1. Introduction
Acipenser stellatus (Pallas 1771), also called stellate sturgeon, belongs to an ancient group of fishes that appeared in the Jurassic period. This sturgeon species lives and eats in Black Sea and migrates in the Danube River in order to reproduce [
1]. The stellate sturgeon possesses an outstanding scientific value because it remarkably survived two mass extinctions and several Ice Ages, being considered a “living fossil”. Moreover,
A. stellatus has a high economical value due to its roe that is intensively used in the caviar industry. Unfortunately, because of its great economical value, the stellate sturgeon has been massively captured. Overfishing, alongside the construction of the Iron Gates Dams over the Danube River that impaired the upstream migration and spawning of
A. stellatus, has led to an alarming decline of this sturgeon population in the Black Sea [
2]. As a consequence,
A. stellatus became extinct in the Upper and Middle Danube and it is considered critically endangered in the Lower Danube River [
3,
4]. Therefore, stellate sturgeon is intensively raised in fish farms, the individuals bred in aquaculture being used either in restocking programs or for the production of caviar, aiming to discourage poaching and overfishing of wild individuals.
Because a large part of sturgeon farming is devoted to caviar production, long production cycle technology is applied. In order to keep the activity within sustainability limits, fish farmers aim to optimize the feeding management and, in this pursuit, they are currently trying to introduce food deprivation periods in the feeding practice. This is performed for multiple reasons. First of all, this would increase the profitability of fish farming, given that feeding represents at least 50% of the production cost [
5]. This represents the highest cost in an intensive aquaculture system [
6]. Secondly, food deprivation is sometimes performed in fish farming in order either to reduce water pollution and to decrease mortality caused by disease outbreaks [
7] or to improve preservation of fish stock before marketing and slaughtering [
8].
Fishes alternate fasting with feeding periods in their natural existence because of seasonal variations in food availability from natural habitats and due to reproduction and migration habits; therefore, fishes are well adapted to starvation [
9,
10]. They adopt different strategies in order to survive starvation periods; precisely they reduce the energetic demands either by decreasing the mass of the tissues with high turnover rates or by lowering the metabolic rates [
11]. Based on the idea that fishes are adapted to food deprivation in natural environments, several studies have been conducted to assess if a regime based on a starvation period followed by refeeding affects the growth performance and the welfare of the fishes reared in aquaculture conditions.
Morphometric, hematological, biochemical, metabolic parameters and oxidative stress biomarkers were determined mainly in fish juveniles, but also in adults subjected to different starvation/refeeding regimes. Siberian sturgeon (
Acipenser baerii) [
12], Persian sturgeon (
Acipenser persicus) [
13] and Chinese sturgeon (
Acipenser sinensis) [
14] presented a complete compensatory growth as a response to short-term starvation, while
A. sinensis [
14] and
A. persicus [
13] showed a partial compensation when subjected to long-term starvation periods. The mechanism of compensatory growth in animals has been described as involving the growth hormone, insulin and insulin-like growth factor [
15]. However, beluga (
Huso huso) did not present a complete catch-up growth when subjected to different starvation/refeeding regimes, although the growth rate of the individuals was high [
16]. In contrast to the above mentioned species, other fish species, such as channel catfish (
Ictalurus punctatus) [
17] and red porgy (
Pagrus pagrus) [
7] failed to present a compensatory growth response when subjected to different starvation/refeeding regimes.
Adaptive responses to short or long-term starvation regarding hematological, biochemical or metabolic parameters were found in
A. baerii [
18], Adriatic sturgeon (
Acipenser naccarii) and rainbow trout (
Oncorhynchus mykiss) [
19], European eel (
Anguilla anguilla) [
20], tench (
Tinca tinca) [
21], common dentex (
Dentex dentex) [
22] and European sea bass (
Dicentrarchus labrax) [
23].
Moreover, starvation/refeeding regimes were proved to enhance cell protective mechanisms, such as antioxidant defense mechanisms and heat shock protein (Hsp) expression in
D. labrax [
24]. Even though the reported results differ amongst species, it was mostly observed that fishes do present a metabolic adjustment to minimize the energy expenditure during starvation and an adaptive response to cope with oxidative stress that may be induced by a starvation/refeeding regime.
