Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis niloticus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Black Soldier Fly Larvae Meal (BSFLM) Preparation
2.2. Experimental Diets
2.3. Experimental Procedure
2.4. Water Quality Measurement
2.5. Sample Collections
2.5.1. Blood Collection and Hematological Parameters
2.5.2. Skin Mucus Preparation
2.6. Growth Parameter Calculations
2.7. Fish Morphometric Indices
2.8. Digestible Efficiency Measurement
2.9. Mucosal Immune Responses
2.9.1. Skin Mucus Lysozyme Assay
2.9.2. Skin Mucus Peroxidase Assay
2.10. Statistical Analysis
3. Results
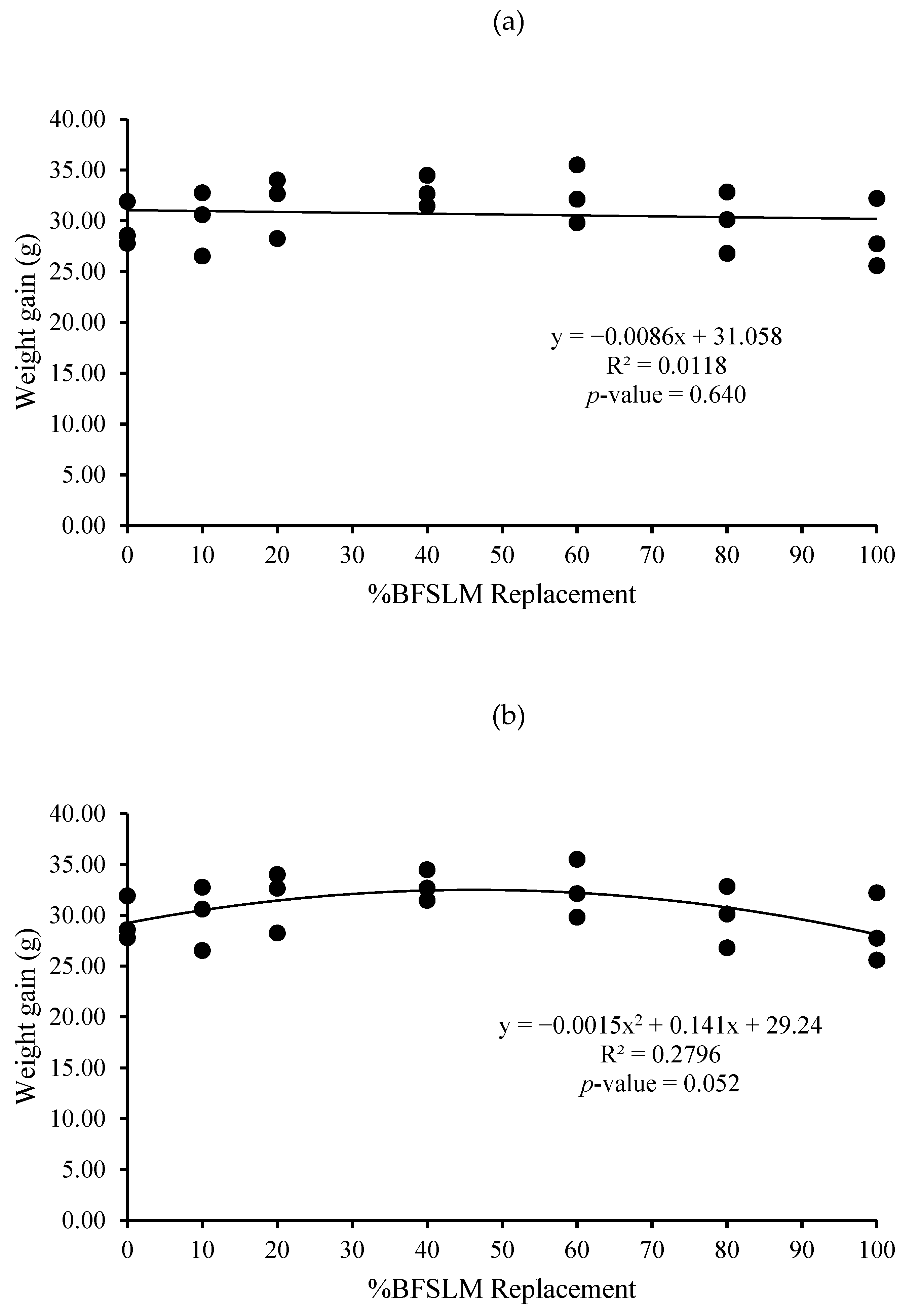
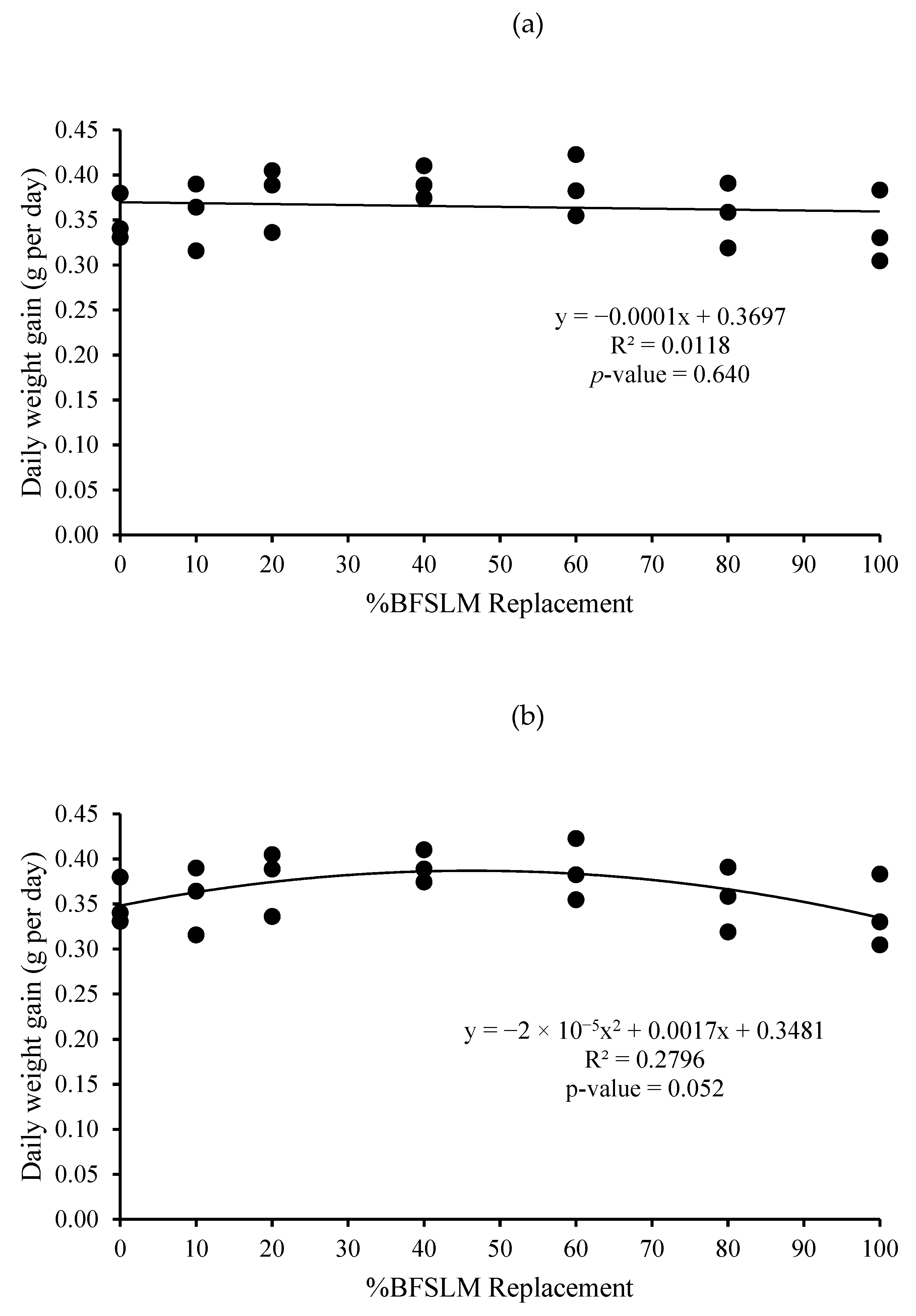
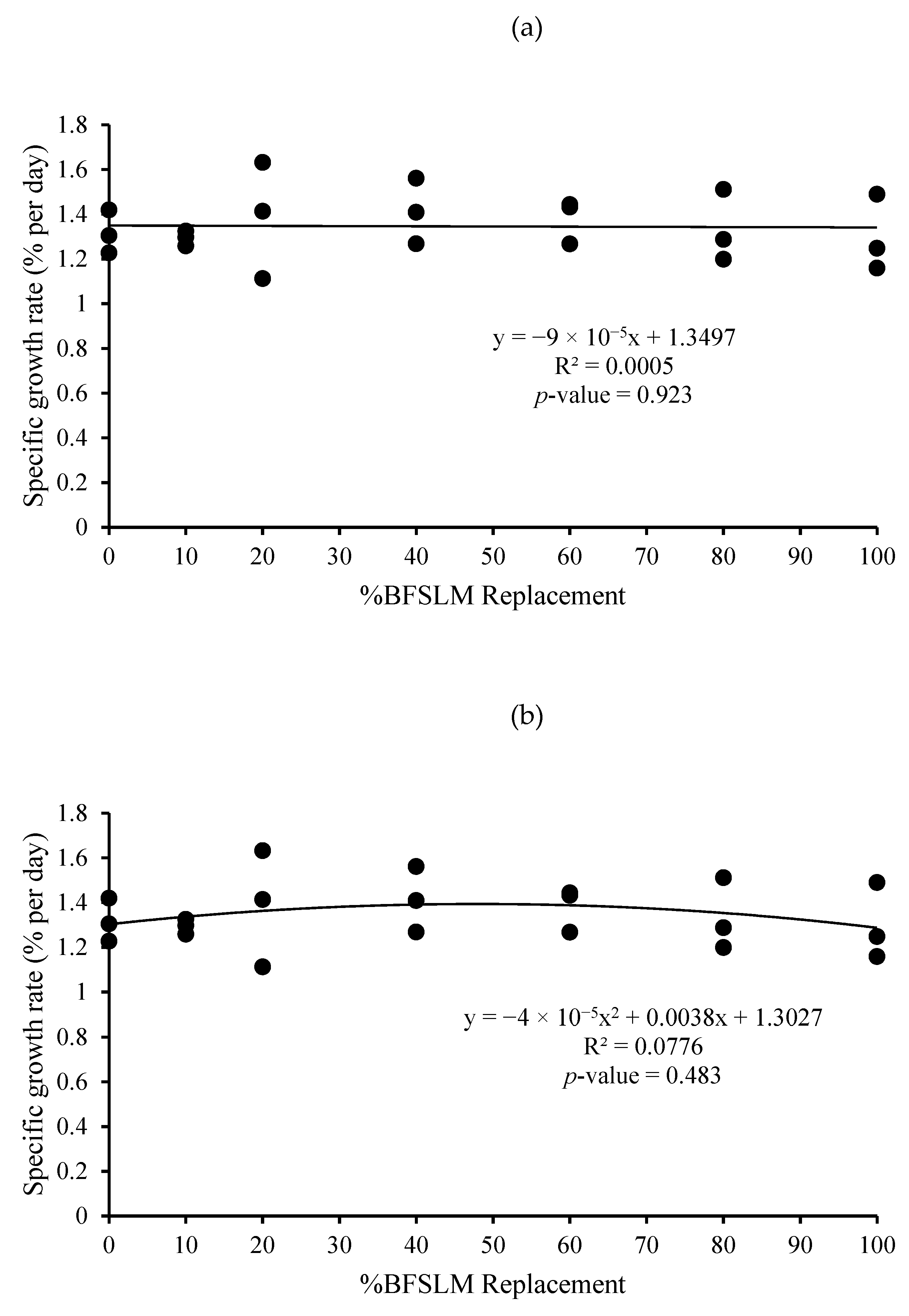
3.1. Growth Performance
3.2. Blood Parameters
3.3. Skin Mucus Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2020. [Google Scholar]
- Guedes, A.C.; Sousa-Pinto, I.; Malcata, F.X. Chapter 8—Application of Microalgae Protein to Aquafeed. In Handbook of Marine Microalgae; Academic Press: Boston, MA, USA, 2015; pp. 93–125. [Google Scholar]
- Cashion, T.; Le Manach, F.; Zeller, D.; Pauly, D. Most fish destined for fishmeal production are food-grade fish. Fish Fish. 2017, 18, 837–844. [Google Scholar] [CrossRef]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the Future of Aquaculture Nutrition: Realigning Perspectives to Reflect Contemporary Issues Related to Judicious Use of Marine Resources in Aquafeeds. N. Am. J. Aquac. 2018, 81, 13–39. [Google Scholar] [CrossRef]
- Kobayashi, M.; Msangi, S.; Batka, M.; Vannuccini, S.; Dey, M.M.; Anderson, J.L. Fish to 2030: The Role and Opportunity for Aquaculture. Aquac. Econ. Manag. 2015, 19, 282–300. [Google Scholar] [CrossRef] [Green Version]
