, F.; Dehnhard, M.; Hildebrandt, T.B.; Naidenko, S.V.; Sanchez, I.; Muñoz, M.A.Q.; Jewgenow, K. Hormone-induced luteolysis on physiologically persisting corpora lutea in Eurasian and Iberian lynx (Lynx lynx and Lynx pardinus). Theriogenology 2014, 82, 557–562. [Google Scholar] [CrossRef]
- Hryciuk, M.M.; Braun, B.C.; Bailey, L.D.; Jewgenow, K. Functional and Morphological Characterization of Small and Large Steroidogenic Luteal Cells From Domestic Cats Before and During Culture. Front. Endocrinol. 2019, 10, 724. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Gonzalez, L.; Müller, K.; Jewgenow, K.; Zahmel, J. Felid-gamete-rescue within EAZA—Efforts and results in biobanking felid oocytes and sperm. J. Zoo Aquar. Res. 2019, 7, 1. [Google Scholar] [CrossRef]
- Dehnhard, M.; Naidenko, S.; Frank, A.; Braun, B.; Göritz, F.; Jewgenow, K. Non-invasive Monitoring of Hormones: A Tool to Improve Reproduction in Captive Breeding of the Eurasian Lynx. Reprod. Domest. Anim. 2008, 43, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.C.; Vargas, A.; Jewgenow, K. The molecular detection of relaxin and its receptor RXFP1 in reproductive tissue of Felis catus and Lynx pardinus during pregnancy. Reproduction 2012, 143, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zschockelt, L.; Amelkina, O.; Siemieniuch, M.J.; Köster, S.; Jewgenow, K.; Braun, B.C. Corpora lutea of pregnant and pseudopregnant domestic cats reveal similar steroidogenic capacities during the luteal life span. J. Steroid Biochem. Mol. Biol. 2014, 144, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Arikan, Ş.; Yigit, A.; Kalender, H. Size Distribution of Luteal Cells during Pseudopregnancy in Domestic Cats. Reprod. Domest. Anim. 2009, 44, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Arikan, S.; Yigit, A.A. Effects of cholesterol and cAMP on progesterone production in cultured luteal cells isolated from pseudopregnant cat ovaries. Anim. Reprod. Sci. 2009, 115, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.E.; McLean, M.P.; Jayatilak, P.G.; Gibori, G. Isolation, characterization, and culture of cell subpopulations forming the pregnant rat corpus luteum. Endocrinology 1992, 130, 954–966. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J.; Wathes, D.C. Characteristics of bovine luteal cells in culture: Morphology, proliferation and progesterone secretion in different media and effects of LH, dibutyryl cyclic AMP, antioxidants and insulin. J. Endocrinol. 1985, 104, 355–361. [Google Scholar] [CrossRef]
- Fitz, T.A.; Mayan, M.H.; Sawyer, H.R.; Niswender, G.D. Characterization of Two Steroidogenic Cell Types in the Ovine Corpus Luteum. Biol. Reprod. 1982, 27, 703–711. [Google Scholar] [CrossRef]
- Friden, B.E.; Hagström, H.-G.; Lindblom, B.; Sjoblom, P.; Wallin, A.; Brannstrom, M.; Hahlin, M. Cell characteristics and function of two enriched fraction of human luteal cells prolonged culture. Mol. Hum. Reprod. 1999, 5, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Lemon, M.; Loir, M. Steroid release in vitro by two luteal cell types in the corpus luteum of the pregnant sow. J. Endocrinol. 1977, 72, 351–359. [Google Scholar] [CrossRef]
- Lemon, M.; Mauléon, P. Interaction between two luteal cell types from the corpus luteum of the sow in progesterone synthesis in vitro. Reproduction 1982, 64, 315–323. [Google Scholar] [CrossRef]
- Harrison, L.M.; Kenny, N.; Niswender, G.D. Progesterone production, LH receptors, and oxytocin secretion by ovine luteal cell types on Days 6,10 and 15 of the oestrous cycle and Day 25 of pregnancy. Reproduction 1987, 79, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, T.C.; Montali, R.J.; Wildt, D.E. Follicle-oocyte atresia and temporal taphonomy in cold-stored domestic cat ovaries. Mol. Reprod. Dev. 1997, 46, 190–200. [Google Scholar] [CrossRef]
- Kalender, H.; Arikan, Ş.; Simsek, O. The effects of LH on progesterone production by cell subpopulations isolated from early and late luteal phase goat corpora lutea. Turk. J. Vet. Anim. Sci. 2014, 38, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Simsek, O.; Arikan, S. Effects of cholesterol, FSH and LH on steroidogenic activity of cat granulosa cells cultured in vitro. Anim. Reprod. 2015, 12, 931–938. [Google Scholar]