In this context, the present study aimed to determine if
A. stellatus juveniles raised in aquaculture have the ability to adapt to a starvation/refeeding regime by assessing the effects of this alternative type of feeding on oxidative stress biomarkers and antioxidant defense mechanisms in the intestine. The specific activities of the major antioxidant enzymes were analyzed, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione S-transferase (GST) and glucose 6-phosphate dehydrogenase (G6PDH). Additionally, the content of reduced glutathione (GSH) was determined alongside the level of several oxidative stress biomarkers: malondialdehyde (MDA), protein thiol groups (PTG), advanced oxidation protein products (AOPP) and protein carbonyl groups (PCG). The present work extends a previous study conducted by our research group on stellate sturgeon liver regarding the adaptability of this species to a starvation/refeeding regime [
25]. There is a strong necessity to expand the study on other organs in order to assess the real adaptability of this species to a starvation/refeeding regime. No other similar studies were conducted before on
A. stellatus individuals, so the results regarding the adaptability of stellate sturgeon individuals to starvation and refeeding are novel and expand the knowledge of stellate sturgeon physiology. Moreover, the oxidative stress biomarkers from the small intestine of sturgeon were not studied before under food deprivation conditions. This study is of great interest for the aquaculture field given that the results represent a starting point in optimizing the feeding regime of
A. stellatus juveniles from fish farms. A regime based on starvation and refeeding periods could decrease the costs of raising juveniles and eventually increase the profitability of fish farms, stimulating aquaculture practice. In the long run, this could enhance the efforts to conserve
A. stellatus, and therefore, this study is important from both economical and conservation perspectives.
As it will be seen further on, the study concluded that several antioxidant defense mechanisms were enhanced by the starvation/refeeding regimes in the intestine of A. stellatus juveniles; overall, proteins were spared by the oxidative damage. Lipid peroxidation was induced significantly only in the intestine of the juveniles subjected to a longer starvation period. A. stellatus possess a potential to adapt to a starvation/refeeding regime, the 7-day starvation period followed by 21 days of refeeding being better tolerated than the 14-day starvation period followed by 21 days of refeeding. This alternative type of feeding is worth to be further researched and optimized so that it could be eventually applied in stellate sturgeon fish farming. As mentioned above, the results presented in this study must be regarded as a starting point in optimizing the feeding regime of A. stellatus juveniles due to several limitations we confronted with. Precisely, the number of individuals available for performing the study was relatively low. We experienced the problem of assessing numerically small groups of individuals due to the fact that A. stellatus is a critically endangered species. Moreover, the high costs of raising stellate sturgeon juveniles in fish farms and the difficulty of attaining a successful reproduction of sexually mature individuals made even harder to obtain and study a high number of juveniles. Therefore, for both ethical and economical purposes the number of the juveniles was reduced as much as it was scientifically possible. As a consequence the study presents two issues. Firstly, the treatments were not replicated and the fact that the individuals subjected to a particular treatment were reared together raises the issue of pseudo replication. Secondly, fish in different treatments were sampled on different days and there was no control group for each sampling point. We were obliged to use only one control group due to the limited number of fish. By highlighting these critical aspects we underline the preliminary nature of the data presented in the study.
4. Discussion
Free radicals, such as reactive oxygen species (ROS), are generated in physiological conditions, being by-products of the aerobic cellular metabolism [
37,
38]. ROS are highly reactive molecules that contain an impaired electron, and therefore they have a major oxidant effect [
37,
38]. There are several enzymatic and non-enzymatic antioxidants that neutralize ROS, either by direct reduction of ROS or by repairing the ROS-mediated damage. An imbalance between the production of ROS and the antioxidant defense mechanisms leads to oxidative stress [
37,
38,
39]. This consists of high level of ROS that was not counteracted by the antioxidant mechanisms and in turn, causes oxidative damage to proteins, lipids and other biomolecules. CAT and SOD are two major antioxidant enzymes that represent a primary defense mechanism against ROS. SOD catalyzes the dismutation of O
2− into H
2O
2, which is further decomposed in the reaction catalyzed by CAT into water and oxygen, preventing formation of the hydroxyl ion. The activities of these enzymes are considered biomarkers for oxidative stress [
37,
38,
39,
40].