- Konar, M.; Qiu, S.; Tougher, B.; Vause, J.; Tlusty, M.; Fitzsimmons, K.; Barrows, R.; Cao, L. Illustrating the hidden economic, social and ecological values of global forage fish resources. Resour. Conserv. Recycl. 2019, 151, 104456. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Fisher, H.; Collins, S.A.; Hanson, C.; Mason, B.; Colombo, S.; Anderson, D. Black soldier fly larvae meal as a protein source in low fish meal diets for Atlantic salmon (Salmo salar). Aquaculture 2020, 521, 734978. [Google Scholar] [CrossRef]
- Gasco, L.; Acuti, G.; Bani, P.; Zotte, A.D.; Danieli, P.P.; De Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G.; et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Belghit, I.; Lock, E.-J. Krogdahl, Åshild Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar). Aquaculture 2020, 520, 734967. [Google Scholar] [CrossRef]
- Arru, B.; Furesi, R.; Gasco, L.; Madau, F.; Pulina, P. The Introduction of Insect Meal into Fish Diet: The First Economic Analysis on European Sea Bass Farming. Sustainability 2019, 11, 1697. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef] [Green Version]
- Basto, A.; Matos, E.; Valente, L.M. Nutritional value of different insect larvae meals as protein sources for European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2020, 521, 735085. [Google Scholar] [CrossRef]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2018, 11, 1080–1103. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; The national Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Wang, G.; Peng, K.; Hu, J.; Yi, C.; Chen, X.; Wu, H.; Huang, Y. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture 2019, 507, 144–154. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Eljack, R.; Schrimsher, C.; Beck, B.H. Use of dietary frass from black soldier fly larvae, Hermetia illucens, in hybrid tilapia (Nile x Mozambique, Oreocromis niloticus x O. mozambique) diets improves growth and resistance to bacterial diseases. Aquac. Rep. 2020, 17, 100373. [Google Scholar] [CrossRef]