- Ursely, J.; Leymarie, P. Varying response to luteinizing hormone of two luteal cell types isolated from bovine corpus luteum. J. Endocrinol. 1979, 83, 303–310. [Google Scholar] [CrossRef]
- Yuan, W.; Connor, M.L.; Buhr, M.M. Responsiveness of porcine large and small luteal cells to luteotropic or luteolytic hormones and cell morphologic changes during the estrous cycle and pregnancy. J. Anim. Sci. 1993, 71, 481–491. [Google Scholar] [CrossRef]
- Hoffman, Y.M.; Peegel, H.; Sprock, M.J.E.; Zhang, Q.-Y.; Menon, K.M.J. Evidence that Human Chorionic Gonadotropin/Luteinizing Hormone Receptor Down-Regulation Involves Decreased Levels of Receptor Messenger Ribonucleic Acid*. Endocrinol. 1991, 128, 388–393. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Wang, Z.; Luo, X.; Zhang, H.; Du, Q.; Chang, L.; Zhao, X.; Tong, D. Establishment and characterization of a telomerase immortalized porcine luteal cells. Theriogenology 2017, 94, 105–113. [Google Scholar] [CrossRef]
- Nishimura, R.; Shibaya, M.; Skarzynski, D.J.; Okuda, K. Progesterone Stimulation by LH Involves the Phospholipase-C Pathway in Bovine Luteal Cells. J. Reprod. Dev. 2004, 50, 257–261. [Google Scholar] [CrossRef] [Green Version]




| Species | Age of Animal (Years) | Sample Origin | Number of CL Per Animal | Number of Biological Replicates for Cell Cultures | Total Number of Technical Replicates | |
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | |||||
| African lion (Panthera leo leo) | 7 | Givskud Zoo in Denmark | 3 | 1 | Control: 6 LH: 14 | Control: 3 LH: 11 |
| Domestic cat (Felis catus) | * | Tierheim Berlin | * | 5 * | Control: 22 LH: 22 | Control: 20 LH: 20 |
| 3 * | Control: 18 LH: 18 | Control: 9 LH: 9 | ||||
| Javan leopard (Panthera pardus melas) | 13 | Tierpark Berlin | 3 | 1 | Control: 6 LH: 14 | Control: 3 LH: 11 |
| Sumatran tiger (Panthera tigris sondaica) | 18 | Zoo Frankfurt | 2 | ** | ** | ** |
| Asiatic golden cat (Catopuma temminckii) | 11 | Allwetterzoo Münster | 1 | 1 | Control: 6 LH: 9 | Control: 3 LH: 6 |
| Asiatic lion (Panthera leo persica) | 12 | Aalborg Zoo in Denmark | undefined | 1 | Control: 2 LH: 12 | Control: 0 *** LH: 10 |
| Margay cat (Leopardus wiedii) | 10 | Tierpark Berlin | 1 | ** | ** | ** |
| Antibody | Host | Type | Dilution | Source |
|---|---|---|---|---|
| anti-HSD3B | mouse | primary | 1:500 | Santa Cruz Biotechnology Inc., Heidelberg, Germany; sc-100466 |
| ImmPRESS™ VR REAGENT Anti-Mouse IgG Kit | goat | secondary | ready to use | BIOZOL Diagnostica Vertrieb GmbH, Eching, Germany; PEROXIDASECat. No. MP-6402 |
| Species | Average Diameter of Isolated Cells ± Standard Deviation (µm) | Number of Counted Cells | Percentage of SLC in Isolated Population of Cells (%) | CL Stage Based on Histology |
|---|---|---|---|---|
| African lion (Panthera leo leo) | 14.5 ± 4.6 | 172 | 83.7 | formation (corpus hemorrhagicum) [f] |
| Domestic cat (Felis catus) | 11.5 ± 2.3 | 253 | 99.6 | development/maintenance [dm] |
| Javan leopard (Panthera pardus melas) | 11.5 ± 2.3 * 37.5 ± 5.6 * | 100 11 | ** | development/maintenance [dm] |
| Asiatic golden cat (Catopuma temminckii) | 10.5 ± 4.5 | 136 | 94.1 | regression [er] |
| Asiatic lion (Panthera leo persica) | 13.3 ± 4.1 | 115 | 93.3 | regression [lr] |
| Margay cat (Leopardus wiedii) | 10.8 ± 3.1 *** | 52 | 100.0 | regression [lr] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hryciuk, M.M.; Jewgenow, K.; Braun, B.C. Luteinizing Hormone Effect on Luteal Cells Is Dependent on the Corpus Luteum Stage in Felids. Animals 2021, 11, 179. https://doi.org/10.3390/ani11010179
Hryciuk MM, Jewgenow K, Braun BC. Luteinizing Hormone Effect on Luteal Cells Is Dependent on the Corpus Luteum Stage in Felids. Animals. 2021; 11(1):179. https://doi.org/10.3390/ani11010179
Chicago/Turabian StyleHryciuk, Michał M., Katarina Jewgenow, and Beate C. Braun. 2021. "Luteinizing Hormone Effect on Luteal Cells Is Dependent on the Corpus Luteum Stage in Felids" Animals 11, no. 1: 179. https://doi.org/10.3390/ani11010179
APA StyleHryciuk, M. M., Jewgenow, K., & Braun, B. C. (2021). Luteinizing Hormone Effect on Luteal Cells Is Dependent on the Corpus Luteum Stage in Felids. Animals, 11(1), 179. https://doi.org/10.3390/ani11010179