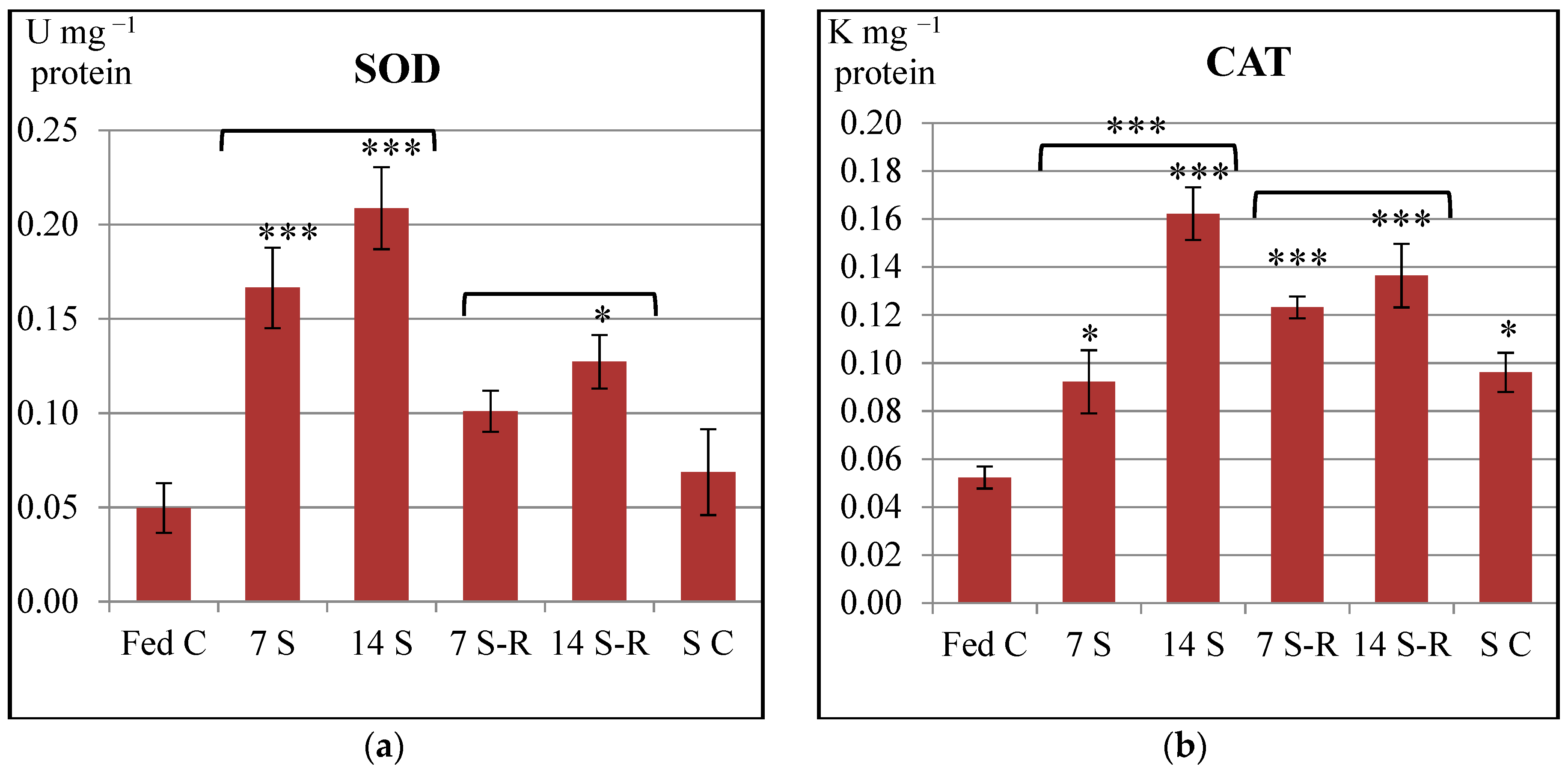
In our study, SOD and CAT specific activities were increased in the intestine of stellate sturgeon juveniles subjected to both 7-day and 14-day starvation periods in comparison to constantly fed juveniles. Therefore, starvation induced an overproduction of ROS, precisely O
2− and H
2O
2 that triggered an enhancement of both SOD and CAT activities in the intestine. Similar to our results, both SOD and CAT activities increased in the intestine of one year old
Dicentrarchus labrax (European sea bass) starved for three weeks [
24], in the liver of sexually immature
Dentex dentex (common dentex) starved for five weeks [
41] as well as in the liver and gills of
Salmo trutta (brown trout) starved for 49 days [
42]. Additionally, CAT activity was reported to increase in the liver of
Gadus morhua (Atlantic cod) after a 12-week starvation period [
43].