- Foysal, J.; Fotedar, R.; Tay, C.-Y.; Gupta, S.K. Dietary supplementation of black soldier fly (Hermetica illucens)meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ 2019, 7, e6891. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Huang, Y.; Tang, T.; Zhong, L.; Chu, W.; Dai, Z.; Chen, K.; Hu, Y. Effect of partial black soldier fly (Hermetia illucens L.) larvae meal replacement of fish meal in practical diets on the growth, digestive enzyme and related gene expression for rice field eel (Monopterus albus). Aquac. Rep. 2020, 17, 100345. [Google Scholar] [CrossRef]
- El Asely, A.; Amin, A.; El-Naby, A.S.A.; Samir, F.; El-Ashram, A.; Dawood, M.A. Ziziphus mauritiana supplementation of Nile tilapia (Oreochromis niloticus) diet for improvement of immune response to Aeromonas hydrophila infection. Fish Physiol. Biochem. 2020, 46, 1561–1575. [Google Scholar] [CrossRef]
- El Asely, A.M.; Reda, R.M.; Salah, A.S.; Mahmoud, M.A.; Dawood, M.A. Overall performances of Nile tilapia (Oreochromis niloticus) associated with using vegetable oil sources under suboptimal temperature. Aquac. Nutr. 2020, 26, 1154–1163. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Khanongnuch, C.; Kanpiengjai, A.; Unban, K.; Van Kim, V.; Srichaiyo, S. Host-associated probiotics boosted mucosal and serum immunity, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 491, 94–100. [Google Scholar] [CrossRef]
- AOAC. Official methods of analysis of AOAC International, 16th ed.; AOAC International: Arlington, MA, USA, 1995; Volume 1. [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1998.