On one hand, SOD activity increased, while CAT activity decreased in the liver of sexually immature
Sparus aurata (gilthead sea bream) subjected to partial or total food deprivation for 46 days [
44]. On the other hand, SOD activity was not affected by starvation, while CAT activity increased in the blood of
Mesopotamichthys sharpeyi fingerlings starved for 16 days [
45]. In contrast to our results, both SOD and CAT activities decreased in the liver of one year old
Acipenser naccarii (Adriatic sturgeon) and
Oncorhynchus mykiss (rainbow trout) starved for 72 days [
46].
Further on, our study demonstrated a tendency of returning the enzymatic activity of SOD to a control level during refeeding. Eventually, SOD activity reached the control level during 7/21-day starvation/refeeding regime. However, the enzymatic activities of both SOD and CAT remained still higher than the control level during 14/21-day starvation/refeeding regime. Therefore, it might be considered that the production level of ROS observed after refeeding could have been diminished in comparison to the level induced by starvation, but remained higher than the physiological ROS level produced by constant feeding.
In accordance to our results, SOD and CAT activities remained still higher than the control level in the intestine of
Dicentrarchus labrax refed for two weeks [
24] and in the liver and gills of
Salmo trutta refed for 21 days [
42].
In contrast to our results, SOD and CAT activities were recovered and returned to control values after refeeding in the liver of
Dentex dentex [
41], in the blood of
Mesopotamichthys sharpeyi fingerlings [
45] and in the liver of
Sparus aurata [
44]. Additionally, SOD activity was recovered after two months of refeeding in the liver of
Acipenser naccarii and
Oncorhynchus mykiss, while CAT activity was recovered in the latter [
46].
GPx catalyzes peroxides removal by oxidizing GSH, while GR recycles the resulting oxidized glutathione (GSSG) by reducing the disulfide bond, thus producing two molecules of GSH. GPx substrates are both lipid peroxides and H
2O
2, the latter being also removed by CAT [
38,
39,
47].
GSH represents an important non-enzymatic cellular antioxidant. It is a tripeptide involved not only in the GPx mediated removal of lipid peroxides and H
2O
2, but also in the reduction of oxidized thiol groups of proteins [
47]. It is also used by GST to conjugate electrophilic compounds in order to render less chemically active compounds and to ensure their clearance from the organism [
48].
In our study, GPx and GR specific activities increased in a time dependent manner in the intestine of starved
Acipenser stellatus juveniles, suggesting that starvation induced peroxides. The increase of GPx activity resulted in a slightly decreased MDA level after 7-day starvation and only a statistically insignificant increase of MDA level was induced after 14-day starvation when compared to constant feeding. Therefore, GPx might have been involved solely in the detoxification of MDA, whereas H
2O
2, the other substrate of GPx might have been removed entirely in the reaction catalyzed by CAT, due to the fact that CAT has a higher value of K
M constant for H
2O
2 compared to GPx [
49]. Therefore, the GPx mediated removal of peroxides and the recycling of glutathione catalyzed by GR were two enzymatic defense mechanisms triggered in the intestine of stellate sturgeon in response to the oxidative stress induced by starvation. Similar to our results, both GPx and GR activities were increased by starvation in the liver of
Salmo trutta [
42] and of
Sparus aurata [
44]. Additionally, GPx activity was enhanced after starvation in the liver of
Gadus morhua [
43] and the blood of
Mesopotamichthys sharpeyi fingerlings [
45]. Furthermore, it was reported an increase of GPx activity and a decrease of GR one in the liver of starved
Dentex dentex [
41]. In contrast to our results, GPx was not affected at all by starvation in the intestine of
Dicentrarchus labrax [
24] and both GPx and GR activities were decreased in the liver of
Acipenser naccarii and
Oncorhynchus mykiss [
46].
In our study, GPx activity returned to control level after refeeding, suggesting that a reduction of peroxides took place during refeeding. Interestingly, GR activity remained higher after refeeding, even though GPx activity was diminished. This could be explained in the perspective that GR was involved in GSH recycling, which in turn acted on its own as a reducing agent. Precisely, GSH was involved in the reduction of the oxidized protein thiol groups. As a consequence, the redox balance of proteins was effectively maintained. This observation is demonstrated by the content of protein thiol groups. The level of GSH was decreased, while PTG was increased at the end of 14-day starvation period compared to constant feeding. And eventually, both GSH, and PTG levels were similar to control levels after subsequent refeeding.
In agreement to our results, GPx activity was recovered and GR continued to be increased after refeeding in the gills of
Salmo trutta [
42]. In addition, GPx activity returned to control level during refeeding in the liver of
Dentex dentex [
41] and the blood of
Mesopotamichthys sharpeyi fingerlings [
45], and GR activity was increased after refeeding in the liver of
Sparus aurata [
44].
In contrast to our results, GPx activity was either decreased in the liver of refed
Sparus aurata [
44] or increased in the intestine of refed
Dicentrarchus labrax [
24] and in the liver of refed
Salmo trutta [
42]. Additionally, GR activity returned to control value in the liver of refed
Dentex dentex [
41].
Further on, it was observed that the high activity of GPx observed during 7-day starvation did not affect the GSH level which remained similar to the control level. It seems that the increase of GR activity observed during 7-day starvation was sufficient to reduce the oxidized glutathione produced by GPx and to supply GSH. Refeeding after 7-day starvation maintained a high activity of GR despite the fact that the activity of GPx returned to control level. This contributed to the accumulation of GSH in the intestine of refed juveniles. Therefore, the 7-day starvation did not affect the GSH-GSSG balance and the subsequent refeeding increased the level of GSH.
The 14-day starvation induced a major enhancement of both GPx and GR activities and a strong diminish of GSH level. Hence, GSH utilization by GPx was strongly enhanced and GR did not manage to maintain a balance between GSH production and its utilization. However, refeeding after 14-day starvation maintained a high activity of GR despite the fact that the activity of GPx returned to control level. This allowed GSH level to reach the control level.
Therefore, 14/21-day starvation/refeeding regime induced a greater mobilization of GSH stock in the intestine of A.stellatus juveniles than 7/21-day starvation/refeeding one.
GST activity was severely reduced by starvation, while subsequent refeeding induced an even greater decrease in the intestine of
Acipenser stellatus juveniles. It was reported that starvation usually enhances the innate enzymatic capacity of detoxification by up-regulating the expression of genes that are related to antioxidant mechanisms, especially GST [
50]. In starved individuals, GPx activity was highly increased compared to constantly fed ones and GSH reserves might have been used with priority as cofactor for GPx than for GST enzyme during starvation. Therefore, it might have been thought that starvation negatively impacted the enzymatic capacity of cellular detoxification mediated by GST. However, even though GPx activity decreased and returned to fed control level after refeeding, GSH content increased instead of being used by GST. So, despite GSH availability, GST activity decreased even more after refeeding compared to fed control. Therefore, we concluded that the diminished GST activity observed during starvation/refeeding regimes did not reflect an incapacity of cellular enzymatic detoxification, but rather a diminished level of electrophilic compounds and that GST had a minor role in the cellular detoxification, whereas GPx was involved in MDA detoxification.
Similar to our results, GST activity decreased after a 49-day starvation period in the liver of
Salmo trutta, however, in contrast to our results the activity was recovered after refeeding [
42]. In contrast to our results, GST activity raised in the liver of starved
Gadus morhua [
43]. Another study reported that GST activity initially increased after a 7-day starvation period and decreased afterwards at the end of a 28-day starvation period in the liver of
Sparus aurata. Finally, after refeeding GST activity increased and reached a higher level than control values [
44].