- Brown, B. Routine hematology procedures. Hematol. Princ. Proced. 1988, 198, 7–122. [Google Scholar]
- Van Doan, h.; Lumsangkul, C.; Hoseinifar, S.H.; Hung, T.Q.; Stejskal, V.; Ringø, E.; Dawood, M.A.O.; Esteban, M.Á. Administration of watermelon rind powder to Nile tilapia (Oreochromis niloticus) culture under biofloc system: Effect on growth performance, innate immune response, and disease resistance. Aquaculture 2020, 528, 735574. [Google Scholar]
- Halver, E.; Hardy, R.V. Fish Nutrition; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Lim, H.-A.; Ng, W.-K.; Lim, S.-L.; Ibrahim, C.O. Contamination of palm kernel meal with Aspergillus flavus affects its nutritive value in pelleted feed for tilapia, Oreochromis mossambicus. Aquac. Res. 2001, 32, 895–905. [Google Scholar] [CrossRef]
- Parry, R.M.; Chandan, R.C.; Shahani, K.M. A Rapid and Sensitive Assay of Muramidase. In Proceedings of the Experimental Biology and Medicine; Society for Experimental Biology and Medicine, New York, NY, USA, 1 June 1965; Volume 119, pp. 384–386. [Google Scholar]
- Van Doan, H.; Hoseinifar, S.H.; Jaturasitha, S.; Dawood, M.A.; Harikrishnan, R. The effects of berberine powder supplementation on growth performance, skin mucus immune response, serum immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2020, 520, 734927. [Google Scholar] [CrossRef]
- Cordero, H.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Changes in the levels of humoral immune activities after storage of gilthead seabream ( Sparus aurata ) skin mucus. Fish Shellfish. Immunol. 2016, 58, 500–507. [Google Scholar] [CrossRef]
- Yossa, R.; Verdegem, M.C.J. Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 2015, 437, 344–350. [Google Scholar] [CrossRef]
- Dawood, M.A.; Amer, A.A.; ElBialy, Z.I.; Gouda, A.H. Effects of including triticale on growth performance, digestive enzyme activity, and growth-related genes of Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 528, 735568. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Hansen, J.; Nøkland, D.; Lagos, L.; Rawski, M.; Øverland, M. Full-fat black soldier fly larvae (Hermetia illucens) meal and paste in extruded diets for Atlantic salmon (Salmo salar): Effect on physical pellet quality, nutrient digestibility, nutrient utilization and growth performances. Aquaculture 2021, 530, 735785. [Google Scholar] [CrossRef]
- Rana, K.S.; Salam, M.; Hashem, S.; Islam, M.A. Development of black soldier fly larvae production technique as an alternate fish feed. Int. J. Res. Fish. Aquac. 2015, 5, 41–47. [Google Scholar]
- Ushakova, N.A.; Ponomarev, S.V.; Bakaneva, Y.M.; Fedorovykh, Y.V.; Levina, O.A.; Kotel’Nikov, A.V.; Kotel’Nikova, S.V.; Bastrakov, A.I.; Kozlova, A.A.; Pavlov, D.S. Biological Efficiency of the Prepupae Hermetia illucens in the Diet of the Young Mozambique Tilapia Oreochromis mossambicus. Biol. Bull. 2018, 45, 382–387. [Google Scholar] [CrossRef]
- Dietz, C.; Liebert, F. Does graded substitution of soy protein concentrate by an insect meal respond on growth and N-utilization in Nile tilapia (Oreochromis niloticus)? Aquac. Rep. 2018, 12, 43–48. [Google Scholar] [CrossRef]
- Muin, H.; Taufek, N.; Kamarudin, M.; Razak, S. Growth performance, feed utilization and body composition of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed with different levels of black soldier fly, Hermetia illucens (Linnaeus, 1758) maggot meal diet. Iran. J. Fish. Sci. 2017, 16, 567–577. [Google Scholar]