G6PDH is involved in pentose-phosphate pathway, an alternative metabolic way of glucose oxidation that results in synthesis of NADPH, which is further used by GR to recycle GSSG into GSH when oxidative stress is induced. Therefore, pentose-phosphate shunt is proposed to be the major NADPH source required for the antioxidant defense mechanisms [
51]. In our study, G6PDH specific activity was not affected by either starvation or refeeding in the intestine of
A. stellatus. The activity of G6PDH was increased after the 14-day starvation period in comparison to 7-day starvation suggesting that the pentose-phosphate shunt was intensified during the 14-day starvation, probably due to induction of gluconeogenesis. Therefore, it is likely that the production of glucose through gluconeogenesis maintained an active pentose-phosphate shunt and thus, G6PDH activity might have been unaffected by glucose deprivation even during 14/21-day starvation/refeeding regime. NADPH, which is produced during pentose-phosphate shunt, was most probably used by GR in the reaction of GSH recycling. This is reflected by the significant enhancement of GR activity during 14/21-day starvation/refeeding regime.
In contrast to our results, G6PDH activity was decreased after starvation and recovered after refeeding in the liver of
Dentex dentex [
41] and the liver and gills of
Salmo trutta [
42].
To conclude, the 7/21-day starvation/refeeding regime did not influence pentose-phosphate shunt in a significant manner, while the 14/21-day starvation/refeeding regime probably induced gluconeogenesis, which sustained this metabolic pathway. Hence, the intestine of stellate sturgeon was able to metabolically adapt to both starvation/refeeding regimes, but the 7/21-day starvation/refeeding regime was better tolerated.
MDA is an iconic biomarker of lipid peroxidation that is produced after ROS mediated oxidation of the polyunsaturated fatty acids (PUFA). The long chains of PUFA are prone to sequential oxidations that could lead to cleavage and render small end products, such as MDA [
37,
48]. The content of MDA suggests that both starvation/refeeding regimes induced oxidative stress that led to lipid peroxidation. Even though starvation did not induce a great amount of MDA level compared to constant feeding, refeeding induced a higher MDA level than constant feeding. Therefore, the lipid peroxidation was provoked by refeeding. Interestingly, SOD and CAT enzymes presented a highly increased level of activity during refeeding compared to that observed during constant feeding. High levels of SOD and CAT activities should have been prevented the formation of hydroxyl ion which is responsible for lipid peroxidation, and therefore, the lipid peroxidation level should have been maintained to a low value.
Even though the antioxidant defense mechanisms were enhanced, some ROS managed to escape the scavenging enzymatic systems and induced peroxidation of lipids from the intestine of
Acipenser stellatus juveniles. The metabolic pathways leading to ATP generation such as the mitochondrial electron transport chain might have been diminished during starvation as an adaptive response to the lack of food. The ATP generation might have been enhanced by refeeding in order to compensate for the previous reduction; therefore, a higher amount of ROS might have been induced during refeeding, leading to lipid peroxidation. The intestine of stellate sturgeon juveniles seems to be more prone to lipid peroxidation mediated by ROS that were induced during starvation/refeeding regimes than the liver subjected to the same regimes. The MDA level initially decreased after starvation periods and recovered after refeeding periods in the liver of stellate sturgeon [
25].
In agreement to our results observed for the intestine of stellate sturgeon, the MDA level was increased during starvation and remained increased after refeeding in the liver and blood of
Acipenser naccarii and
Oncorhynchus mykiss [
46] and the liver and gills of
Salmo trutta [
42]. In contrast to our results, even though starvation induced a high level of MDA, the level was recovered and returned to control values after refeeding in the liver of
Dentex dentex [
41], the blood of
Mesopotamichthys sharpeyi [
45] and the liver of
Sparus aurata [
44].
The oxidation of protein thiol groups represents a reversible protein modification; the disulfide bonds of oxidized protein can be reduced by GSH compound, and therefore the redox status of the proteins can be restored [
52]. PCG and AOPP represent hallmarks of the oxidative stress and are irreversible changes in the proteins [
37,
53]. PCG consists of aldehydes and ketones that are produced during interaction between the hydroxyl ions and amino acids, such as lysine, arginine, proline and threonine [
54]. AOPP are considered to be cross-linked products of proteins that contain dityrosine [
53]. Additionally, they could be small products resulting from cleavage of a long polypeptidic chain due to protein oxidation mediated by the hypochlorous acid [
52,
55]. In our study, the oxidation of protein was induced during starvation periods, as reflected by both AOPP and PCG levels. However, refeeding reduced the carbonylation level that returned to the control one, and decreased the AOPP level that was slightly higher than control level. The oxidized thiol groups of proteins were reduced by GSH during starvation/refeeding regimes, and therefore the redox balance of protein was sustained by the antioxidant defense mechanisms.