- Aini, N.; Nugroho, R.A.; Hariani, N. Growth and Survival Evaluation of Oreochromis Sp fed Hermetia illucens Larva and Manihot esculenta leaves Meal. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Devic, E.; Leschen, W.; Murray, F.; Little, D.C. Growth performance, feed utilization and body composition of advanced nursing Nile tilapia (Oreochromis niloticus ) fed diets containing Black Soldier Fly (Hermetia illucens ) larvae meal. Aquac. Nutr. 2017, 24, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Toriz-Roldan, A.; Ruiz-Vega, J.; García-Ulloa, M.; Hernández-Llamas, A.; Fonseca-Madrigal, J.; Rodríguez-González, H. Assessment of Dietary Supplementation Levels of Black Soldier Fly, Hemertia illucens1, Pre-Pupae Meal for Juvenile Nile Tilapia, Oreochromis niloticus. Southwest. Entomol. 2019, 44, 251–259. [Google Scholar]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.-J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Truzzi, C.; Giorgini, E.; Marcellucci, C.; Vargas-Abúndez, J.A.; Zimbelli, A.; Annibaldi, A.; Parisi, G.; Tulli, F.; et al. A six-months study on Black Soldier Fly (Hermetia illucens) based diets in zebrafish. Sci. Rep. 2019, 9, 8598. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar]
- Caimi, C.; Renna, M.; Lussiana, C.; Bonaldo, A.; Gariglio, M.; Meneguz, M.; Dabbou, S.; Schiavone, A.; Gai, F.; Elia, A.C.; et al. First insights on Black Soldier Fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture 2020, 515, 734539. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef] [Green Version]
- Fontes, T.V.; Oliveira, K.R.B.; Almeida, I.L.G.; Orlando, T.M.M.; Rodrigues, P.B.; Da Costa, D.V.; Rosa, P.V.E. Digestibility of Insect Meals for Nile Tilapia Fingerlings. Animals 2019, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Jeuniaux, C. Chitinolytic systems in the digestive tract of vertebrates: A review. Chitin Enzymol. 1993, 1, 233–244. [Google Scholar]
- Ikeda, M.; Shirase, D.; Sato, T.; Ueda, M.; Hirabayashi, S.; Matsumiya, M. Primary Structure and Enzymatic Properties of Chitinase Isozymes Purified from the Stomach of the Marbled Rockfish Sebastiscus marmoratus. J. Chitin Chitosan Sci. 2014, 2, 106–116. [Google Scholar] [CrossRef]
- Ikeda, M.; Miyauchi, K.; Matsumiya, M. Purification and Characterization of a 56 kDa Chitinase Isozyme (PaChiB) from the Stomach of the Silver Croaker,Pennahia argentatus. Biosci. Biotechnol. Biochem. 2012, 76, 971–979. [Google Scholar] [CrossRef]
- Krogdahl, A.; Hemre, G.-I.; Mommsen, T. Carbohydrates in fish nutrition: Digestion and absorption in postlarval stages. Aquac. Nutr. 2005, 11, 103–122. [Google Scholar] [CrossRef]
- Cottrell, M.T.; Moore, J.A.; Kirchman, D.L. Chitinases from Uncultured Marine Microorganisms. Appl. Environ. Microbiol. 1999, 65, 2553–2557. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Ghany, H.M.; Salem, M.E. Effects of dietary chitosan supplementation on farmed fish; a review. Rev. Aquac. 2019, 12, 438–452. [Google Scholar] [CrossRef]
- Nawaz, A.; Javaid, A.B.; Irshad, S.; Hoseinifar, S.H.; Xiong, H. The functionality of prebiotics as immunostimulant: Evidences from trials on terrestrial and aquatic animals. Fish Shellfish. Immunol. 2018, 76, 272–278. [Google Scholar] [CrossRef]
- Zhou, Z.; Karlsen, Ø.; He, S.; Olsen, R.E.; Yao, B.; Ringø, E. The effect of dietary chitin on the autochthonous gut bacteria of Atlantic cod (Gadus morhua L.). Aquac. Res. 2013, 44, 1889–1900. [Google Scholar]