The present work was conducted on the intestine of stellate sturgeon juveniles and therefore, it is hard to properly interpret the results of our study in the perspective of previous studies because most of them were conducted on other organs, mainly liver and blood. As it can be seen, studies reported various results regarding the effects of starvation/refeeding regimes on oxidative stress biomarkers in fish because the species and the age of the individuals, the periods of starvation and refeeding and the methods used are highly different.
Overall, our study proved that starvation/refeeding regimes enhanced the major antioxidant enzymatic systems in the intestine of stellate sturgeon juveniles raised in aquaculture, precisely the 7/21-day starvation/refeeding regime enhanced CAT and GR activities, while the 14/21-day starvation/refeeding regime enhanced SOD, CAT and GR activities. Only, GPx activity returned to control level in both starvation/refeeding regimes. Generally, starvation periods induced higher enzymatic activities than refeeding periods. Therefore, ROS were induced during starvation/refeeding regimes and antioxidant defense mechanisms were activated as an adaptive response. Similarly, the induction of antioxidant cell protective mechanisms is regarded as an “adaptation strategy related to the fact that many fish experience periods of starvation as part of their natural life cycle” [
24]. Moreover, only GST activity was significantly reduced both by starvation and refeeding, suggesting that this antioxidant enzyme was not important in the detoxification of electrophilic compounds. Unfortunately, even though both SOD-CAT and GPx-GR antioxidant defense mechanisms were activated during starvation and the activities of several enzymes remained high after refeeding (e.g., CAT and GR), a certain amount of ROS managed to escape the enzymatic scavenging systems and affected some biomolecules. Lipids were more susceptible to ROS mediated oxidation than proteins. The lipid peroxidation did not return to control level after refeeding, while protein oxidation biomarkers almost reached the control levels or were similar to them after refeeding. Therefore, the starvation period might have been too long or the refeeding period might have been too short to allow a complete recovery of the oxidative stress biomarkers and antioxidant enzymes in the intestine of stellate sturgeon. Similar to our results, it was reported an increase of lipid peroxidation level and of all antioxidant enzymes excepting GST in the liver of starved
Salmo trutta, and the increase trend was still observed at the end of a 21-day refeeding period. Therefore, in agreement to our results observed for the intestine of
Acipenser stellatus, total food deprivation induced oxidative stress and the effects of food restriction did not disappear even after a 21-day refeeding period in
Salmo trutta liver [
42]. Furthermore, starvation induced oxidative stress reflected as an increase of lipid peroxidation level in the liver of
Acipenser naccarii and
Oncorhynchus mykiss. This modification was accompanied by a decreasing trend in the enzymatic activities of CAT, SOD, GPx and GR. The stress was not removed after subsequent refeeding, thus the liver of these two fish species showing an “incapacity to meet the stress situation provoked by 72 days of starvation, leading to the oxidation of the biomolecules” [
46]. Additionally, it was reported an enhancement of the total antioxidant capacity of the blood in
Acipenser sinensis in the first 19 days of starvation and after that period a reduction was observed [
56].
In contrast to our results, lipid peroxidation level indicated that the oxidative stress disappeared and the antioxidant enzymatic activities returned to control values after refeeding thus showing a “compensatory capacity” in
Dentex dentex [
41],
Mesopotamichthys sharpeyi [
45] and
Sparus aurata [
44].