- Qin, C.; Zhang, Y.; Liu, W.; Xu, L.; Yang, Y.; Zhou, Z. Effects of chito-oligosaccharides supplementation on growth performance, intestinal cytokine expression, autochthonous gut bacteria and disease resistance in hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂. Fish Shellfish. Immunol. 2014, 40, 267–274. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gini, E.; Iannini, F.; Gasco, L.; Terova, G. The Effects of Dietary Insect Meal from Hermetia illucens Prepupae on Autochthonous Gut Microbiota of Rainbow Trout (Oncorhynchus mykiss). Animals 2019, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Kamilya, D.; Khan, M.I.R. Chapter 24—Chitin and chitosan as promising immunostimulant for aquaculture. In Handbook of Chitin and Chitosan; Elsevier: New York, NY, USA, 2020; pp. 761–771. [Google Scholar]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- Barragan-Fonseca, K.; Dicke, M.; Van Loon, J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed. 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Erbland, P.; Alyokhin, A.; Perkins, L.B.; Peterson, M. Dose-Dependent Retention of Omega-3 Fatty Acids by Black Soldier Fly Larvae (Diptera: Stratiomyidae). J. Econ. Èntomol. 2020, 113, 1221–1226. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Lu, R.; Chen, Y.; Yu, W.; Lin, M.; Yang, G.; Qin, C.; Meng, X.; Zhang, Y.; Ji, H.; Nie, G. Defatted black soldier fly (Hermetia illucens) larvae meal can replace soybean meal in juvenile grass carp (Ctenopharyngodon idellus) diets. Aquac. Rep. 2020, 18, 100520. [Google Scholar] [CrossRef]
- Dawood, M.A.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A. Evaluation of fermented date palm seed meal with Aspergillus oryzae on the growth, digestion capacity and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2020, 26, 828–841. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, S.; Ji, H.; Yu, H. Effect of replacing dietary fish meal with black soldier fly larvae meal on growth and fatty acid composition of Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2018, 24, 424–433. [Google Scholar] [CrossRef]
- Yano, T. The Nonspecific Immune System: Humoral Defense. In The Fish Immune System: Organism, Pathogen and Environment; Iwama, G., Nakanishi, T., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 105–157. [Google Scholar]
- Van Doan, H.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; El-Haroun, E.; Paolucci, M. Dietary inclusion of chestnut (Castanea sativa) polyphenols to Nile tilapia reared in biofloc technology: Impacts on growth, immunity, and disease resistance against Streptococcus agalactiae. Fish Shellfish. Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Bruni, L.; Pastorelli, R.; Viti, C.; Gasco, L.; Parisi, G. Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture 2018, 487, 56–63. [Google Scholar] [CrossRef]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]



| Ingredients (g/kg DM) | Diets | ||||||
|---|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | Diet 7 | |
| Fish meal (FM) | 100 | 90 | 80 | 60 | 40 | 20 | 0 |
| BSFLM 1 | 0 | 10 | 20 | 40 | 60 | 80 | 100 |
| Corn meal | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Soybean meal | 450 | 440 | 435 | 415 | 400 | 380 | 365 |
| Wheat flour | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Rice bran | 150 | 160 | 165 | 185 | 200 | 220 | 235 |
| Cellulose | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Soybean oil | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Premix 2 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Vitamin C 3 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Proximate composition | |||||||
| Dry matter (%) | 97.15 | 97.05 | 96.95 | 96.95 | 97.10 | 96.85 | 96.95 |
| Crude protein (%) | 30.21 | 29.98 | 29.90 | 29.83 | 29.79 | 29.18 | 28.81 |
| Crude fiber (%) | 3.10 | 2.90 | 2.43 | 3.01 | 3.14 | 3.22 | 2.52 |
| Crude lipid (%) | 4.78 | 4.79 | 4.78 | 4.83 | 4.75 | 4.85 | 4.73 |
| Ash (%) | 6.92 | 6.62 | 6.17 | 6.16 | 5.65 | 5.39 | 4.95 |
| Ca (%) | 0.98 | 0.87 | 0.87 | 0.75 | 0.56 | 0.39 | 0.25 |
| P (%) | 1.00 | 1.03 | 0.97 | 1.00 | 0.90 | 0.92 | 0.93 |
| Gross energy (kJ/g) | 17.37 | 17.37 | 17.29 | 17.37 | 17.37 | 17.29 | 17.37 |
| Composition | Amount |
|---|---|
| Energy (Kcal) | 461 |
| Water (%) | 23.33 |
| Protein (%) | 26.12 |
| Fat (%) | 36.47 |
| Carbohydrate (%) | 7.24 |
| Dietary fiber (%) | 3.62 |
| Ash (%) | 6.84 |
| Lauric acid (%) | 17.25 |
| Total vitamin A (µg/g) | 0 |
| Vitamin B1 (mg/g) | 0.26 |
| Omega-3 (mg/g) | 244.71 |
| Omega-6 (mg/g) | 2835.37 |
| Omega-9 (mg/g) | 4100.19 |
| Diets | IBW | FBW | DWG | WG | SGR | RGR | HPA | SR |
|---|---|---|---|---|---|---|---|---|
| 1 | 14.62 ± 1.90 | 44.04 ± 3.52 | 0.35 ± 0.03 | 29.42 ± 2.19 | 1.32 ± 0.10 | 66.85 ± 2.67 | 0.88 ± 0.07 | 100 |
| 2 | 15.31 ± 2.24 | 45.25 ± 5.40 | 0.36 ± 0.04 | 29.95 ± 3.17 | 1.29 ± 0.03 | 66.25 ± 0.95 | 0.91 ± 0.11 | 100 |
| 3 | 14.72 ± 3.36 | 46.35 ± 0.71 | 0.38 ± 0.04 | 31.63 ± 3.02 | 1.39 ± 0.26 | 68.28 ± 7.02 | 0.93 ± 0.01 | 100 |
| 4 | 14.66 ± 2.82 | 47.52 ± 3.86 | 0.39 ± 0.02 | 32.86 ± 1.51 | 1.41 ± 0.15 | 69.32 ± 3.75 | 0.95 ± 0.08 | 100 |
| 5 | 14.91 ± 2.06 | 47.38 ± 4.22 | 0.39 ± 0.03 | 32.47 ± 2.87 | 1.38 ± 0.10 | 68.57 ± 2.66 | 0.95 ± 0.08 | 100 |
| 6 | 14.67 ± 2.61 | 44.58 ± 4.40 | 0.36 ± 0.04 | 29.91 ± 3.02 | 1.33 ± 0.16 | 67.16 ± 4.31 | 0.89 ± 0.09 | 100 |
| 7 | 14.51 ± 2.06 | 43.02 ± 3.15 | 0.34 ± 0.04 | 28.50 ± 3.37 | 1.30 ± 0.17 | 66.19 ± 4.70 | 0.86 ± 0.06 | 100 |
| p-value | 1.000 | 0.734 | 0.442 | 0.442 | 0.937 | 0.946 | 0.734 | 100 |
| Means overall | 14.77 ± 2.09 | 45.45 ± 3.61 | 0.37 ± 0.03 | 30.68 ± 2.82 | 1.35 ± 0.14 | 67.52 ± 3.64 | 0.91 ± 0.07 | 100 |
| Diet | Total Feed Intake (kg) | Rates of Feed Intake (g/fish/day) | Feed Conversion Ratio | Feed Efficiency (%) | Total Digestibility (%) | Apparent Protein Digestibility Coefficient (%) |
|---|---|---|---|---|---|---|
| 1 | 1.96 ± 0.13 | 1.25 ± 0.08 | 2.22 ± 0.17 | 45.08 ± 3.32 | 47.65± 0.39 | 75.22 ± 0.52 d |
| 2 | 1.93 ± 0.07 | 1.24 ± 0.05 | 2.15 ± 0.27 | 46.92 ± 5.39 | 48.19± 0.37 | 76.20 ± 0.16 c |
| 3 | 1.99 ± 0.06 | 1.28 ± 0.04 | 2.15 ± 0.10 | 46.63 ± 2.03 | 47.83± 0.81 | 76.17 ± 0.30 c |
| 4 | 2.02 ± 0.12 | 1.29 ± 0.08 | 2.14 ± 0.31 | 47.38 ± 6.39 | 47.92± 0.00 | 77.45 ± 0.23 b |
| 5 | 2.03 ± 0.21 | 1.30 ± 0.13 | 2.16 ± 0.42 | 47.25 ± 8.31 | 48.72± 0.37 | 78.04 ± 0.27 b |
| 6 | 1.92 ± 0.06 | 1.23 ± 0.04 | 2.16 ± 0.17 | 46.53 ± 3.83 | 48.18± 1.14 | 77.38 ± 0.45 b |
| 7 | 1.91 ± 0.02 | 1.23 ± 0.01 | 2.23 ± 0.15 | 44.92 ± 2.90 | 48.19± 0.37 | 82.84 ± 0.60 a |
| p-value | 0.757 | 0.758 | 0.998 | 0.993 | 0.687 | 0.000 |
| Means overall | 1.96 ± 0.10 | 1.26 ± 0.07 | 2.17 ± 0.21 | 46.39 ± 4.31 | 48.09 ± 0.55 | 77.61 ± 2.41 |
| Diet | Initial Total Length (cm) | Final Total Length (cm) | CF | HSI (%) | VSI (%) |
|---|---|---|---|---|---|
| 1 | 9.73 ± 0.80 | 14.69 ± 0.75 b | 1.60 ± 1.60 | 1.67 ± 0.34 | 7.48 ± 1.52 |
| 2 | 9.84 ± 0.65. | 15.30 ± 0.59 ab | 1.57 ± 0.19 | 1.63 ± 0.43 | 6.50 ± 1.29 |
| 3 | 9.40 ± 0.89 | 15.55 ± 0.72 a | 1.60 ± 0.06 | 1.68 ± 0.57 | 6.33 ± 1.15 |
| 4 | 9.71 ± 1.03 | 14.75 ± 0.74 b | 1.54 ± 0.08 | 1.39 ± 0.30 | 8.01 ± 2.28 |
| 5 | 9.51 ± 0.87 | 15.17 ± 0.88 ab | 1.60 ± 0.09 | 1.62 ± 0.38 | 6.10 ± 1.20 |