In addition, the present work extends a previous study conducted by our research group on stellate sturgeon liver regarding the adaptability of this species to a starvation/refeeding regime. Our previous data had shown that
A. stellatus juveniles subjected to 7/21-day starvation/refeeding regime presented a complete compensatory growth and were able to efficiently counteract the oxidative stress by enhancing activities of the antioxidant enzymes in their liver [
25]. Only the 7 S-R group presented a complete recovery of the weight loss during refeeding, as reflected by the final weight, weight gain and specific growth rate. All of the morphometric parameters suggested that 7 S-R group reached the same weight of the fed control and that the 7/21-day starvation/refeeding regime did not affect the growth performance of
A. stellatus juveniles [
25]. Additionally, ROS were induced only after 14 days of starvation, leading to enhanced activity of antioxidant enzymes such as: CAT, GR and GST in the liver of
A. stellatus. However, ROS were entirely neutralized by the antioxidant enzymes and even more, since the lipid peroxidation decreased and proteins were spared from oxidation during starvation [
25]. Refeeding induced ROS to a greater extent than starvation and gradually activated almost all antioxidant enzymes in the liver, so the lipid peroxidation increased, but did not surpass the control level, while protein oxidation occurred only when juveniles were refed after 14-day starvation [
25].
In the present study, it was observed that 7/21-day starvation/refeeding regime induced oxidative stress to a smaller extent in the intestine of stellate sturgeon than 14/21-day starvation/refeeding regime did. Based on our previous data and on the fact that the antioxidant enzymatic mechanisms were enhanced and the fact that the raise of lipid peroxidation level was statistically insignificant in the intestine of the juveniles subjected to 7/21-day starvation/refeeding regime, we concluded that this alternative feeding regime is worth to be further researched and optimized. Therefore, Acipenser stellatus juveniles possess a potential to adapt to a short starvation period introduced in the feeding schedule.
Moreover,
Acipenser stellatus juveniles might be able to adapt to long-term starvation. A peculiar observation regarding our data is that the level of some oxidative stress biomarkers (SOD, GSH, MDA, PTG, PCG and AOPP) was not statistically different between the fed and starved controls. These findings were also observed and reported in our previous study conducted on stellate sturgeon liver [
25]. These results could be interpreted as an adaptation of the juveniles to long-term starvation. Mature
A. stellatus individuals possess the ability to cope with starvation in the wild. These are capable to survive months without feeding during the migration and reproductive seasons because they accumulate energy stores during feeding season [
1]. Additionally, fish alternate feeding with starvation periods because of seasonal variations in temperature and food availability [
10]. This observation has not been made before for juveniles that need energy resources for growth and development, and that are supposed to present a continuous feeding behavior. However, wild juveniles might experience short periods of food deprivation because of the food availability, so they might not eat on a daily basis. Therefore,
A. stellatus juveniles might possess an innate capacity to cope with starvation for longer periods.
Our study must be regarded as a starting point in optimizing stellate sturgeon feeding in aquaculture due to some limitations we confronted with. One limitation of our study consists in large values of standard deviation and standard error of the mean due to a high degree of variability amongst individuals. It seems that adaptive responses to starvation/refeeding regimes differ to a high degree between individuals. Unfortunately, there was no possibility to raise the number of individuals per group due to the necessity to protect the species which is on the verge of extinction. In addition, it is very expensive to raise stellate sturgeon juveniles in aquaculture and it is very hard to successfully reproduce sexually mature individuals. Due to the need to spare sacrification of large number of individuals and to reduce the number of animals used for ethical purpose we lowered the number of individuals as much as it was scientifically possible and used only one control group for all treatments. In consequence, the experiment was not repeated to validate the results. Therefore the issue of pseudo replication and the issue of using a unique control group represent the second limitation of the study. This study has to be extended if possible and it represents, as mentioned above, a starting point in optimizing the feeding practice of Acipenser stellatus.
One future research direction of our study consists in analyzing Hsp stress response to starvation/refeeding regimes. Additionally, it is our desire to extend the study on other organs. Furthermore, it is highly important to conduct this experiment in such a manner that it could test the ability of stellate sturgeon to adapt to long-term applied starvation/refeeding regimes.
A starvation/refeeding regime has certain economical and scientific benefits. If applied in aquaculture, such a regime could lower the costs of raising juveniles and enhance the profitability of fish farms without harming the juveniles. In the long term, an enhanced profitability might lead to an increased number of fish farms, which could stimulate the conservation of the stellate sturgeon. Therefore, a starvation/refeeding regime represents a strategy aimed to improve the sustainability of fish farming. This alternative type of fish feeding is worth taking into consideration for both the socio-economical implications and conservation of the stellate sturgeon.