| 6 | 9.55 ± 0.78 | 14.69 ± 0.52 b | 1.61 ± 0.10 | 1.79 ± 0.50 | 7.23 ± 1.74 |
| 7 | 9.70 ± 0.60 | 14.91 ± 0.99 b | 1.62 ± 0.11 | 1.69 ± 0.48 | 6.67 ± 1.63 |
| p-value | 0.785 | 0.011 | 0.576 | 0.330 | 0.137 |
| Means overall | 9.63 ± 0.80 | 15.01 ± 0.80 | 1.59 ± 0.11 | 1.64 ± 0.44 | 6.90 ± 1.64 |
| Diet | RBC | Hb | HCT | MCV | MCH | MCHC | RDW-CV | PLT |
|---|---|---|---|---|---|---|---|---|
| 106/µL | g/dL | % | fl | pg | g/dL | % | 103/µL | |
| 1 | 1.46 ± 0.33 | 5.18 ± 1.37 | 23.24 ± 6.33 | 158.42 ± 19.69 | 35.36 ± 3.22 | 22.44 ± 1.53 | 12.41 ± 3.56 | 34.75 ± 43.05 |
| 2 | 0.77 ± 0.42 | 3.88 ± 2.42 | 12.90 ± 7.89 | 159.40 ± 22.48 | 48.82 ± 20.39 | 29.98 ± 9.14 | 10.45 ± 1.34 | 26.00 ± 4.18 |
| 3 | 1.29 ± 0.29 | 4.89 ± 1.29 | 20.17 ± 3.75 | 157.94 ± 18.11 | 38.23 ± 8.99 | 24.00 ± 3.10 | 11.68 ± 3.49 | 17.00 ± 12.73 |
| 4 | 1.23 ± 0.43 | 4.61 ± 1.57 | 20.03 ± 7.05 | 163.67 ± 21.82 | 37.95 ± 5.71 | 23.16 ± 0.75 | 14.30 ± 4.26 | 33.22 ± 37.68 |
| 5 | 1.01 ± 0.68 | 4.11 ± 1.92 | 16.10 ± 9.54 | 167.81 ± 19.46 | 48.93 ± 19.99 | 28.56 ± 8.95 | 16.97 ± 7.31 | 25.64 ± 25.49 |
| 6 | 1.24 ± 0.63 | 5.89 ± 2.70 | 22.40 ± 12.86 | 174.06 ± 23.98 | 48.01 ± 11.76 | 27.68 ± 6.04 | 15.01 ± 2.96 | 22.11 ± 11.50 |
| 7 | 1.07 ± 0.63 | 4.34 ± 2.58 | 17.03 ± 10.96 | 157.64 ± 20.84 | 43.13 ± 16.48 | 27.04 ± 8.23 | 11.17 ± 2.20 | 34.75 ± 34.34 |
| p-value | 0.340 | 0.547 | 0.378 | 0.639 | 0.236 | 0.171 | 1.69 | 0.85 |
| Means overall | 1.18 ± 0.52 | 4.78 ± 2.04 | 19.28 ± 9.09 | 163.11 ± 20.70 | 42.65 ± 13.52 | 25.92 ± 6.28 | 13.20 ± 3.88 | 27.97 ± 28.00 |
| Diet | WBC | Neutrophil | Lymphocyte | Monocyte |
|---|---|---|---|---|
| 103/µL | % | % | % | |
| 1 | 3.59 ± 1.36 | 34.00 ± 26.25 | 50.78 ± 26.88 | 14.33 ± 15.51 |
| 2 | 3.05 ± 1.04 | 47.40 ± 24.94 | 36.80 ± 23.95 | 13.80 ± 5.45 |
| 3 | 3.58 ± 1.01 | 39.14 ± 19.34 | 47.00 ± 21.49 | 13.43 ± 9.52 |
| 4 | 3.87 ± 1.28 | 24.90 ± 13.64 | 63.03 ± 22.10 | 11.09 ± 12.49 |
| 5 | 4.25 ± 0.96 | 22.29 ± 18.95 | 63.00 ± 17.26 | 13.00 ± 8.87 |
| 6 | 3.59 ± 2.02 | 47.22 ± 33.32 | 40.33 ± 24.96 | 11.33 ± 10.91 |
| 7 | 3.69 ± 0.92 | 39.88 ± 21.57 | 45.25 ± 28.93 | 13.13 ± 10.43 |
| p-value | 0.838 | 0.261 | 0.260 | 0.996 |
| Means overall | 3.68 ± 1.28 | 35.95 ± 23.97 | 50.06 ± 24.59 | 12.77 ± 10.72 |
| Skin Parameters | Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | Diet 7 |
|---|---|---|---|---|---|---|---|
| SMLA | 0.99 ± 0.04 c | 1.45 ± 0.05 b | 1.86 ± 0.07 b | 2.59 ± 0.12 a | 2.35 ± 0.09 a | 1.55 ± 0.21 b | 1.76 ± 0.13 b |
| SMPA | 0.09 ± 0.006 c | 0.12 ± 0.002 b | 0.13 ± 0.007 b | 0.18 ± 0.005 a | 0.17 ± 0.003 a | 0.12 ± 0.002 b | 0.11 ± 0.005 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Doan, H.V.; Paolucci, M. Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis niloticus. Animals 2021, 11, 193. https://doi.org/10.3390/ani11010193
Tippayadara N, Dawood MAO, Krutmuang P, Hoseinifar SH, Doan HV, Paolucci M. Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis niloticus. Animals. 2021; 11(1):193. https://doi.org/10.3390/ani11010193
Chicago/Turabian StyleTippayadara, Nisarat, Mahmoud A. O. Dawood, Patcharin Krutmuang, Seyed Hosseini Hoseinifar, Hien Van Doan, and Marina Paolucci. 2021. "Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis niloticus" Animals 11, no. 1: 193. https://doi.org/10.3390/ani11010193
APA StyleTippayadara, N., Dawood, M. A. O., Krutmuang, P., Hoseinifar, S. H., Doan, H. V., & Paolucci, M. (2021). Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis niloticus. Animals, 11(1), 193. https://doi.org/10.3390/ani11010193










